Introduction
The Prochilodus genus comprises 13 described species (Reis et al., Reference Reis, Kullander and Ferraris2003), occurring in nearly every major South American watershed (Castro & Vari, Reference Castro and Vari2004). All species of the genus Prochilodus are iteroparous and exhibit a single seasonal spawning event, high fecundity, external fertilization and an absence of parental care (Lowe-McConnell, Reference Lowe-McConnell1999). The grumatã (Prochilodus vimboides Kner 1859) occurs between the Jucuru and Paraíba do Sul Rivers, the Uruguai River and the headwaters of both the tributaries of the upper Paraná river and the São Francisco River (Castro & Vari, Reference Castro and Vari2004). The conservation status of P. vimboides is vulnerable despite showing a wide geographic distribution. To date, there is no available ecological information regarding the initial and adult forms of the species. Studying early life stages is key for successful conservation programs, and morphological description is an important tool for distinguishing congeneric or sympatric species (Oliveira et al., Reference Oliveira, Bialetzki, Gomes, Santim and Taguti2012). The great morphological similarity amongst congeneric species in early life stages (Snyder, Reference Snyder1981) and the lack of taxonomic keys are obstacles to the identification of material collected in the natural environment (Bialetzki et al., Reference Bialetzki, Sanches, Baumgartner and Nakatani1998). Ahlstrom & Moser (Reference Ahlstrom and Moser1976) highlighted the importance of biological collections of ichthyoplankton because they enable the determination of life history characteristics, linking larval history to the adult phase. Such links are made mainly through meristic characters, also using a number of osteological characters, morphometric characters and pigmentation patterns. The species indicated above is an ecologically relevant species with a delicate population status. Thus, this study aims to describe the initial ontogeny of P. vimboides using morphological and meristic characters of the eggs and larvae and to determine allometric patterns of larval growth.
Material and methods
The breeding individuals of P. vimboides used to obtain egg and larvae samples were derived from the exsitu bank of endangered species from the Paraíba do Sul River watershed maintained by the non-governmental organisation the Piabanha Project, at Itaocara/Rio de Janeiro/Brazil (21°38′6.33′′S 42°1′59.25′′W). The breeding stock for this species was composed of individuals captured in the Imbé River basin. We used three females and six males at approximately 2 years of age. During the pre-experimental period, the fish were housed in a density of 1 individual/5 m2. Reproduction was induced using pituitary extract, followed by scheduled egg and larvae sampling, which occurred in November 2011 in the incubators of the Piabanha Project. The observed fertilization rate was approximately 80%. The induced reproduction method followed the technique described by Woynarovich & Horváth (Reference Woynarovich and Horváth1983). The temperature, dissolved oxygen, pH and conductivity of the water were monitored at the time of the second dose until the pituitary time of extrusion of the oocytes, using, respectively, oximeter accuracy of 0.01 mg/l water (YSI 550), electronic pH meter accurate to two decimal places (YSI PH 100) and electronic conductivity with 1 mS accuracy YSI – EC300.
The egg and larvae samples in the incubators were obtained following the protocol used by Romagosa et al. (Reference Romagosa, Narahara and Fenerich-Verani2001), which consisted of sampling eggs every hour after fecundation (HAF) until hatching. Beginning on the second day, the methodology was changed. Samples were collected every 2 h on the second day, every 3 h on the third day, and every 4 h on the fourth day. The samples ceased to be collected from the incubators when the beginning of exogenous feeding became clear. Thereafter, the larvae were transferred to a 1700 m2 ground tank that was previously prepared and provided with abundant natural food, receiving daily commercial feed powder (50% crude protein). For the next 15 days, daily samples were collected from the tank using a net with a 500-micron mesh opening, all at the same time of day. The sampling procedures ceased on the 19th day, when the individuals started to exhibit a shape similar to the adults. The hour-degrees were recorded (sum of the water temperature values in degrees centigrade, measured during the incubation and nursery processes) using the thermometer of an oximeter (brand YSI 550) during the sampling procedures for early developmental stages. In total, 266 individuals, comprising embryos (108) and larvae (158), were sampled and measured.
