Introduction
In contrast with most other mammals, the formation, activation and development of ovarian follicles occurs entirely postnatally in rabbits (Hutt et al., Reference Hutt, McLaughlin and Holland2006). Germ cells first appear in the ovary as oogonium, arranged in cell nests that disappear late in development (Ricken and Viebahn, Reference Ricken and Viebahn2002; Takagi et al., Reference Takagi, Yamada, Miki, Umegaki, Nishimura and Sasaki2007). Folliculogenesis starts 2 weeks after birth. By that time, primordial follicles have already developed (Daniel-Carlier et al., Reference Daniel-Carlier, Harscoët, Thépot, Auguste, Pailhoux and Jolivet2013). Primary follicles and growing primary follicles are visible at the fourth week of age (Hutt et al., Reference Hutt, McLaughlin and Holland2006). Secondary follicles with clearly distinct theca cell layers are first identified at 6 weeks of age, while growing and antral follicles are noticed in the ovaries of 8-week-old and 12-week-old rabbits (Hutt et al., Reference Hutt, McLaughlin and Holland2006). The interstitial glands are typical steroid-secreting cells that were first demonstrated in the rabbit ovary at 8 weeks of age (Abd-Elkareem, Reference Abd-Elkareem2010). These glands are mostly derived from the theca interna cells of the atretic antral follicle (Abd-Elkareem, Reference Abd-Elkareem2010).
Ovarian follicle development is a complex process that is controlled by the integration of endocrine, cellular and molecular mechanisms (Wear et al., Reference Wear, McPike and Watanabe2016) and its dynamic regulation is an integral part of the reproductive process (Wu et al., Reference Wu, Han, Cao, Zhang, Li, Liu, Liu, Li, Pan, Chen and Liu2015). This process is highly orchestrated by endocrine signalling from the hypothalamus and the pituitary gonadotropins (Jin et al., Reference Jin, Won, Shin, Kim, Lee and Bae2017). However, the effects of gonadotropins on ovarian development depend not only on the level of circulation, but also on the expression of their respective receptors (FSHR and LHR) (Mehl et al., Reference Mehl, Khalid, Srisuwatanasagul, Swangchan-uthai and Sirivaidyapong2017). These receptors belong to the superfamily of G-protein coupled receptors that are characterized by the presence of a large extracellular domain (Narayan et al., Reference Narayan, Ulloa-Aguirre and Dias2018). Pituitary gonadotropins are necessary for promoting ovarian development, maturation of the follicles and development of granulose cells through interaction with their receptors (Dias et al., Reference Dias, Cohen, Lindau-Shepard, Nechamen, Peterson and Schmidt2002; Zachos et al., Reference Zachos, Billiar, Albrecht and Pepe2003; Hunter et al., Reference Hunter, Robinson, Mann and Webb2004; Durlej et al., Reference Durlej, Knapczyk-Stwora, Duda, Galas and Slomczynska2011). Genetic mutation of these receptors can result in pathological conditions in which follicular growth is arrested and the female becomes infertile (Narayan et al., Reference Narayan, Ulloa-Aguirre and Dias2018).
Regarding the onset of FSHR gene expression, some investigators have found earlier expression before the formation of histologically distinct primordial follicles in hamster ovaries (Chakraborty and Roy, Reference Chakraborty and Roy2015). In contrast, other studies have reported a later onset of expression at the beginning of mouse folliculogenesis (Lei et al., Reference Lei, Jin, Mayo and Woodruff2010) or granulose cells proliferation (Saraiva et al., Reference Saraiva, Celestino, Araújo, Chaves, Almeida, Lima-Verde, Duarte, Silva, Martins, Bruno, Matos, Campello, Silva and Figueiredo2011). An in situ hybridization-based study showed restricted FSHR expression in granulose cells (Hillier, Reference Hillier2001). Whereas, LHR was mainly expressed in the thecal cells (Zhang et al., Reference Zhang, Shi, Segaloff and Van Voorhis2001) and partially in corpus luteal cells (Ziecik et al., Reference Ziecik, Kaczmarek, Blitek, Kowalczyk, Li and Rahman2007).
