Introduction
Somatic cell nuclear transfer (SCNT) has been applied widely in different animals including pigs; however, the efficiency of SCNT in pigs still remains low (Polejaeva et al., Reference Polejaeva, Chen, Vaught, Page, Mullins, Ball, Dai, Boone, Walker, Ayares, Colman and Campbell2000; Hyun et al., Reference Hyun, Lee, Kim, Kim, Lee, Nam, Jeong, S., Yeom, Kang, Han, Lee and Hwang2003; Park et al., Reference Park, Cho, Park, Lee, Choi, Kwon, Son, Seo and Kim2004; Pratt et al., Reference Pratt, Sherrer, Reeves and Stice2006). One of the main factors accounting for this low efficiency is the oocytes (Hyun et al., Reference Hyun, Lee, Kim, Kim, Lee, Nam, Jeong, S., Yeom, Kang, Han, Lee and Hwang2003; Ikeda & Takahashi, Reference Ikeda and Takahashi2003; Chen et al., Reference Chen, Li, Zhang, Han, Zhao, Wu and Huwang2007), as the recipient oocyte exerts a key role in remodelling of the chromatin introduced with the somatic nucleus. Previous studies have demonstrated that the volume of cytoplasm in the final reconstructed embryos is one important factor related to the nuclear transfer efficiency in mice (Kishigami & Wakayama, Reference Kishigami and Wakayama2007) and cattle (Peura et al., Reference Peura, Lewis and Trounson1998; Tecirlioglu et al., Reference Tecirlioglu, Cooney, Lewis, Korfiatis, Hodgson, Ruddock, Vajta, Downie, Trounson, Holland and French2005; Ribeiro et al., Reference Ribeiro, Gerger, Ohlweiler, Ortigari, Mezzalira, Forell, Bertolini, Rodriques, Ambrósio, Miglino, Mezzalira and Bertolini2009). Removal of cytoplasm will lead to problems in later development, as embryos with initially reduced cytoplasm often posses fewer cells at the morula or blastocyst stage (Evsikov et al., Reference Evsikov, Morozova and Solomko1990), but the success rates can be improved by addition of supplementary oocyte cytoplasm during nuclear transfer (Wakayama et al., Reference Wakayama, Kishigami, Nguyen, Ohta, Hikichi, Mizutani, Bui, Miyake and Wakayama2008).
For SCNT, two different enucleation techniques have been applied: traditional cloning by using a micromanipulator (Polejaeva et al., Reference Polejaeva, Chen, Vaught, Page, Mullins, Ball, Dai, Boone, Walker, Ayares, Colman and Campbell2000; Boquest et al., Reference Boquest, Grupen, Harrison, McIlfatrick, Ashman, d’Apice and Nottle2002; Kurome et al., Reference Kurome, Ishikawa, Tomii, Ueno, Shimada, Yazawa and Nagashima2008; Li et al., Reference Li, Liu, Dai, Xing, Fang, Zhang, Shi, Zhang and Chen2010) and hand-made cloning (HMC) by using a bisection blade (Du et al., Reference Du, Kragh, Zhang, Li, Schmidt, Bogh, Zhang, Purup, Jorgensen, Pedersen, Villemoes, Yang, Bolund and Vajta2007; Kragh et al., Reference Kragh, Nielsen, Li, Du, Lin, Schmidt, Bogh, Holm, Jakobsen, Johansen, Purup, Bolund, Vajta and Jorgensen2009; Li et al., Reference Li, Villemoes, Zhang, Du, Kragh, Purup, Xue, Pedersen, Jorgensen, Jakobsen, Bolund, Yang and Vajta2009; Ribeiro et al., Reference Ribeiro, Gerger, Ohlweiler, Ortigari, Mezzalira, Forell, Bertolini, Rodriques, Ambrósio, Miglino, Mezzalira and Bertolini2009). In the latter technique, the cytoplasm reduction is compensated for by using two fusion steps, each with one half cytoplasm (Li et al., Reference Li, Du, Zhang, Kragh, Purup, Bolund, Yang, Xue and Vajta2006), so the resulting cloned embryos usually attain approxi-mately 100–125% of the original cytoplasm volume and demonstrate good developmental capacity (Du et al., Reference Du, Kragh, Zhang, Li, Schmidt, Bogh, Zhang, Purup, Jorgensen, Pedersen, Villemoes, Yang, Bolund and Vajta2007; Kragh et al., Reference Kragh, Nielsen, Li, Du, Lin, Schmidt, Bogh, Holm, Jakobsen, Johansen, Purup, Bolund, Vajta and Jorgensen2009). In contrast, similar good results have not been achieved by studies in mice (Wakayama et al., Reference Wakayama, Kishigami, Nguyen, Ohta, Hikichi, Mizutani, Bui, Miyake and Wakayama2008), perhaps because of species differences. Only a few studies regarding the modification of cytoplasm of reconstructed embryos have been performed in pigs (Terashita et al., Reference Terashita, Sugimura, Kudo, Amano, Hiradate and Sato2011). The amount of cytoplasm of reconstructed embryos might affect the developmental kinetics in for example the timing of cleavage and blastocyst formation and the blastocyst cell numbers (Evsikov et al., Reference Evsikov, Morozova and Solomko1990; Feng & Gordon, Reference Feng and Gordon1997), so more detailed studies of the effect of cytoplasm volume on the developmental kinetics during in vitro culture are needed.