The swimming behaviour of the larvae was first recorded in the incubator soon after hatching and persisted until the pre-flexion stage. The records of swimming behaviour were subsequently correlated with the type of fish displacement and morphological changes observed in the larvae. Samples of embryos, larvae and juveniles were stored in numbered Eppendorf tubes with a buffered 4% formalin solution. The numbers represented the sequence of egg and larva sampling. Morphological descriptions and measurements were performed using a Bell Photonics stereomicroscope fitted with a digital camera (5 megapixel) and the software EUREKAM 10.0. The method proposed by Ahlstrom & Moser (Reference Ahlstrom and Moser1976), modified by Nakatani et al. (Reference Nakatani, Agostinho, Baumgartner, Bialetzki, Sanches, Makrakis and Pavanelli2001) was employed to describe the initial biometrics and ontogeny of the eggs and larvae originating from the artificial induction process. The following measurements (in millimetres; mm) were obtained for the eggs: egg area (EgA), egg perimeter (EgP), egg radius (ER), embryo area (EmA), embryo perimeter (EmP) and embryo radius (EmR).
The size of the perivitelline space was categorised in regard to its contribution to the total volume of the egg according to the recommendations made by Nakatani et al. (Reference Nakatani, Agostinho, Baumgartner, Bialetzki, Sanches, Makrakis and Pavanelli2001), as follows: restricted (0–9.9%), moderate (10–19.9%), wide (20–29.9%) and very wide (≥30.0%).
The following larval measurements were performed (mm): total length (TL), standard length (SL), yolk length (YL), yolk height (YH), head length (HL), head height (HH), rostrum length (RL), body height (BH), eye perimeter (EP), eye diameter (ED), snout–dorsal fin distance (SDFD) and snout–anal fin distance (SAFD).
The embryonic stages were categorised into developmental stages following Ahlstrom & Ball (Reference Ahlstrom and Ball1954) and Kendall et al. (Reference Kendall, Ahlstrom, Moser, Moser, Richards, Cohen, Fahay, Kendall and Richardson1984) and the modification made by Nakatani et al. (Reference Nakatani, Agostinho, Baumgartner, Bialetzki, Sanches, Makrakis and Pavanelli2001). The developmental stages were as follows: early cleavage (EC; when the first cells are formed); early embryo (EE; when the embryo is differentiated); free-tailed embryo (FT; when the tail is released from the yolk); and final embryo (FE); when the embryo is completely formed and ready for eclosion).
After eclosion, the larvae were classified into five categories according to notochord flexion and to the development of the caudal fin and its supporting elements (following Ahlstrom & Ball, Reference Ahlstrom and Ball1954; Kendall, et al., Reference Kendall, Ahlstrom, Moser, Moser, Richards, Cohen, Fahay, Kendall and Richardson1984; Nakatani et al., Reference Nakatani, Agostinho, Baumgartner, Bialetzki, Sanches, Makrakis and Pavanelli2001). The categories were as follows: yolk-sac larva (YL; stage between hatching and the first exogenous feeding); pre-flexion (PF; from the beginning of exogenous feeding until the beginning of notochord flexion, when supporting elements of the caudal fin appear); flexion (FL; from the beginning of notochord flexion, when supporting elements of the caudal fin appear, until complete flexion of the notochord, the appearance of the pelvic fin bud and the beginning of dorsal and anal fin ray segmentation); post-flexion (POF; from full notochord flexion, the appearance of the pelvic fin bud and the beginning of dorsal and anal fin ray segmentation until the full formation of the pectoral fin rays, absorption of the embryonic fin and the appearance of scales) and juvenile (JU; regarded as small adults, characterized by the complete formation of the fin rays and the appearance of scales, until the first indication of sexual maturity).
The following traits were emphasised in the larval ontogeny: the appearance of pigmentation, the appearance and regression of the embryonic fin, the appearance of branchial arches, the formation of the digestive tract, the emergence of gill slits and the operculum, the appearance and quantity of fin rays, the quantity of myomeres, the position of the mouth area, the appearance of the olfactory hole, the appearance of the gas bladder and the appearance of the operculum.