Ovarian expression of the LHR and FSHR genes has been detailed in the mouse, rat, hamster, horse, cat, and duck (Rannikki et al., Reference Rannikki, Zhang and Huhtaniemi1995; O’Shaughnessy et al., Reference O’Shaughnessy, Dudley and Rajapaksha1996, Reference O’Shaughnessy, McLelland and McBride1997; Ni et al., Reference Ni, Zhou, Lu, Grossmann and Zhao2007; Chakraborty and Roy, Reference Chakraborty and Roy2015; Scarlet et al., Reference Scarlet, Walter, Hlavaty and Aurich2015; Mehl et al., Reference Mehl, Khalid, Srisuwatanasagul, Swangchan-uthai and Sirivaidyapong2017). However, this expression at transcriptional and translational levels has not yet been elucidated in rabbit. Therefore, this study aimed to investigate the changes in FSHR and LHR gene and protein expression in rabbit ovary from birth to maturity, in addition to pregnant rabbits. In addition, we correlated these changes with granulosa and theca layer thickness to improve the understanding of the expression underlying ovarian follicle development, which is considered a fundamental step in reproduction success, as it can be utilized in rabbit farming as predictors of disrupted reproductive capabilities in rabbits.
Materials and methods
Tissue sample collection
Thirty healthy female New Zealand rabbits at birth (0 day), neonate (2 week), cub (4 week), maturity (16 week), and pregnancy (18 days post-coitum, 18dpc) (n = 6 animals/group) were obtained from the animal house, Faculty of Veterinary Medicine, Benha University. Their body weights were 43.19 ± 2.51, 225.00 ± 31.05, 404.75 ± 11.87, 2275 ± 515, and 2635 ± 523 g, respectively. The animals were housed in individual cages at a controlled temperature (23 ± 2°C) under a 12 h:12 h, light:dark cycle and provided with free access to water and food. The animals were euthanized by intramuscular injection of sodium pentobarbitone (140 mg/kg) in compliance with recommendations of the animal care committee of the Faculty of Veterinary Medicine, Benha University (approval number: BUFVTM 07–3–21). Right ovaries were kept in Bouin’s fluid for no longer than 24 h for the histological technique, while the left ovaries were stored at −80°C until RNA and protein extraction.
Histological preparation
Fixed ovaries were dehydrated in a graded series of ethanol and embedded in paraffin wax. Ovaries were sectioned (5–7 µm) and stained with haematoxylin and eosin for general histological structure and Masson’s trichrome stain for staining collagen fibres, as described by Feldman and Wolfe (Reference Feldman and Wolfe2014).
Histomorphometric measurements
Morphometrical studies were applied to images of stained histological sections of ovaries at all developmental stages using ImageJ software for measuring granulosa and theca layer thickness as previously described (Lan et al., Reference Lan, Liu, He, Chen, Liu, Shi, Liu, Yoshimura and Zhang2014). Ovarian follicles were classified according to Hutt et al. (Reference Hutt, McLaughlin and Holland2006).
Quantitative real- time PCR
Total RNA from 15 rabbit ovarian samples (n = 3/group) was extracted using a Total RNA Purification Kit following the manufacturer’s guidelines (iNtRON Biotechnology, easy-REDTM Total RNA Extraction Kit) as previously described (Abd-Allah et al., Reference Abd-Allah, Shalaby, Abd-Elbary, Saleh and El-Magd2015). cDNA was prepared from 5 µg of total RNA using the M-MuLV reverse transcriptase enzyme following the protocol of the manufacturer (Thermo Scientific, Fermentas, #EP0451). Quantitative PCR was conducted using the 2X Maxima SYBR Green/ROX qPCR Master Mix following the manufacturer’s protocol (Thermo Scientific, USA, #K0221). PCR cycling conditions were as follows: 95°C for 10 min, 40 cycles of 95°C for 15 s (denaturation step) and 60°C for 1 min (annealing step). The melting curve was produced by increasing the temperature from 60 to 95°C. RT-PCR was performed using three sets of primers, FSHR (forward, 5′-GAGGAATGCCATTGAACTGAGG-3 and reverse, 5′-GTTGGAGAACACATCTG-3′); LHR (forward, 5′-CTGGAGAAGATGCACAATGG-3′ and reverse, 5′-AATTAGCCTCTGAATGGACTC-3′) and GAPDH (forward, 5′-TGTTTGTGATGGGCGTGAA-3′ and reverse, 5′-CCTCCACAATGCCGAAGT-3′).