Finally, it has been reported that the interaction between nucleus and cytoplasm is of critical importance in determining the outcome of oocyte maturation and embryonic development (Fulka et al., Reference Fulka, First and Moor1998; Moor et al., Reference Moor, Dai, Lee and Fulka1998). Therefore, it will be necessary to consider the effect of changes from not only the cytoplasm but also from the nucleus in the reconstructed embryos. Hence, parthenogenetic activated (PA) embryos with the original nucleus from the matured oocytes and HMC embryos with somatic donor cells were used in the present study.
Therefore, the objectives of the current study were: (i) to investigate the influence of cytoplasm volume on in vitro development of cloned and PA embryos; (ii) to use time-lapse observation of the different cell cycles of HMC and PA embryos to monitor the effect of the cytoplasm volume on the developmental kinetics of these embryos.
Materials and methods
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA), unless otherwise stated. All manipulations were performed on a heated stage adjusted to 39°C, unless otherwise indicated.
Oocyte collection and in vitro maturation
In vitro oocyte maturation was performed as described earlier (Du et al., Reference Du, Kragh, Zhang, Li, Schmidt, Bogh, Zhang, Purup, Jorgensen, Pedersen, Villemoes, Yang, Bolund and Vajta2007). Briefly, cumulus–oocyte complexes (COCs) were aspirated from 2–6 mm follicles of slaughterhouse-derived sow ovaries with an 18-gauge needle using vacuum suction. The COCs were selected according to their morphological characteristics, i.e. at least three layers of compact cumulus and even cytoplasm. After washing twice in HEPES-buffered Tissue Culture Medium 199 (TCM-199, Invitrogen, Carlsbad, California, USA) plus 0.3% heparin, 5% amphotericin and 10% cattle serum (CS; DTU-VET, Frederiksberg, Denmark), the COCs were cultured in groups of 50–60 for 42–44 h in 4-well dishes (Nunc, Roskilde, Denmark) with bicarbonate-buffered TCM-199 supplemented with 10% (v/v) CS, 10% (v/v) pig follicular fluid, 10 IU/ml pregnant mare's serum gonadotropin (PMSG) and 5 IU/ml human chorionic gonadotropin (hCG) at 38.5°C with 5% CO2 in air with maximum humidity.
Somatic cell nuclear transfer–hand-made cloning
All handling medium drops were 20 μl in volume and were covered with mineral oil.
Oriented bisection
The oriented enucleation procedure was as previously described by Li et al. (Reference Li, Villemoes, Zhang, Du, Kragh, Purup, Xue, Pedersen, Jorgensen, Jakobsen, Bolund, Yang and Vajta2009). Briefly, after the removal of cumulus cells of the matured oocytes and partial digestion of zona pellucida using 3.3 mg/ml pronase in T33, oocytes were washed in T2 and T20 drops (TCM-199 with CS; numbers refer to volume ratio of CS, here 33, 2 and 20%, respectively). Oocytes were lined up in a T2 drop supplemented with 2.5 μg/ml cytochalasin B (CB) and were rotated to identify the polar body (PB). Oriented bisection, according to the PB position, was performed manually with a microblade (AB Technology, Pullman, WA, USA) under a stereomicroscope. The halves without the PB were selected for use in first and second fusion and were transferred into a T2 drop to be used as cytoplasm, while some of the halves with PB were randomly collected and used later for activation.
First fusion
Porcine fetal fibroblast cells were established and prepared from a Danish large white pig as described previously by Du et al. (Reference Du, Kragh, Zhang, Li, Schmidt, Bogh, Zhang, Purup, Jorgensen, Pedersen, Villemoes, Yang, Bolund and Vajta2007). Confluence of the donor cells was achieved after 4–5 days in vitro culture, and the cells (passages 5–9) were trypsinized before being stored in T2 at room temperature (22–25 °C) until use for first fusion.