Growth patterns in different larval stages were assessed by fitting four growth models to the relationship between TL and hour-degrees (HD): linear, von Bertalanffy, Gompertz and Logistic (Katsanevakis, Reference Katsanevakis2006; Katsanevakis & Maravelias, Reference Katsanevakis and Maravelias2008), separating the curves for yolk-sac larvae and peri-flexion larvae (combining pre-flexion, flexion and post-flexion stages). The linear model presents the simplest growth pattern, with a constant growth rate, whereas the remaining models decelerate growth towards an asymptotic body size. Model fitting was assessed by information criteria (AIC), and the model with the smallest AIC value was selected as best fitting.
The allometric patterns during different larval stages were modelled based on the SL power function, and the allometric coefficients were calculated with the power function Y = aX b , where Y is the dependent variable (measured characteristic); X the independent variable (SL – standard length); a is the intercept; and b is the allometric coefficient. Isometric growth occurred when b = 1, positive allometric growth when b > 1 and negative allometric growth when b < 1. The morphometric variables (response variables) were plotted against SL, and the relationships were described using regression models for log-transformed variables (Kováč et al., Reference Kováč, Copp and Francis1999).
Results
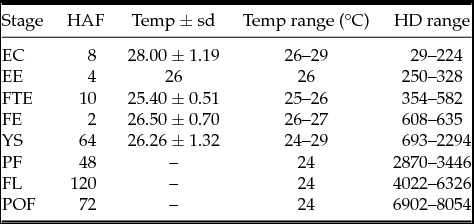
The extrusion occurred 288.9 hour-degrees (HD) after hormonal induction. Under the following physicochemical average water temperature (°C), pH, dissolved oxygen (mg/l) and electrical conductivity (μS): 28.89 ± 0.44°C; pH 6.7 ± 0.43; 4.88 ± 144 mg/l; and 73.71 ± 0.65 μS. The number of hours elapsed after fecundation (per stage), the incubation water temperature and the recorded HD are described in Table 1.
Table 1 Environmental variables measured throughout the incubation of Prochilodus vimboides eggs and larvae

EC: early cleavage, EE: Early embryo, FTE: free-tailed embryo, FE: final embryo, YS: Yolk-sac larva, PF: pre-flexion, FL: flexion, POF: post-flexion, HAF: hours after fecundation per stage; Temp ± sd: temperature ± standard deviation; Temp range °C: temperature range in degrees centigrade; HD range: hour-degree range.
The description of early development was divided into embryonic and larval stages. Six stages were observed within the embryonic development stage. The six stages lasted 24 h at an average temperature of 26.48 ± 1.41°C. The most relevant events in early development are shown in Table 2.
Table 2 Description of morphological events occurring after the fertilization of Prochilodus vimboides eggs, according to developmental stages.

EC: early cleavage; EE: early embryo; FE: final embryo; FTE, free-tailed embryo; HAF: hours after fecundation; HD: hour-degrees.
The eggs were spherical, exhibited a yellowish yolk and were non-adhesive and pelagic AF and hydration. There were no significant differences in the egg perimeter observed amongst the cleavage stages (CL) until the FE stage. The eggs presented a diameter range from 3.3–4.1 mm (mean ± standard deviation: 3.7 ± 0.19 mm) and an egg perimeter ranging from 10.21–12.84 mm (mean ± standard deviation: 11.52 ± 0.60 mm). The embryos exhibited an area of 1.88 ± 0.26 mm2 (mean ± standard deviation) and occupied 17.30 ± 2.46% of the inner volume (a 12.98–23.07 range) of the egg (egg area was 10.95 ± 1.06 mm). Therefore, the perivitelline space was categorised as ‘very wide’ (Nakatani et al., Reference Nakatani, Agostinho, Baumgartner, Bialetzki, Sanches, Makrakis and Pavanelli2001).
Early cleavage
The reorganisation of the cytoplasm into the animal pole and vegetal pole occurred in the first hour, along with the early cleavages (Fig. 1 A, B). Early cleavage ended in the second hour. The ‘blastocyst’ stage began in the third hour, when the blastoderm exhibited a domed shape (Fig. 1 C). ‘Gastrulation’ occurred between the fourth and eighth hour, when epiboly increased, and the animal pole covered 40% (Fig. 1 D), 60% (Fig. 1 E), 80% (Fig. 1 F) and 90% (Fig. 1 G) of the animal pole during the fourth, fifth, sixth and seventh hour, respectively. The animal pole completely covered the vegetal pole in the eighth hour, corresponding to the blastopore closure stage (Fig. 1 H). At this point, the embryo area ranged from 1.49–1.84 mm2 (1.66 ± 0.14 mm2).