The ΔΔCT method was used to calculate the data with GAPDH as an internal control (Livak and Schmittgen, Reference Livak and Schmittgen2001). Three replicates of RT-PCR assays were performed for each sample
Western blot
In RIPA buffer, 15 ovarian samples (n = 3/group) were homogenized as previously described (Yang et al., Reference Yang, Wang, Shen and Roy2004). In total, 20 μg proteins were segregated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, USA). The membrane was blocked with 3% bovine serum albumin in Tween-20/Tris-buffered saline (TPST) at room temperature for 1–2 h. After blocking, the membranes were incubated with primary antibodies: anti-β-actin (1:1000, ab8226, Abcam), anti-FSHR (1:500, ab75200, Abcam), and anti-LHR (1:500, ab179780, Abcam). PVDF membranes were then incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody that was diluted to 1:5000 in blocking solution at room temperature. Densitometry analysis of protein bands was carried out using ImageJ software. The density of each band was normalized to β-actin. Three replicates were conducted for each protein sample.
Statistical analysis
Data were assessed using SPSS 16.0 software and one-way variance analysis (ANOVA). Duncan’s multi-range test was used to assess differences in the mean values. Correlations between granulosa and theca layers thickness of different types of ovarian follicles and mRNA as well as protein expression of FSHR and LHR were calculated using Pearson’s correlation coefficient (r). P-values < 0.05 or P < 0.01 were considered statistically significant. All data were presented as mean ± standard error of the mean (SEM).
Results
Ovarian follicle development
At birth, the ovarian cortex mainly contained germ cells, not yet assembled into primordial follicles and arranged in cell nests. Each cell nest was surrounded by a continuous basal lamina and elongated flattened stromal cells, and formed of germ cells of large size and spherical shape, and somatic cells, which by comparison were smaller and slightly oblong in shape (Figure 1A). At 2 weeks of age, the cell nests were completely resolved, as each oocyte individually acquired follicular cells and was organized into newly formed primordial follicles that appeared for the first time in the inner portion of the cortex. These follicles consisted of an oocyte surrounded by a one-cell layer of flattened follicular cells (Figure 1B). By the fourth week, a pool of primordial follicles occupied the majority of the ovarian cortex. In addition, primary and secondary follicles were often first observed near the interface of the cortex and medulla (Figure 1C). Each of these primary follicles consisted of an oocyte surrounded by a single cell layer of cuboidal follicular cells, while the secondary follicles were occasionally enclosed by a theca-cell-like layer (Figure 1D). At week 16 (age of maturity), all ovarian follicle types (primordial, primary, growing, and antral) were observed in the ovarian cortex. Primordial and primary follicles were located peripherally, whereas the growing and antral follicles were located centrally (Figure 1E). The antral follicle was distinguished by the formation of a fluid-filled cavity adjacent to the oocyte known as the antrum. This follicle was formed of an oocyte surrounded by acidophilic zona pellucida. A layer of columnar follicular cells was organized radially around the zona pellucida, forming the corona radiata. The follicular cavity was lined by stratified epithelium that constituted the mural granulosa layer. Cumulus oophorous cells connected the ovum and its surrounding cells with mural granulosa cells. Well organized theca folliculi surrounded the large antral follicles from outside. The theca folliculi were differentiated into theca interna that was more cellular and less fibrous, while the theca externa was more fibrous and less cellular. The cells of the theca interna were polyhedral or elongated in shape. (Figure 1F). A prominent and characteristic feature of the mature rabbit ovary was the presence of fully developed interstitial glands that were represented by groups of polyhedral or rounded cells separated from each other by connective tissue (Figure 1F). At the pregnancy stage (18 days post-coitum), the rabbit ovary was mostly occupied by corpora lutea with a low number of ovarian follicles at different developmental stages (Figure 2A,B).