After a short equilibration in the fusion medium (0.3 M mannitol supplemented with 0.01% [w/v] polyvinyl alcohol (PVA)), each cytoplast was transferred to 1 mg/ml of phytohemagglutinin (PHA) for 2–3 s, dropped over a single somatic cell sedimented at the bottom of a 20 μl T2 drop with a total of about 200 somatic cells. Cytoplasm–fibroblast pairs were aligned to one wire in a fusion chamber (BTX, San Diego, CA, USA) using an alternating current (AC) of 0.6 kV/cm during the whole aligning progress, then fused with a single direct current (DC) impulse of 2.0 kV/cm for 9 μs by using an electrofusion machine (BLS, Budapest, Hungary).
Second fusion and activation
After 1 h incubation in a T10 drop, fused pairs were selected and equilibrated alongside with cytoplasts in activation medium (0.3 M mannitol, 0.1 mM MgSO4 and 0.1 mM CaCl2 supplemented with 0.01% [w/v] PVA). The fused pair and another one or two cytoplasts (according to the experimental design) were aligned to one wire of the fusion chamber using an AC of 0.6 kV/cm, and fusion was initiated using a single DC pulse of 0.86 kV/cm for 80 μs. For chemical activation, the reconstructed embryos were transferred into one well of a 4-well dish in 400 μl porcine zygote medium 3 (PZM-3; Yoshioka et al., Reference Yoshioka, Suzuki, Tanaka, Anas and Iwamura2002) supplemented with 5 μg/ml CB and 10 μg/ml cycloheximide (CX) and covered with 400 μl mineral oil for culture at 38.5°C in 5% CO2, 5% O2 and 90% N2 with maximum humidity for the 4 h chemical activation treatment. In the routine system, the final volume of such reconstructed embryos is around 125% of one oocyte.
In vitro culture of the cloned embryos
Microwells (WOWs; approx. depth 130 μm, width 150 μm; Du et al., Reference Du, Kragh, Zhang, Li, Schmidt, Bogh, Zhang, Purup, Jorgensen, Pedersen, Villemoes, Yang, Bolund and Vajta2007) were made by repeated pressing of an unheated darning needle into the bottom of the dish. In each well of the 4-well dish, 25–30 microwells were made. After the chemical activation described above, the embryos were washed thoroughly and transferred: one embryo into each microwell. Each well of the 4-well dish was covered with 400 μl PZM-3 medium and 400 μl mineral oil, and the dish was then incubated in the Galaxy R CO2 incubator (RSBiotech, UK) at 38.5°C in 5% CO2, 5% O2 and 90% N2 with maximum humidity for 6 days.
Parthenogenetic activation–zona pellucida (ZP)-free oocytes
An optimized activation protocol applied for ZP-free oocytes was used as described previously (Kragh et al., Reference Kragh, Du, Corydon, Purup, Bolund and Vajta2005) but with slight modifications. Briefly, after cumulus cell removal by hyaluronidase, oocytes with a visible PB, indicating successful nuclear maturation, were selected to be used for PA and were sorted into either non-sorted (the common way for doing PA, used as control), large or small oocytes depending on their size. Then, the sorted oocytes were used for fusion and/or activation as described in the section on ‘experimental design’.
After a short equilibration, the ZP was removed by incubating the oocytes in 3.3 mg/ml pronase in T33 for 1 min. The ZP-free oocytes and some cytoplasts with half cytoplasm volume achieved from ‘Oriented bisection’ as described above were then moved into T10 medium until activation. The electrical stimulation was delivered with the electrofusion machine to the chamber overlaid with activation medium (0.3 M mannitol, 0.05 mM CaCl2, 0.1 mM MgSO4 and 0.01% PVA, pH adjusted to 7.8). After a short equilibration in the activation medium, 10–15 ZP-free oocytes, one non-sorted cytoplast without PB or ZP-free oocyte–cytoplast pairs were transferred into the chamber in each working round (as described in the section on ‘experimental design’). A single DC pulse with 0.86 kV/cm was applied for 80 μs. The process for the post-activation and in vitro incubation of these PA embryos was the same as described above.
Embryo evaluations
On day 0 (the day of cloning), the diameter of the reconstructed embryos (HMC and PA) collected randomly from different groups was evaluated after the chemical activation and before in vitro culture in the incubator by using the EmbryoScope (Unisense FertiliTech A/S, Denmark); an example with PA embryos is shown in Fig. 1. Fusion rate (the number of cytoplasts fused with donor cells divided by the total number of cytoplasts used for fusion with donor cells) of the reconstructed hemicytoplast with donor cell was assessed by microscopic examination at 1 h after the first fusion and before the second fusion. On days 2 and 6, rates of cleavage (the number of reconstructed embryos that had cleaved divided by the number of total reconstructed embryos that had been cultured in vitro) and blastocysts (the number of embryos that had developed into blastocysts divided by the number of total reconstructed embryos that had been cultured in vitro), respectively, were recorded.