Figure 1 Early developmental stages of Prochilodus vimboides. (A, B) Cleavage, first and second hour after fecundation (HAF); (C) Blastula, third HAF; (D) Gastrula, fourth HAF (40% epiboly); (E) Gastrula, fifth HAF (60% epiboly); (F) Gastrula, sixth HAF (80% epiboly); (G) Gastrula, seventh HAF (90% epiboly); (H) Gastrula, eighth HAF (blastopore closure). Scale bars represent 0.28 mm.
Early embryo
Embryo differentiation began 9 h AF, when the cranial region and the tail of the embryo could be distinguished (Fig. 2 A). The optic vesicle was clear in the 10th hour, and the embryo displayed 18 somites at the 12th hour.

Figure 2 Early developmental stages of Prochilodus vimboides. (A) Early embryo, 9 hours after fecundation (HAF); (B) Free-tailed embryo, 19 HAF; (C) Final embryo, 24 HAF. Scale bars represent 1.0 mm. Abbreviations: cr – cranium; t – tail; ys – yolk sac; pf – primordial fin.
Free-tailed embryo
The most relevant change in this stage occurred in the 13th hour, when the tail of the embryo was detached from the yolk sac. The yolk sac expanded parallel to the embryo's body at the 14th hour. Twenty somites were observed in the 15th hour, and the formation of otoliths began in the following hour. Another remarkable body change was observed only in the 19th hour, when the embryonic membrane of the primordial fin developed on the periphery of the caudal region of the larvae (Fig. 2 B).
Final embryo
This stage occurred in 2 h (23rd and 24th) (Fig. 2 C), during which no relevant body changes occurred. However, the embryo ruptured the chorion at the end of this stage.
Larval development
Larval development lasted 302 h, including 62 h in the yolk larval stage, 48 h in the pre-flexion stage, 120 h in the flexion stage and 160 h in the post-flexion stage. The most relevant events, morphometric data, meristic data and development times of the larval phases are shown in Tables 3 and 4.
Table 3 Description of the main ontogenetic events in Prochilodus vimboides larvae

E: stages = YS: yolk-sac larva, PF: pre-flexion, FL: flexion, POF: post-flexion, HAF: hours after fecundation, HD: hour-degrees. Variables = BH: body height, ED: eye diameter, EP: eye perimeter, ER: eye radius, HL: head length, HH: head height, RL: rostrum length, SDFD, snout–dorsal fin distance, SAFD: snout–anal fin distance, TL: total length, SL: standard length, YH: yolk height, YL: yolk length.
Table 4 Mean (x), standard deviation (sd) and range of variation (range) of the morphometric and meristic variables obtained from Prochilodus vimboides larvae (n: number of individuals evaluated, YS: yolk-sac larva, PF: pre-flexion, FL: flexion, POF: post-flexion)

TL: total length; SL: standard length; YL: yolk length; YH: yolk height; RL: rostrum length; ED: eye diameter; HL: head length; HH: head height; BH: body height; SDFD: snout–dorsal fin distance; SAFD: snout–anal fin distance; BH: body height; P: pectoral fin; L: pelvic fin; D: dorsal fin; A: anal fin; C: caudal fin; dv: difficult visualization; na: not available; Myom: myomeres; Pra: pre-anal; Poa: post-anal. The absence of measurements is a consequence of the incomplete development of the larvae.
Yolk-sac larva
This stage showed the greatest number of bodily changes. The larvae spent most of the time resting and clustered in the bottom of the incubator after hatching. The larvae performed vertical movements leading to the surface or near the surface in a short time span, then passively returned to the bottom with their head turned downward. The following structures developed in this stage: pectoral fins (without rays), the swimming bladder, and the optical and brain vesicles. In addition, the number of myomeres increased along the torso; the respiratory system (composed of the arches, gill filaments and opercula) developed; and the heart and nasal opening began to be formed.
