Introduction
It is widely acknowledged that, during folliculogenesis, a controlled programme of intercellular communication and support between the oocyte and the granulosa/cumulus cells occurs via the gap junctions of the zona pellucida and the release of paracrine soluble factors. Several studies on animal models have demonstrated that this ‘oocyte–cumulus cell bidirectional dialogue’ has key reciprocal metabolic implications such as the supply of cholesterol, pyruvate or alanine from the oocyte, and the oocyte-triggered promotion of estradiol synthesis by granulosa cells. Therefore, the follicle must be regarded as a single physiological unit whose compartments cooperate as the initial transition from a primary to a secondary stage, to the final event of ovulation (Zuccotti et al., Reference Zuccotti, Merico, Cecconi, Redi and Garagna2011; Agnello et al., Reference Agnello, Bosco, Chiarelli, Martino, Roccheri and Ntuli2015; El-Hayek and Clarke, Reference El-Hayek and Clarke2016; Bosco et al., Reference Bosco, Roccheri, Martino, Chiarelli, Lispi and Ruvolo2017a).
Dealing with human in vitro fertilization (IVF), the correct realization of this bidirectional molecular transfer is required for the acquisition of oocyte competence, which leads to a good quality metaphase II (MII) gamete endowed with a positive effect on embryo development, implantation and clinical outcome. Due to the still limited knowledge of the biochemical processes that are involved in the process of oogenesis, the identification of non-invasive markers that may help the selection of the gametes to be submitted to intracytoplasmic sperm injection (ICSI) procedure is a fundamental issue and a still-open field in assisted reproduction research. In this regard, cumulus and granulosa cells, which are easily available as a waste product of the manipulation procedures before ICSI, have been used to evaluate the fertility potential of individual oocytes via non-invasive approaches. In fact, in light of the close structural and physiological connections occurring between the oocyte and the surrounding cells, the latter may reflect the quality and competence acquisition of the gamete when tested for the expression of markers of cell survival/death (Ruvolo et al., Reference Ruvolo, Bosco, Pane, Morici, Cittadini and Roccheri2007).
Previous studies (Ruvolo et al., Reference Ruvolo, Fattouh, Bosco, Brucculeri and Cittadini2013; Bosco et al., Reference Bosco, Chiarelli, Roccheri, Matranga and Ruvolo2017b) have been focused on evaluation of the DNA fragmentation index (DFI) and the intracellular accumulation of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) and serine/threonine kinase AKT (or protein kinase B). ERK1/2 isoforms belong to the mitogen-activated protein kinase (MAPK) superfamily and are involved, once activated by phosphorylation, in the control of cell proliferation and apoptosis via the Ras–Raf–MEK–ERK signalling cascade. AKT, one of the key components in the AKT/PI3K/PTEN signalling pathway, once phosphorylated transduces signals that promote cell survival and interfere with the onset of apoptosis (Duronio, Reference Duronio2008; Mebratu and Tesfaigzi, Reference Mebratu and Tesfaigzi2009; Librizzi et al., Reference Librizzi, Chiarelli, Bosco, Sansook, Gascon, Spencer, Caradonna and Luparello2015). From the analysis of individual cumulus–oocyte complexes (COCs), cumulus cells associated with the oocytes able to produce blastocysts were shown to possess a higher pAKT/DFI ratio than cumulus cells related to embryos arrested during in vitro culture (Bosco et al., Reference Bosco, Chiarelli, Roccheri, Matranga and Ruvolo2017b). In addition, a significant direct correlation was found between pAKT and pERK1/2 accumulation, suggesting a cooperative action as survival factors in the model system under study.
The role played by ERK1/2 in apoptosis regulation in the ovary is controversial, according to data in published literature (Stanciu and De Franco, Reference Stanciu and De Franco2002; Shiota et al., Reference Shiota, Sugai, Tamura, Yamaguchi, Fukushima, Miyano and Miyazaki2003).
The results presented here extend previous observations on cumulus cell markers associated with the clinical outcome of the embryo, through the parallel immunocytochemical evaluation of intracellular accumulation of both pAKT and pERK1/2, with a specific focus on the intranuclear localization of the latter. The data obtained on the two cytological markers were also related to the percentage of DNA fragmentation examined via the terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labelling (TUNEL) assay.