Figure 1 Diameter measurement of porcine parthenogenetic (PA) embryos by the EmbryoScope.
On day 6, the total cell number per blastocyst was determined. Blastocysts were collected randomly and transferred into a 20 μl T2 droplet containing 5 μg/ml of Hoechst 33342 and incubated for 15 min in total darkness. After washing three times in T10, embryos were placed on a glass slide in 5 μl glycerol and covered with a 10 × 10 mm cover glass that subsequently was carefully pressed to assure that the cells from each embryo were not overlapping each other. After overnight storage in a dark place at room temperature, the slides were observed with an epifluorescence microscope and UV-2A filter (Leica DMIRB, Cambridge, UK). Pictures were taken of each embryo, and their cell numbers were counted from the microscopic pictures with the RunImageCount software (our own design). Briefly, the microscopic picture of each embryo was opened by RunImageCount software and each cell in the embryo over the picture was marked with a red star once it was counted by clicking on it. The total cell number per embryo was counted accordingly.
Experimental design
The present work was performed over a period of several months, but both before and during this period the laboratory performed SCNT (Kragh et al., Reference Kragh, Nielsen, Li, Du, Lin, Schmidt, Bogh, Holm, Jakobsen, Johansen, Purup, Bolund, Vajta and Jorgensen2009; Li et al., Reference Li, Villemoes, Zhang, Du, Kragh, Purup, Xue, Pedersen, Jorgensen, Jakobsen, Bolund, Yang and Vajta2009; Schmidt et al., Reference Schmidt, Kragh, Li, Du, Lin, Liu, Bøgh, Winther, Vajta and Callesen2010; Liu et al., Reference Liu, Østrup, Li, Vajta, Kragh, Purup and Callesen2011; Luo et al., Reference Luo, Li, Liu, Lin, Du, Li, Yang, Vajta, Callesen, Bolund and Sørensen2011; Al-Mashhadi et al., Reference