These structural acquisitions occurred along with the initiation of horizontal swimming (72 h after fecundation). The digestive system developed; the mouth was terminal and open; the digestive tract was elongated, and the anus was open; and there was a reduction of the yolk reserve, which was nearly exhausted at this point. Pigments were present in several body parts (Fig. 3 A).

Figure 3 Early developmental stages of Prochilodus vimboides. (A) Yolk-sac larva, 42 HAF; (B) Pre-flexion, 136 HAF; (C) Flexion, 256 HAF; (D) Post-flexion, 304 HAF. Scale bars represent 1.0 mm. Abbreviations: dt – digestive tube; ys – yolk sac; m – macula; n – flexed notochord; od – developed operculum.
Pre-flexion
The punctate and dendritic chromatophores increased progressively on the snout, the back of the head and the sides of the body, extending to the early portion of the anal region during the pre-flexion stage of the notochord. The number of myomeres along the torso increased, and the height of the hyaline fin was reduced along with the development of the caudal (with rays) and anal fins. In the pre-flexion stage, the larvae undergo more active movement in all directions in the water column. Food was observed in the digestive tract lumen based on transparency, along with the exhaustion of the yolk reserve. Notochord flexion began at the end of the pre-flexion stage (Fig. 3 B). The numbers of pre-anal and post-anal myomeres (24–25 and 12–13 myomeres, respectively) were the same in the yolk-sac and pre-flexion stages (Table 3). In the flexion and post-flexion stages, it was not possible to quantify the number of myomeres throughout the body due to body growth and the occurrence of additional pigment.
Flexion
Flexion of the tip of the notochord began at the flexion stage. A high concentration of pigments was observed in the eyeballs, around the borders of the myomeres and on the dorsal fin, beginning the formation of a macula. Hours later, pigmentation intensified in the flanks forming transverse bands. The opercula were more developed. The caudal, anal and dorsal fins started to show rays, and the pelvic fin bud began to form (Fig. 3 C).
Post-flexion
This was the longest larval development stage, lasting 160 h. The regression of the hyaline fin in the dorsal area, except for in the caudal peduncle region, and adipose fin formation occurred in this stage. Fin development, considering the presence of rays, occurred in the following order: pectoral, caudal, dorsal, anal, pelvic and adipose. At the end of the post-flexion phase, the caudal, dorsal, anal and pelvic fins already exhibited rays. The quantification of pectoral fin rays was difficult because these rays were difficult to see. The adipose fin does not present rays at any life stage in this species. Finally, scales appeared, and complete regression of the hyaline fin occurred. The hyaline fin still exists in the caudal peduncle and in the early juvenile stage, when individuals begin to resemble adults in shape (Fig. 3 D).
Growth and allometry
Two larval growth stages, alternating with a stabilisation stage, were observed after the hatching of P. vimboides eggs. The first growth stage occurred throughout most of the yolk-sac larva phase (except the last hours), 88 HAF or 2.294 HD. In this first stage, the non-linear growth models were all equivalent according to AIC criteria (Gompertz AIC = 13.44, Logistic AIC = 14.61, von Bertalanffy AIC = 16.37) as the difference between the lowest AIC and the remaining ones is <3. The linear model performed significantly worse (linear AIC = 208.68), suggesting a deceleration of growth towards the asymptotic length of 6.73 mm (sd = 0.0693 mm) (Fig. 4 A). There was no significant growth once the yolk was nearly consumed (Fig. 4 B). The second growth phase combined the period before and after notochord flexion, and the fit of the models were all equivalent (Linear AIC = 99.60, Logistic AIC = 101.21, Gompertz AIC = 101.35, von Bertalanffy AIC = 105.84). In this case, we parsimoniously selected the linear model as the best fit and assume a constant growth rate of 0.00144 mm/hour-degree (Fig. 4 A) throughout the flexion stages, all the way to the juvenile stage. Because of the biphasic growth, allometric coefficients were calculated for each larval stage separately (Table 5 and Fig. 5).