Materials and Methods
Study design and patients
Twenty-six women, attending assisted reproductive treatment (ART) because of fertility problems, and included in a programme of blastocyst embryo transfer at the Centro di Biologia della Riproduzione (CBR, Palermo, Italy), were enrolled in the present study and signed an informed consent form to allow the use of any discarded cumulus cells for apoptosis rate assessment. The Internal Review Board of CBR considered this research ethically acceptable (27 June 2012) as it uses cumulus/granulosa cells that are usually discarded after decoronization of the COCs prior to performing ICSI. Therefore, all procedures performed were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments. The inclusion criteria were: normoresponder status with a minimum of six oocytes collected at pick-up, age ≤ 38 years [mean ± standard deviation (SD): 34.3 ± 3.5], normal follicle stimulating hormone (FSH) basal level (<12 UI/ml), and body mass index (BMI) <28 kg/m2 (mean ± SD: 22 ± 3.18). Azoospermia or severe oligoasthenospermia (motile sperms < 0.5 × 106/ml) were considered as an exclusion criterion.
Ovarian stimulation
All patients were subjected to administration with the gonadotropin-releasing hormone (GnRH) agonist buserelin (Suprefact, Sanofi-Aventis, Italy; 0.2 ml/day) starting on day 21 of the previous cycle. Administration of 150 IU r-FSH/day (Gonal-f, Merck, Rome, Italy) was started at day 8 after buserelin treatment and follicular growth was monitored every 2 days using ultrasound and serum estradiol E2 levels, starting on day 6 of stimulation, modifying the dose of r-FSH as a consequence. A dose of 10,000 IU hCG (Ovitrelle; Merck, Rome, Italy) was administered when at least three follicles showed a diameter ≥ 18 mm.
Preparation of cumulus cells
Sample preparation was performed as reported by Bosco et al. (Reference Bosco, Ruvolo, Luparello, Ferrari, Valerio, Santi, Piomboni, Sarcina, Lispi and Roccheri2017c). Essentially, after hyaluronidase treatment of the COCs, cumulus cells were fixed in 3.7% paraformaldehyde for 60 min, harvested by centrifugation at 2000 rpm for 7 min and resuspended in phosphate-buffered saline (PBS). Slides were prepared through cytospin centrifugation at 1000 rpm for 5 min on polylysine-coated glass slides. Cell permeabilization was performed at 4°C in 0.1% Triton X-100 plus 0.1% sodium citrate in PBS before immunostaining and TUNEL assay. Oocytes were transferred to fertilization medium (SAGE IVF; Irvine Scientific, California, USA) and incubated at 37°C and 6% CO2 until ICSI.
Fluorescence in situ TUNEL and immunodetection assays
Mcroscopic analyses were performed as reported by Bosco et al. (Reference Bosco, Chiarelli, Roccheri, Matranga and Ruvolo2017b). To assess the extent of apoptotic DNA fragmentation, cumulus cells were submitted to TUNEL assay using the DeadEndFluorometric TUNEL System (Promega Italia, Milan, Italy), according to the manufacturer’s instructions, in parallel with a positive and a negative control. The reaction was blocked with saline sodium citrate (SSC) and, after exhaustive washing in PBS the cumulus cells were counterstained with propidium iodide (1 µg/ml).
For in situ immunofluorescence assays, the cell preparations were blocked with 3% bovine serum albumin (BSA)/PBS and co-incubated at 4°C overnight with anti-pAKT polyclonal antibody from rabbit (p-Akt1/2/3 (Ser 473)-R, sc-7985-R, working dilution 1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-pERK1/2 antibody from mouse [p-ERK (E-4), sc-7383, working dilution 1:50, Santa Cruz Biotechnology] in 3% BSA/PBS. The primary antibody was omitted in the negative controls. The samples were then co-exposed for 1 h to the secondary antibodies, i.e. anti-rabbit IgG (whole molecule)–FITC conjugate (F0382, working dilution 1:50, Sigma, St. Louis, MO, USA) for anti-pAKT and anti-mouse IgG (whole molecule) TRITC conjugate (T5393, working dilution 1:50, Sigma) for anti-pERK1/2, counterstained for 10 min with Hoechst 33342 (Invitrogen) and mounted in 10 μl DABCO solution.