Al-Mashhadi, Sørensen, Kragh, Christoffersen, Mortensen, Tolbod, Thim, Du, Li, Liu, Moldt, Schmidt, Vajta, Larsen, Purup, Bolund, Nielsen, Callesen, Falk, Mikkelsen and Bentzon2013) based on sow oocytes almost every week. Because of this regular use and the relative stability of the results obtained, the basic system served as the overall control of the results from the current experimental work. Briefly, controls were therefore matured oocytes with first PB used directly for HMC, where bisection and subsequent fusion of the two halves was made to the same fibroblast cells as used in the experimental work to reach a volume of ~125% of the original oocyte. In all experiments, the developmental ability of the reconstructed embryos was evaluated on day 2 and day 6 after day of cloning (day 0) based on cleavage and blastocyst rates, respectively.
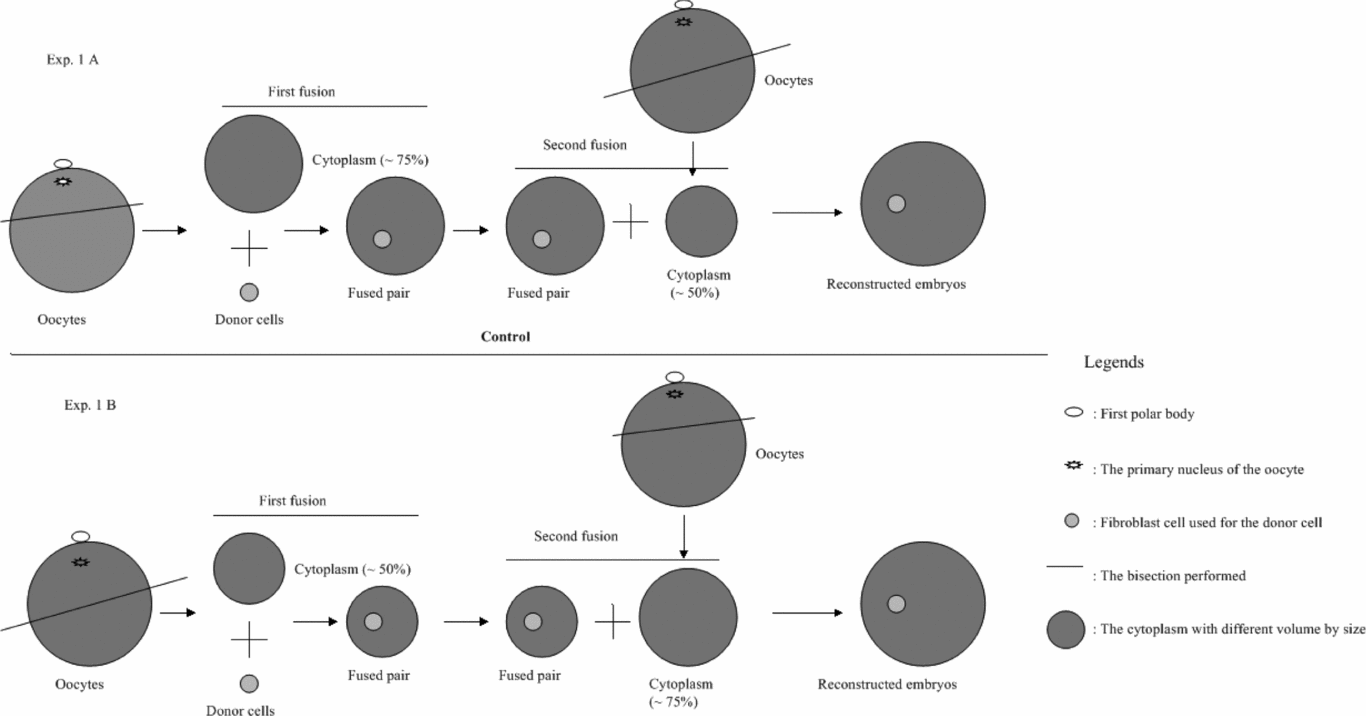
Experiment 1: Effect of different cytoplasm volume used for the first fusion with donor cells on developmental potential of cloned embryos
As described above, the final cytoplasm volume of the reconstructed embryos was around 125% of one oocyte, and this can be achieved in two different ways. Therefore, the possible differences caused by these two methods were compared as shown in Fig. 2. Briefly, after the orientated bisection of matured oocytes (Fig. 3), the selected cytoplasts were sorted into two groups: large cytoplasts (~75% of original cytoplasm volume) and small cytoplasts (~ 50% of original cytoplasm volume). The diameter of the cytoplast with ~75% and ~50% volume was then measured immediately after sorting.

Figure 2 The experimental design for Experiment 1.

Figure 3 Cytoplasm with different volume selected after bisection during hand-made cloning (HMC). PB, polar body.