Table 5 Allometric coefficients (regression on standard length) for the different larval growth stages of Prochilodus vimboides

The 95% lower (LL) and upper (UL) confidence limits based on a t distribution. The peri-flexion stage is the sum of the pre-flexion, flexion and post-flexion stages. HL: head length; HH: head height; RL: rostrum length; BH: body height; ED: eye diameter; SAFD: snout–anal fin distance; SDFD: snout–dorsal fin distance; NS: not significant.

Figure 4 Larval growth and development of Prochilodus vimboides. (A) Growth curves for the different developmental stages, indicating the models with the best fit. The Gompertz model (curved line) showed the best fit (AIC criterion) in the yolk-sac larval stage, and the linear model (straight line) showed the best fit in the flexion, pre- and post-flexion (peri-flexion) stages. (B) Scatter plot showing the decrease in yolk length with hour-degrees in Prochilodus vimboides during the yolk-sac larva phase.

Figure 5 Allometric regression lines between log-transformed variables. (A) Head height, (B) head length, (C) eye diameter, (D) rostrum length, (E) body height and (F) snout–dorsal fin distance relative to standard length of Prochilodus vimboides during the yolk-sac larva stage (to the left of the vertical dotted line) and pre-flexion, flexion and post-flexion stages (peri-flexion, to the right of the dotted line), a total of 19 days after fecundation.
Among the yolk-sac larva stage variables, only HL exhibited positive allometry relative to SL (Table 5, Fig. 5 B). During the yolk-sac larva stage, HL proportion represented on average 16.37% of the total size of the larvae (Table 6). The positive allometric growth in HL persisted throughout the latter stages (Table 5 and Fig. 5 B) and the HL proportion reached approximately 30% of body length (Table 6). Head height switched from negative to positive allometry between phases (Table 5 and Fig. 5 A), also reached approximately 30% BP in the post-flexion stage (Table 6). Rostrum length switched between no growth to isometric growth between phases (Table 5 and Fig. 5 D). Given that HL was partially determined by RL, its positive allometry must be due to the growth of the braincase. Eye diameter (ED) exhibited negative allometry in all phases (Table 5 and Fig. 5 C), decreasing its relative proportion relative to the head (Table 6). The allometric coefficient of BH was not significant (actually a negative relation) in the larval yolk phase (Table 5 and Fig. 5 E). This negative association is due to the reduction of the yolk sac, causing a ‘decrease’ in BH as the length increases. In later stages, BH displayed a positive allometric coefficient, causing an increase in the body proportion for this variable (Table 6). The distances from the snout to the dorsal and anal fins were both isometric (Table 5 and Fig. 5 F), but could only be measured in the peri-flexion stages.
Table 6 Body proportions (%), means (x), standard deviations (sd) and range of variation (range) of the morphometric and meristic variables obtained from Prochilodus vimboides larvae

n: number of individuals evaluated; BP: body proportions; YS: yolk-sac larva; PF: pre-flexion; FL: Flexion; POF: post-flexion; SL: standard length; YL: yolk length; YH: yolk height; RL: rostrum length; HL: head length; HH: head height; SDFD: snout–dorsal fin distance; SAFD: snout–anal fin distance; ED: eye diameter; na: measurement not available. The absence of measurements is a consequence of the incomplete development of the larvae.