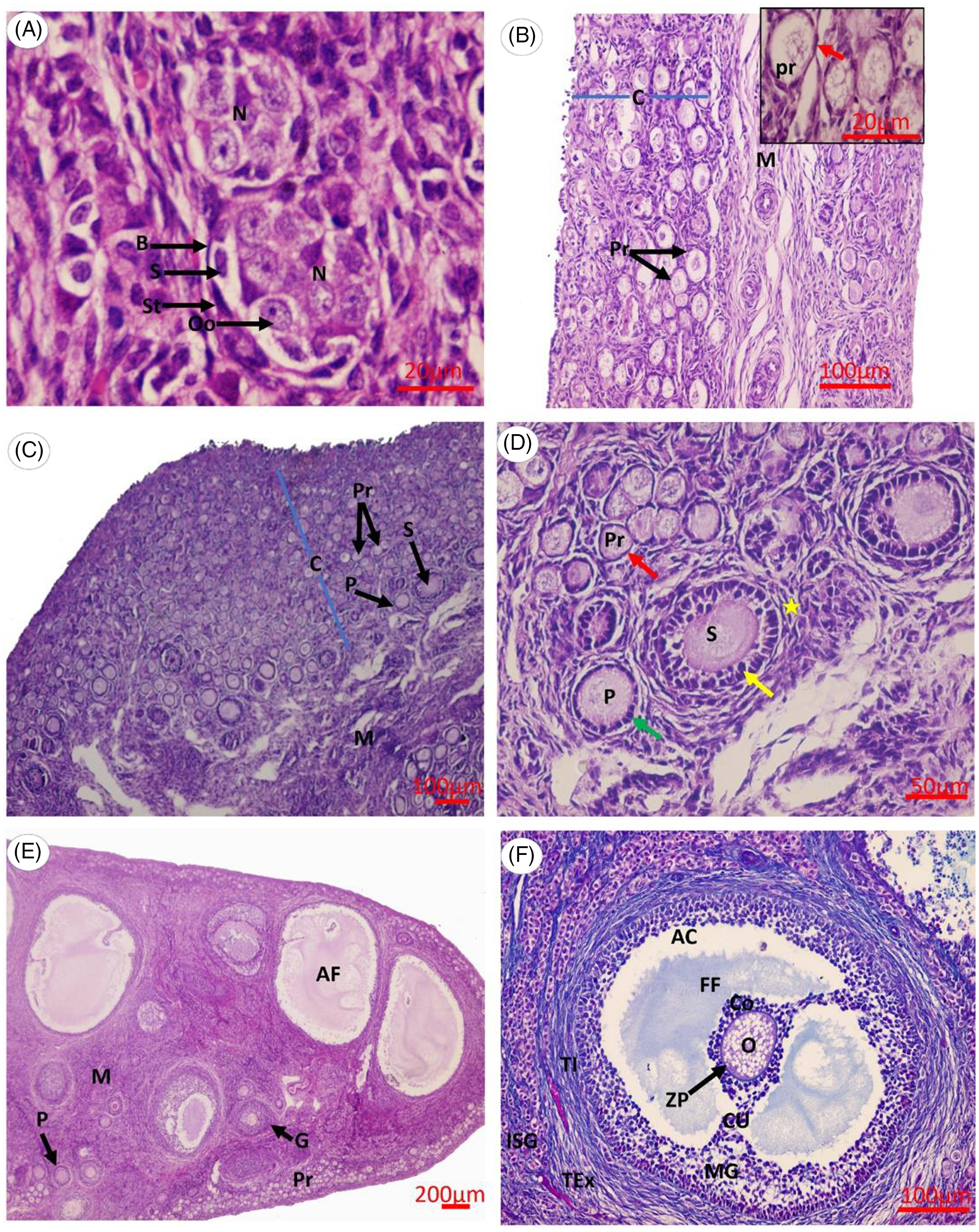
Figure 1. Photomicrograph of the rabbit ovary at different postnatal development stages. (A) 0 day, (B) 2 weeks, (C, D) 4 weeks, (E, F) 16 weeks. Abbreviations: AC, antrum cavity; AF, antral follicle; B, basal lamina; C, cortex; Co, corona radiata; CU, cumulus oophorous cells; FF, follicular fluid; G, growing follicle; ISG, interstitial gland; M, medulla; MG, mural granulosa cells; N, oogonial nest; O, primary oocyte; Oo, oogonia; P, primary follicle; Pr, primordial follicle; S, secondary follicle/somatic cells; St, stromal cells; TEx, theca externa; TI, theca interna. Red arrow, flattened follicular cells; green arrow, cuboidal follicular cells; yellow arrow, granulosa cells; yellow asterisk, theca cells. Scale bars: 20 μm (A), 100 μm (B, C, F), 50 μm (D), 200 μm (E). (A–E) Haematoxylin and eosin stain. (F) Masson’s trichrome stain.

Figure 2. Photomicrograph of 18 day pregnant rabbit ovary. (A, B) Pregnant rabbit ovary containing AF, antral follicle; CL, corpus luteum; M, ovarian medulla; P, primary follicle; Pr, primordial follicle. Scale bars: 200 μm (A), 100 μm (B). Masson’s trichrome stain.