First fusion was then performed in two combinations as shown in Fig. 2: (a) ~50% cytoplasm fused with donor cells, followed by the ~75% during the second fusion step; and (b) ~75% cytoplasm fused with donor cells, followed by the ~50% during the second fusion (used as control group). After reconstruction, the fusion rate of the cytoplast with donor cells was recorded before in vitro culture for 6 days. Cleavage and blastocyst rates were registered as well as the total cell number per blastocyst.
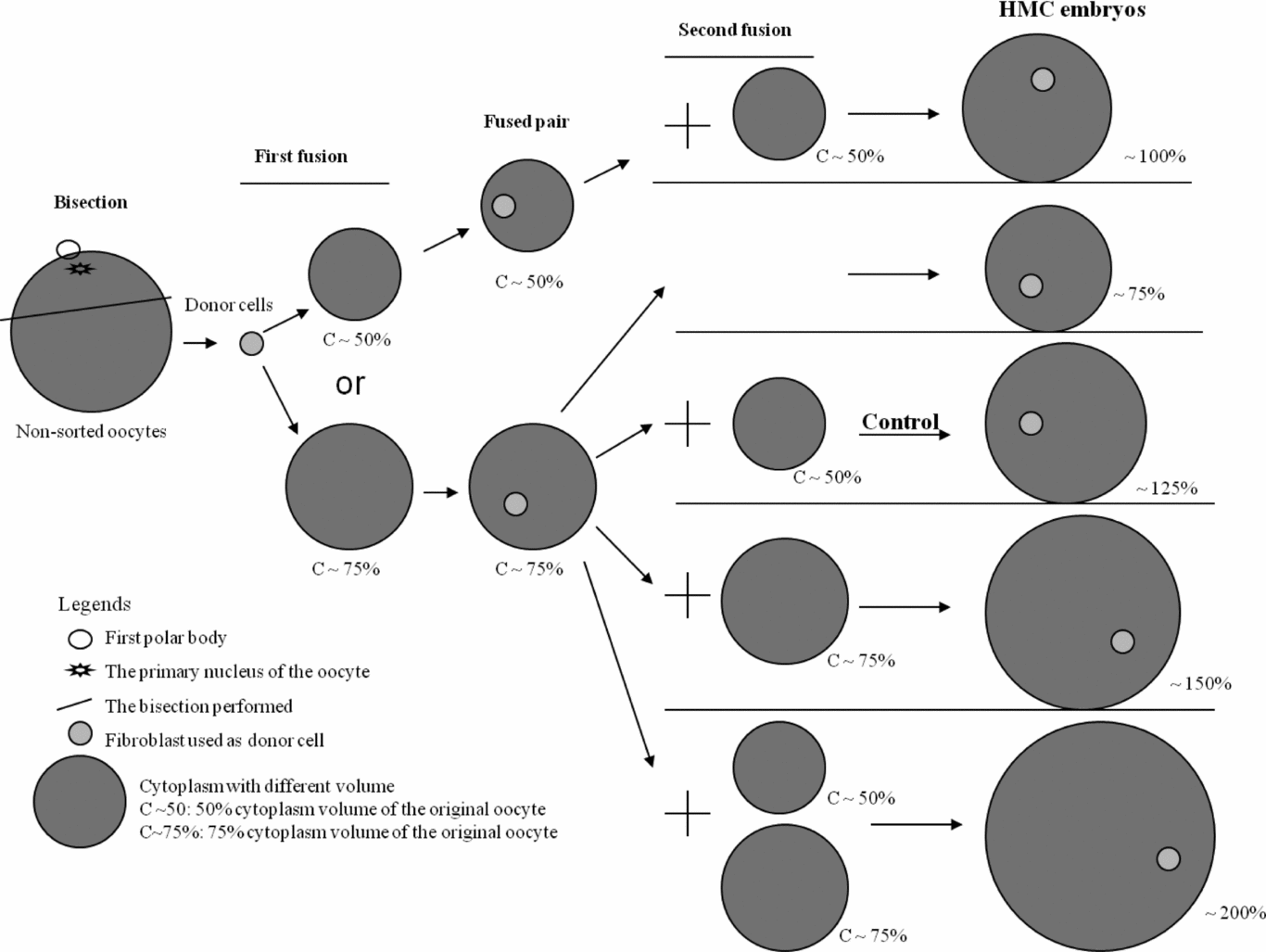
Experiment 2: Effect of different cytoplasm volume in final reconstructed embryos
Based on the results from Experiment 1, the ~75% cytoplasm was used for the first fusion, and the different cytoplasm volume was then applied in the second fusion as shown in Fig. 4 to achieve cloned embryos with different final cytoplasm volume (~75%, ~100%, ~125%, ~150% and ~200%). The ~125% group was considered as control. In each group, 12 reconstructed embryos in three replicates were randomly collected and their diameters were measured. Developmental ability including rates of cleavage and blastocyst as well as total cell numbers were evaluated as described in Experiment 1.

Figure 4 The experimental design for Experiment 2. HMC, hand-made cloning.
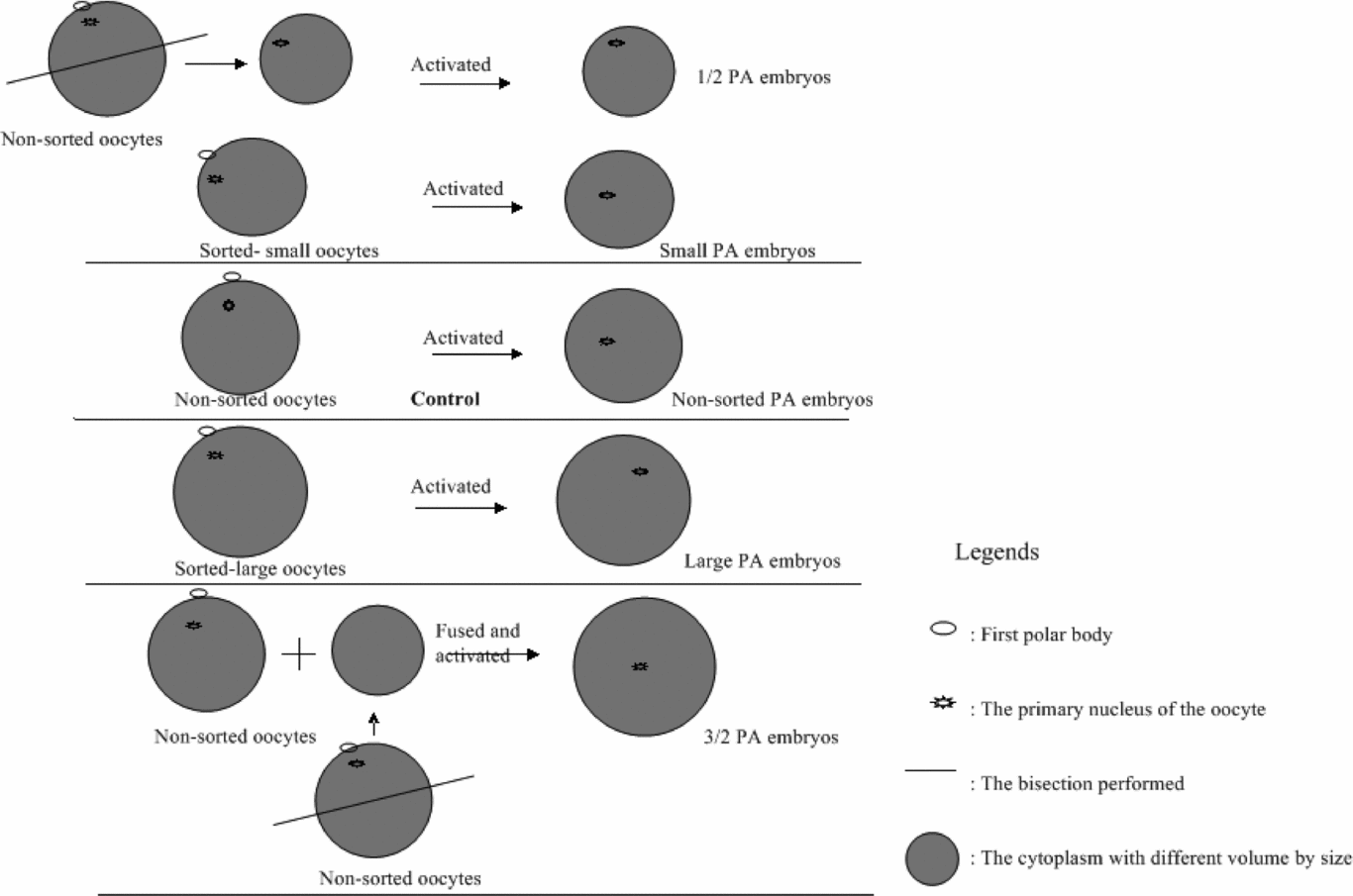
Experiment 3: Effect of different cytoplasm volume on the development of the PA oocytes (Fig. 5)
Matured oocytes were sorted into three groups according to their morphological size, observed under the microscope: non-sorted oocytes (the common way for doing PA and used as control), large oocytes and small oocytes. The activation was carried out on these groups of oocytes as follows: The small, non-sorted and large oocytes were activated as described above. For the other two groups (½ oocyte group and 1½ oocyte group), the procedure was as follows: the non-sorted oocyte was bisected as described above. In order to create the ½ oocyte, the halves containing the PB were selected and used for activation, while to create the 1½ oocyte, one oocyte was fused with one half cytoplast without PB, which had been collected after bisection. Subsequently, the diameter and developmental ability of all activated oocytes were evaluated as described above.