Discussion
Both the duration of the embryonic period and the early development pattern vary considerably among different fish species (Balon, Reference Balon1981). Water temperature is the environmental factor that most strongly influences development in these organisms (Chambers & Leggett, Reference Chambers and Leggett1987; Souza, Reference Souza2004; Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006) and higher temperatures speed the early development process. Longer photoperiods during incubation also accelerates the initial development and egg hatching (Hernández Cuadrado, Reference Hernández Cuadrado2013). P. vimboides showed a slightly longer hatching time (24 hours after fecundation – HAF) at an average temperature of 26.5 ± 1.41°C when compared with the hatching times of other species belonging to the same family (Characidae) at similar or lower temperatures, such as Leporinus frederici 13 HAF at 27°C (Sanches et al., Reference Sanches, Baumgartner, Bialetzki, Suiberto, Gomes, Nakatani and de Campos Barbosa2001), L. piau 21 HAF at 24°C (Borçato et al., Reference Borçato, Bazzoli and Sato2004), Prochilodus magdalenae 14 HAF at 24°C (Arias-Gallo et al., Reference Arias-Gallo, Jiménez-Segura and Dorado2010), and P. lineatus 18 HAF at 25°C (Botta et al., Reference Botta, Sciaraa, Arranja, Musgas, Pereira and Oberlender2010; Hernández Cuadrado, Reference Hernández Cuadrado2013). Although egg size in P. vimboides lies well within the range of other species in the genus (2.23–4.25 mm) (Nakatani et al., Reference Nakatani, Agostinho, Baumgartner, Bialetzki, Sanches, Makrakis and Pavanelli2001; Arias-Gallo et al., Reference Arias-Gallo, Jiménez-Segura and Dorado2010), the yolk-sac larvae are larger (mean = 5.8 mm) than in other Prochilodus species (Arias-Gallo et al., Reference Arias-Gallo, Jiménez-Segura and Dorado2010). Hatching times can be adaptive as part of life history strategies, as they can be related to environmental particularities and species-specific developmental rates (Fuiman, Reference Fuiman, Fuiman and Werner2002). The delayed hatching in P. vimboides might be adaptive, as their larvae hatch with larger size than other Prochilodus species. It is, however, difficult to assess the relative influence of environmental factors other than temperature (light, pH, oxygen) in this comparison.
P. vimboides larvae hatch with a large yolk sac in relation to their body length (~30%), becoming quite heavy and spending much of the time at the bottom of the incubator. A change in swimming patterns from vertical to horizontal was observed during the larval development. Initially, the larvae performed only vertical ascending movements until reaching the water surface and then returned passively to their original position. After the development of sense organs and pectoral fins (though without rays), the individuals became capable of swimming both vertically and horizontally. Mouth opening and filling of the swimming bladders occurred simultaneously, as larvae were able to swallow air to inflate the gas bladder (Pinder & Gozlan, Reference Pinder and Gozlan2004). The development of fins facilitates balance and direction in the water column, being also responsible for the swimming behaviour changes in other species (Santos, Reference Santos1992; Santos & Godinho, Reference Santos and Godinho2002; Beerli et al., Reference Beerli, Logato and Freitas2004; Mukai et al., Reference Mukai, Tuzan, Lim and Yahaya2010).
In the pre-flexion stage (101 HAF), a transition occurred from endogenous to exogenous feeding. The mouth, digestive tract and anus became functional, enabling the larvae to capture, digest and excrete food. The same pattern of anatomical development is observed in the pre-flexion stage of the congeneric species P. magdalenae, 106 HAF, when the yolk reserve becomes depleted (Arias-Gallo et al., Reference Arias-Gallo, Jiménez-Segura and Dorado2010). Similar transitions have been described for Engraulis mordax (Lasker et al., Reference Lasker, Feder, Theilack and May1970); Cyprinus carpio, Clarias gariepinus (Van Snik et al., Reference van Snik, van den Boogaart and Osse1997); Danio rerio (Jardine & Litvak, Reference Jardine and Litvak2003); Leporinus piau (Borçato et al., Reference Borçato, Bazzoli and Sato2004) and Steindachneridion parahybae (Honji et al., Reference Honji, Tolussi, Mello, Caneppele and Moreira2012).
Yolk depletion and the food transition proved to be the time of stabilisation in the growth of P. vimboides larvae. Yolk consumption in teleost fish occurs in three distinct stages, the first of which is the FE stage, while the other two occur during the yolk-sac larva stage (Heming & Buddington, Reference Heming, Buddington, Hoar and Randall1988; Peña & Dumas, Reference Peña and Dumas2009). In contrast, P. vimboides undergoes two separate growth stages, before and after the beginning of the pre-flexion stage. The first at the expense of yolk consumption, with a decelerating growth rate. The second, based on exogenous feeding, with a constant growth rate (at least for the period measured). This switching of growth phases is critical from a nutritional point of view and may kill the larvae if they do not find exogenous food (Bailey & Houde, Reference Bailey and Houde1989). According to Blaxter (Reference Blaxter, Hoar and Randall1988), the larvae must have a functional mouth and intestine and developed eyes after absorbing the yolk to be able to seek exogenous dietary sources (e.g., plankton and/or organic particles). The allometric positive growth of head dimensions is associated with changes in feeding habits, with brain mass development and with the development of bones in the oral region (Kováč et al., Reference Kováč, Copp and Francis1999), indicating that many relevant morphological changes with ecological importance are accomplished even before they are functionally needed (Gisbert, Reference Gisbert1999). The mouth must be open, and all organs related to prey capture (eyes, sensory organs, fins) and food intake (absorption, digestion and assimilation) must be formed for development to continue (Yúfera & Darias, Reference Yúfera and Darias2007).