Histomorphometry for different follicular structures
The thickness of the granulosa layer in primordial follicles increased significantly (three-fold) from 1.54 ± 0.16 µm to 4.37 ± 0.44 µm in primary follicles at 2 weeks of age (P < 0.05) and increased significantly (12-fold) from 1.58 ± 0.08 µm in primordial follicles to 18.55 ± 1 µm in secondary follicles at 4 weeks of age (P < 0.05). This thickness increased significantly (35-fold) from the primordial stage (1.84 ± 0.33 µm) to the antral stage (65.19 ± 7.5 µm) at 16 weeks of age (P < 0.05) and ∼38-fold from 1.64 ± 0.6 µm in primordial follicles to 62.34 ± 6.4 µm in antral follicles at pregnancy (P < 0.05). The theca cell layer appeared at the secondary stage in rabbit follicles. Theca cell layer thickness increased significantly (six-fold) from the secondary (13.35 ± 0.6 µm) to the antral stage (85.44 ± 2.9 µm) at 16 weeks of age and six-fold from 13.21 ± 0.65 µm in secondary follicles to 85.39 ± 16.2 µm in antral follicles at pregnancy (P < 0.05) (Table 1).
Table 1. Micromorphometric measurements showed thickness of granulosa and theca layers of different types of ovarian follicles in the rabbit ovary

Data presented in form of mean ± SEM. The same uppercase letters (A) in the same row mean that there was no significant difference (P < 0.05). Values within the same column for the thickness of granulosa and theca layers (µm) and carrying different lowercase letters (a–e) are significantly different at P < 0.05.
Differential expression of FSHR and LHR genes
The data obtained from RT-qPCR revealed that ovarian FSHR gene expression was detectable at a low level in the newly born rabbit around the time of birth (0D) followed by a significant increase by week 2 to week 4 after birth. Thereafter, FSHR gene expression significantly increased (∼11-fold more than the 0D) by week 16, then decreased notably by pregnancy (18 dpc) (Figure 3A). The levels of LHR transcripts remained low from the day of birth (0D) to week 4 and became significantly higher by week 16. At pregnancy, LHR expression increased and reached the maximum level (Figure 3B).

Figure 3. RT-PCR analysis of mRNA levels in 15 rabbit ovarian samples (n = 3 per each group). Three replicates of RT-PCR assays were performed for each sample. (A) Relative FSHR gene expression. (B) Relative LHR gene expression. Different letters above the bars indicate significant differences between different ovarian developmental stages (P < 0.05). Data presented as mean ± SEM. Abbreviations: FSHR, follicular stimulating hormone receptor; LHR, luteinizing hormone receptor. 0D, 0 day; 2W, 2 weeks; 4W, 4 weeks; 16W, 16 weeks; 18 DPC, 18 days post-coitum.
Differential expression of FSHR and LHR proteins
The expression levels of FSHR protein as detected by western blot exhibited a significant gradual increase with highest expression at week 16 (Figure 4A,B). The expression was decreased at pregnancy (18 dpc), but remained higher than at the earlier stages (from 0D to 4 weeks) (Figure 4A, B). In contrast, LHR protein did not show any significant difference from birth to week 4, but at further stages its expression gradually increased until reaching the highest expression at pregnancy (Figure 4A,C).

Figure 4. Western blot analysis of FSHR and LHR protein in 15 rabbit ovarian samples (n = 3 per each group). Three replicates were conducted for each protein sample. (A) Western blots of FSHR, LHR, and β-actin (internal control) at different ages. Densitometric analysis of (B) FSHR and (C) LHR. Different letters above bars indicate significant differences between the different ovarian developmental stages (P < 0.05). Data presented as mean ± SEM. Abbreviations: FSHR, follicular stimulating hormone receptor; LHR, luteinizing hormone receptor; 0D, 0 day; 2W, 2 weeks; 4W, 4 weeks; 16W, 16 weeks; 18 DPC, 18 days post-coitum.
Correlation between genes and proteins expression and ovarian parameters
It was noted from our results that a significant increase in LHR and FSHR gene and protein expression was observed by the appearance of the advanced ovarian follicles that have many layers of granulosa cells and defined thecal layers. Therefore, we investigated the correlation between FSHR and LHR gene and protein expression and granulosa and theca layer thickness. The obtained results showed that FSHR gene and protein expression had a significant positive correlation with increases in granulosa layer thickness (Table 2). LHR gene and protein expression revealed a significant positive correlation with increases in theca layer thickness (Table 3).