All samples were observed under an Olympus BX 50 microscope equipped with a reflected light fluorescent attachment (Olympus), and a ×20/0.40 objective. The densitometric analysis of fluorescent signals was carried out using NIS-Elements BR 3.10 image analyzer software (Nikon) as reported by Choi et al. (Reference Choi, Galán, Kassan, Partyka, Trebak and Matrougui2012).
Statistics
The accumulation levels of pAKT and pERK1/2 and the DFI of oocytes were summarized by means of median and interquartile range. Statistical significance of the difference between the accumulation levels of pAKT and pERK1/2 and the DFI of cumulus cells resulting in transferred versus arrested blastocysts was checked through the non-parametric Kruskal–Wallis test. Spearman’s coefficient correlation was calculated between paired data of pAKT, pERK1/2 and DFI of cumulus cells. Statistical significance of Spearman’s coefficient was assessed using Student’s t-test. A P-value < 0.05 was considered to be statistically significant. Statistical analysis was performed using Stata/SE 14.0 software.
Results and Discussion
In total, 91 MII oocytes were fertilized by ICSI, and the derived embryos had the following evolution: 53 developed to 5–6-day-old blastocysts and were transferred in utero, eight were arrested at different cleavage stages during the 7 days of in vitro culture and were discarded, and the remaining 30 were cryopreserved.
In a first set of assays, cumulus cells from the corresponding COCs were submitted to immunofluorescence microscopy and densitometric analysis of the images for the assessment of the accumulation levels of both total pAKT and intranuclear pERK1/2. A representative microscopic field of cumulus cells, immunolocalized and counterstained with Hoechst 33342 for total nuclei, and the resulting merged image is shown in the panel of Figure 1. In parallel, the DFI was evaluated via the TUNEL assay. A representative microscopic field of cumulus cells showing DNA fragmentation and counterstained with propidium iodide for total nuclei, and the resulting merged image is shown in the panel of Figure 2. For each COC, at least 60 (for immunostaining) or 450 cells (for DFI) were analyzed.

Figure 1. Panel of fluorescence micrographs showing the immunolocalization of pAKT, in green (a) and pERK1/2, in red (b) and total nuclei counterstain with Hoechst 33342, in blue (c) in a representative cumulus cell preparation. The merged image is shown in (d) and the arrows in the enlarged view (e) point to pERK1/2 localization in the nucleus, in fuchsia, due to merging of the blue fluorescence of Hoechst 33342 and the red fluorescence of pERK1/2 (n) and in the cytoplasm, in orange, due to the merging of the green fluorescence of pAKT and the red fluorescence of pERK1/2 (c). Bar, 30 μM.
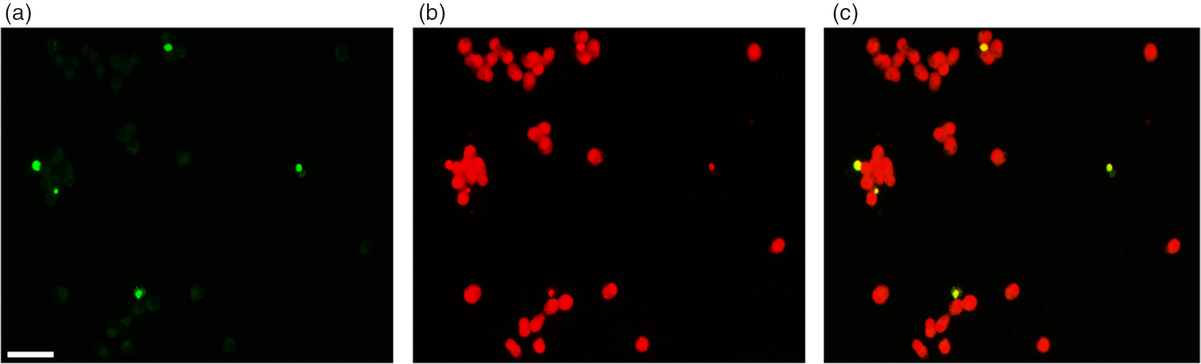
Figure 2. Panel of fluorescence micrographs showing the DNA fragmentation detection by TUNEL assay (a), and total nuclei counterstain with propidium iodide (b) in a representative cumulus cell preparation. The merged image is shown in (c). Bar, 30 μM.