Changes in the oral region began in P. vimboides at the yolk-sac larva stage. In this period, the oral region shifted from a ventral to a terminal position, which is apparently synchronised with the food transition phase. Head allometry was positive throughout all development phases, however, the eye showed negative allometry and progressively decreased its proportion relative to HL after exogenous feeding began. Eye development with positive allometry is usually related to visual acuity (Fuiman, Reference Fuiman, Fuiman and Werner2002) and its relevance to visualize prey and rheotactic behaviour (Gisbert, Reference Gisbert1999; Gisbert et al., Reference Gisbert, Merino, Muguet, Bush, Piedrahita and Conklin2002), and allometric positive growth of ED during the yolk-sac larva phase is common (Gisbert, Reference Gisbert1999, Reference Gisbert, Merino, Muguet, Bush, Piedrahita and Conklin2002; Fuiman, Reference Fuiman, Fuiman and Werner2002; Kupren et al., Reference Kupren, Prusinska, Zarski, Krejszeff and Kucharczyk2014). This pattern suggests that the ability of P. vimboides larvae to capture prey may not depend on visual development.
In the post-flexion stage, body length and depth increased, fin development occurred, and the larvae moved rapidly in the water column. Better performance in propulsion leads to increased efficiency in prey capture, in both quality and quantity, and enables greater agility in escaping predators (Blaxter, Reference Blaxter, Hoar and Randall1988; Bailey & Houde, Reference Bailey and Houde1989; Fuiman, Reference Fuiman, Fuiman and Werner2002). Such abilities should positively influence the survival of P. vimboides larvae, as observed for P. magdalenae and P. lineatus, due to the similarities in the early development of these species.
The external morphology of P. vimboides resembled that of an adult at the end of the post-flexion stage, when the specimens exhibited a fusiform and tall body, long pectoral fins and a developed caudal fin. These morphological characteristics are typical of species inhabiting regions with strong river currents (Alexandre et al., Reference Alexandre, Quintella, Ferreira, Romão and Almeida2014). It may be inferred that the larvae of P. vimboides inhabit marginal lakes that are abundant in natural food, at least in the pre- and post-food transition stages. Cunico et al. (Reference Cunico, Graça, Veríssimo and Bini2002) indicated that marginal lakes represent genuine natural nurseries that meet the feeding needs of growing fish larvae. Therefore, a relationship between species morphology and ecology may occur in the early stage of ontogeny, as the chronology of the early development of the eggs and larvae of P. vimboides appear to be in line with the transition stages from lotic (river channel) to lentic (flooded marginal areas) and again to lotic environments (when the juveniles return to the river channel). The description of larval development presented here allows for the identification of specimens in ecological field studies, where some species breed at the same time. The synchrony of the reproductive period, larval drift and floods ensure maximum food availability in the early stages of development, enabling rapid growth through the early larval stages, which are vulnerable to more intense predation (Fuiman, Reference Fuiman, Fuiman and Werner2002). The connection between fish reproduction and flood cycles through larval development enables the maintenance of viable populations and is relevant for management and decision taking by environmental agencies.
Acknowledgements
The authors thank the Piabanha Centro Socioambiental Project and Agricultural Research Enterprise of Rio de Janeiro State (Empresa de Pesquisa Agropecuária do Estado do Rio de Janeiro – PESAGRO-RIO) for the logistical support provided during the egg and larval sampling periods. The manuscript has been greatly improved by the suggestions of Prof. I. V. Isler and Prof. W. A. Olaniyi.
Financial support
Work by the authors is supported by Mr José Roberto Marinho, Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq, and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ.
Conflicts of interest
None.