Figure 5 The experimental design for Experiment 3.
Experiment 4: Developmental kinetics of HMC and PA embryos by time-lapse
From each of the following groups (Experiment 2: HMC: 75%, 125% and 200%; Experiment 3: PA: ½ oocyte, non-sorted oocytes and 1½ oocyte), embryos were cultured in vitro in the time-lapse system under the same conditions. Because of the limitations on number of embryos that can be observed in each working round (only 40 individual embryos could be observed at one time) and since one working round was time-consuming, taking one week per round, the embryos were observed in different working rounds. In one round, 20 individual embryos from one treatment group were running, so only two treatment groups could be running in one round. In each round, the video graph and the precise time were recorded every 30 mins for each embryo, and the first time for the formation of 2-cell, 3-cell or 4-cell, morula and blastocyst stage were then registered later for each embryo.
Statistics
The data were analysed by the single factor of analysis of variance (ANOVA) analysis. A probability of P < 0.05 was considered to be statistically significant.
Results
Experiment 1: Effect of different cytoplasm volume used for the first fusion with donor cells on developmental potential of cloned embryos
The average diameter of the cytoplasm in the ~75% group (98.4 ± 1.3 μm) was significantly different to that in the ~50% group (87.6 ± 1.0 μm). However, in five replicates, no significant difference was observed either on the fusion rate with donor cells (97.5% (115/118) vs. 99.0% (107/108)) or on the cleavage (91.3% (105/115) vs. 95.4% (102/107)) and blastocyst rates (53.9% (62/115) vs. 57.9% (62/107)).