The accumulation levels of pAKT and pERK1/2 and the DFI of cumulus cells do not show any statistically significant difference between transferred and arrested embryos (Table 1). The statistical correlation among the immune-quantitation, the DFI rate and the clinical outcome of the embryos is shown in Table 2. For a positive clinical outcome, we found that in cumulus cells of the corresponding COCs, nuclear localization of pERK1/2 showed a significant inverse correlation with the DFI value, the latter being an apoptosis hallmark, (rs = −0.39, P = 0.007), and a significant direct correlation with the intracellular accumulation of pAKT (rs = 0.477, P < 0.001). These results were not obtained with cumulus cells from COCs related to negative clinical outcomes of the arrested embryos. In addition, a significant inverse correlation between the intracellular accumulation of pAKT and the DFI value (rs = −0.37, P < 0.001) was observed in all cumulus cells examined.
Table 1. Accumulation levels of pAKT and pERK1/2 and the DFI of cumulus cells: medians and interquartile range

a Data must be considered as the digits × 109.
b P-value from the Kruskal–Wallis test.
Table 2. Correlation between pair of pAKT, pERK1/2 and DFI of cumulus cells by clinical outcome

a P-value from the Student’s t-test for the Spearman’s correlation.
Previous results by Bosco et al. (Reference Bosco, Chiarelli, Roccheri, Matranga and Ruvolo2017b) validated the apoptosis rate and the inverse correlation between DFI and pAKT accumulation in cumulus cells as molecular markers of oocyte competence, suggesting their prognostic meaning with regard to blastocyst formation. In the present study, we extended the previous observations to co-localization analysis of pAKT and pERK1/2 in cumulus cells from single COCs, correlating the results obtained with the DFI and evolution of embryo development. It is known that unphosphorylated ERK is localized in the cytoplasm and on the surface of organelles, where it binds anchoring and scaffold proteins therefore controlling cellular activities such as cell–cell and cell–matrix adhesion, intracellular trafficking and resistance to apoptosis (Casar et al., Reference Casar, Pinto and Crespo2009). Upon phosphorylation, ERK is allowed to translocate into the nucleus via an importin 7-mediated mechanism. Interestingly, ERK activation and nuclear localization was proven to regulate the activity of cell cycle factors, further promoting cell viability, but also to trigger cell death via apoptosis promotion and therefore not being predictive of the subsequent specific cellular response (Mebratu and Tesfaigzi, Reference Mebratu and Tesfaigzi2009; Plotnikov et al., Reference Plotnikov, Flores, Maik-Rachline, Zehorai, Kapri-Pardes, Berti, Hanoch, Besser and Seger2015; Wainstein and Seger, Reference Wainstein and Seger2016). Concerning the COC model system, Du et al. (Reference Du, Fu and Zhou2013) reported that ERK1/2 activity in mouse cumulus cells is essential for their gonadotropin-induced expansion, whereas Xu et al. (Reference Xu, Zhang, Tong and Liu2015) demonstrated that miRNA-mediated downregulation of ERK1 in human granulosa cells may impair their proliferation and differentiation, as well as the production of steroid hormones and follicular development. Our data represent the first evidence of: (i) a potential cell survival effect played by intranuclear pERK1/2 in human cumulus cells that adds to the upregulation of intracellular pAKT; and (ii) a significant correlation of these histochemical signatures with a positive clinical outcome of the embryos.
Taking data from published literature and our results together, we therefore suggest that high levels of nuclear pERK1/2 accumulation, coupled with an increase of pAKT concentration and a low DFI rate value, in the cumulus cells of corresponding COCs may be considered as a marker of oocyte competence, leading to development of embryos of presumed good quality.
Financial Support
The authors are grateful to the host institution: Department STEBICEF of Palermo University. Funding for this study was provided by FFR 2013, University of Palermo, Italy to Professor M.C. Roccheri.
Conflicts of Interests
Authors declare that they have no competing interests.
Ethical Standards
All procedures performed were in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.