Table 2. Correlation between the thickness of the granulosa layer of different types of follicles and the expression of the FSHR gene and protein

r: Pearson’s correlation coefficient.
Statistical significances were set at *P < 0.05 or **P < 0.01.
Table 3. Correlation between the thickness of the theca layer of different types of ovarian follicles and the expression of the LHR gene and protein

r: Pearson’s correlation coefficient.
Statistical significances were set at *P < 0.05 or **P < 0.01.
Discussion
Based on the role of FSHR and LHR in reproduction and the limited knowledge of transcriptional and translational expression of rabbit FSHR and LHR throughout folliculogenesis, we described the expression pattern of FSHR and LHR genes and proteins during the development of rabbit ovary, using folliculogenesis as a model system, and correlated their expression changes with the ovarian histomorphological changes, which would contribute to the creation of a theoretical basis for understanding the potential biological mechanisms of hormone receptors in ovarian development. The present study found that there was a correlation between linear morphological development of the ovarian follicles and the expression profiles of FSHR and LHR.
At birth, the ovarian cortex primarily consisted of germ cells arranged in cell nests. A similar result was obtained by Ricken and Viebahn (Reference Ricken and Viebahn2002) and Picut et al. (Reference Picut, Dixon, Simons, Stump, Parker and Remick2015). The data from RT-qPCR at this age exhibited low FSHR expression, indicating an earlier expression before the start of folliculogenesis. This result is similar to the data obtained by Chakraborty and Roy (Reference Chakraborty and Roy2015) on hamster, but differs from that reported by Lei et al. (Reference Lei, Jin, Mayo and Woodruff2010) and Hu et al. (Reference Hu, Zhu, Wang, Li, He, Hu, Hu and Xia2021) who found subsequent FSHR expression coinciding with the start of folliculogenesis.
By week 2 of age, the FSHR gene significantly increased compared with its level at birth. This is the period during which the newly formed primordial follicles first appeared in the inner part of the ovarian cortex that was a hallmark of initiating folliculogenesis. In line with these findings, Durlej et al. (Reference Durlej, Knapczyk-Stwora, Duda, Galas and Slomczynska2011) also reported a negligible expression of FSHR in porcine ovary at birth, with only an appreciable expression observed when primordial follicles appeared. The increase in FSHR expression at this age could be due to the appearance of follicular cells of the primordial follicles, as these cells showed higher FSHR expression (Rannikki et al., Reference Rannikki, Zhang and Huhtaniemi1995; Hillier, Reference Hillier2001; Durlej et al., Reference Durlej, Knapczyk-Stwora, Duda, Galas and Slomczynska2011).
At week 4, FSHR was significantly upregulated and this elevated expression coincided with the development of primary and secondary follicles (O’Shaughnessy et al., Reference O’Shaughnessy, McLelland and McBride1997). This upregulation could be due to the transformation and proliferation of granulosa cells during the growth phase (Wu et al., Reference Wu, Han, Cao, Zhang, Li, Liu, Liu, Li, Pan, Chen and Liu2015). Similarly, one previous study showed that FSHR was higher in primary follicles than in primordial follicles (Zheng et al., Reference Zheng, Magid, Kramer and Chen1996).
By week 16 (age of maturity), a marked morphological growth of the rabbit ovary has occurred and has become fully developed containing ovarian follicles in all developmental stages including primordial, primary, growing and antral follicles (Al-Saffar and Almayahi, Reference Al-Saffar and Almayahi2018). During this period, the level of the FSHR gene dramatically increased. Notably, this increase coincided with the appearance of growing follicles, and small and large antral follicles that contained numerous layers of granulosa cells (Kishi et al., Reference Kishi, Kitahara, Imai, Nakao and Suwa2018).
In general, this study found a positive correlation between FSHR mRNA and protein expression and thickness of the granulosa layer, as the ovary grows during postnatal life. Therefore, the results of this study supported the hypothesis that FSHR is essential for the proliferation of granulosa cells (Johnson and Woods, Reference Johnson and Woods2009; Donaubauer and Hunzicker-Dunn, Reference Donaubauer and Hunzicker-Dunn2016).
Follicular development and steroidogenic activity are necessary to maintain pregnancy in the rabbit, as estradiol is the luteotropin principle in rabbits (Goodman et al., Reference Goodman, Kugu, Chen, Preutthipan, Tilly, Tilly and Dharmarajan1998), and only follicles are the major source of oestrogen (Niswender et al., Reference Niswender, Juengel, Silva, Rollyson and McIntush2000). The number and size of large follicles in pregnant rabbit ovaries decreased compared with the number and size of follicles in non-pregnant rabbit ovaries, as high progesterone during pregnancy appears to suppress follicular development (Saleh, Reference Saleh2013; Fawzy et al., Reference Fawzy, Ibrahim, Mahmoud, Heleil, Ismail, Almadaly, El-Magd and Ramoun2021). In agreement with previous studies, our results indeed found that the ovarian cortex of the pregnant rabbit ovary was mostly occupied by corpora lutea, with few different types of ovarian follicles. By analyzing the expression of the FSHR gene during this period, we found an expected decrease in the expression of this gene. Therefore, it was speculated that the decrease in the expression of the FSHR gene might be due to the decrease in the number of developing follicles in the pregnant ovary. This reflects that the number of follicles may affect the total level of FSHR expression (Quan et al., Reference Quan, Zheng, Ling, Fang, Chu, Zhang, Liu and Li2019). Furthermore, this result demonstrated that FSHR expression is affected by reproductive status (pregnancy).
Data from RT-PCR showed that LHR transcripts remained low from the day of birth (0D) to week 4. This is the period in which no theca cells are morphologically distinguished, although at week 4 the theca cells surrounding the secondary follicles can be identified. Nonetheless, they were still not well differentiated, which was consistent with previous in situ studies showing that LHR is expressed by differentiating theca cells (Kishi et al., Reference Kishi, Kitahara, Imai, Nakao and Suwa2018). Thereafter, at week 16, we found that LHR expression became significantly higher and coincided with the appearance of growing and antral follicles, which have a defined thecal layer. These results are compatible with in situ hybridization studies in which LHR mRNA was expressed exclusively in ovarian theca cells (Zhang et al., Reference Zhang, Shi, Segaloff and Van Voorhis2001). Also, this study found a significant positive correlation between LHR mRNA, protein expression and the thickness of theca cell layer, suggesting that LHR is essential for the development of the thecal compartment (Kishi et al., Reference Kishi, Kitahara, Imai, Nakao and Suwa2018).
The histological sections of the pregnant rabbit ovary revealed that the rabbit ovary contained several well developed corpora lutea, occupying most of the ovarian cortex in addition to some different ovarian follicles as reported by Saleh (Reference Saleh2013). By following the expression of LHR at this time, we found that the expression of LHR mRNA increased and reached the maximum level. It is expected that the appearance of several corpus lutea in addition to the presence of growing and antral follicles, which have a defined thecal layer leading to an increase in the total level of LHR expression as the earlier studies, showed that LHR was demonstrated to be an integral component of the luteal cells of corpus lutea, theca cells and large follicles in the ovary (Zhang et al., Reference Zhang, Shi, Segaloff and Van Voorhis2001; Ziecik et al., Reference Ziecik, Kaczmarek, Blitek, Kowalczyk, Li and Rahman2007).
Post-transcriptional, translational, and degradation regulation of proteins have recently been shown to be as significant as transcription itself (Vogel and Marcotte, Reference Vogel and Marcotte2012). Therefore, FSHR and LHR expression was analyzed at the protein level in ovarian tissue using western blot. Notably, the protein expression of FSHR and LHR had similar mRNA pattern changes. This compatibility between mRNA and protein profiles supports the hypothesis that both gonadotropin receptors are involved in the regulation of folliculogenesis in rabbits
In conclusion, this is the first study to reveal mRNA and protein expression of FSHR and LHR throughout rabbit folliculogenesis from birth to maturity, as well as in pregnant rabbit, and to correlate their expression changes with the thickness of granulosa and theca layers. These findings indicated that variations in the expression profiles of the FSHR gene and protein are associated with granulosa layer thickness, while LHR expression profiles are associated with theca layer thickness, suggesting that each gene/protein may have a different way to regulate ovary development in rabbits.
Acknowledgements
The authors express their gratitude to the Faculty of Veterinary Medicine/Benha University for supporting this work.
Conflict of interest
None of the authors have any conflict of interest to declare.