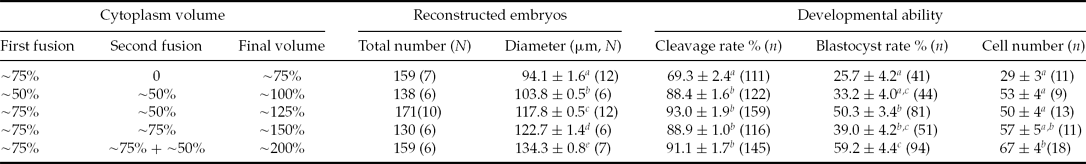
Experiment 2: Effect of different cytoplasm volume in final reconstructed embryos
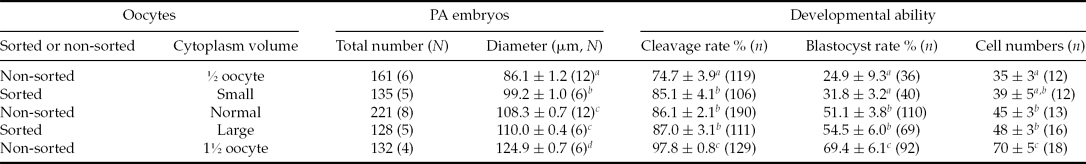
As shown in Table 1, the average diameter of the cloned embryos after reconstruction gradually increased with increasing volume from 75% to 200% after cloning. Also the developmental ability of the cloned embryos was influenced by increasing volume: the 75% group had the lowest values for each parameter (cleavage and blastocyst, total cell numbers per blastocyst), while the 200% had the highest values. The 100 to 150% groups had similar levels for all these values.
Table 1 Developmental potential of reconstructed porcine embryos with different final cytoplasm volume after second fusion of hand-made cloning (HMC)

N indicates the repeats.
n indicates the number of observed embryos.
Different superscripts in the same column indicate significant differences. a ,b,c P < 0.05.
Mean values are shown ± standard error of mean (SEM).
Experiment 3: Effect of different cytoplasm volume on the development of the PA oocytes
As shown in Table 2, the average diameter of the PA embryos gradually increased from the ½ to 1½ group. Also, the developmental ability of the PA embryos was influenced by increasing volume, with the ½ group showing the lowest values for the various parameters (cleavage and blastocyst, total cell numbers per blastocyst), while the 1½ had the highest values. The small, non-sorted and large groups had similar levels for all these values.
Table 2 Developmental potential of porcine parthenogenetic (PA) embryos with different cytoplasm volume in the final activated embryos

N indicates the repeats.
n indicates the number of observed embryos.
Different superscripts in the same column show significant differences. a ,b,c P < 0.05.
Mean values are shown ± standard error of mean (SEM).
Experiment 4: Developmental kinetics of HMC and PA embryos by time-lapse
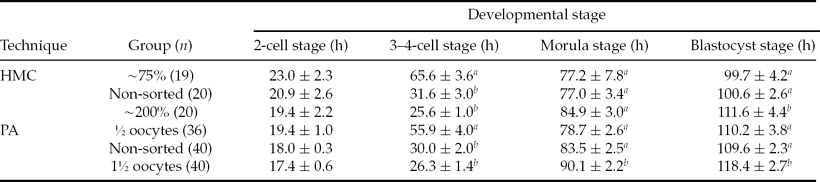
As shown in Table 3, the time for 2-cell and 3–4-cell stages was reduced with increasing cytoplasm volume both in HMC embryos and PA embryos. For both embryo types, a common pattern was observed: the smaller group tended to have a longer time before 2-cell formation and a clearly longer time before the 3–4-cell stage was first seen, while the largest group had the longest time before morula and blastocyst formation, and the non-sorted controls were in between. Therefore, with the increasing volume of cytoplasm in the reconstructed embryos, the time taken for the early stages of cell cycles showed a linear decrease, while that for the later stages showed a linear increase.
Table 3 Start time for the cell cycle of the developmental stage in porcine hand-made cloning (HMC) and parthenogenetic (PA) embryos

Different superscripts in the same column show significant differences. a ,b P < 0.05.
Mean values are shown ± standard error of mean (SEM).
n indicates the number of embryos were observed.
Discussion
The current study is the first report on the effect of volume of cytoplasm on the developmental kinetics of reconstructed porcine embryos evaluated by non-invasive time-lapse technique. The overall results showed that the developmental kinetics of reconstructed embryos were clearly affected by the cytoplasm volume, and the developmental ability of porcine cloned or PA embryos was improved with increasing cytoplasm volume.
One important factor related to the efficiency of the nuclear transfer is the volume of cytoplasm in the final reconstructed embryos. Some factors have been reported to influence the in vitro cleavage rate, such as ooplasm quality, mitochondria and genetic factors (Mateusen et al., Reference Mateusen, Van Soom, Maes, Donnay, Duchateau and Lequarre2005). Increasing volume has been found to be beneficial for development in other species, i.e. in cattle, total cell number was increased by increasing the cytoplasm volume by fusing two or three enucleated oocytes with one hemicytoplast to approximately 150–200% of the embryo size (Peura et al., Reference Peura, Lewis and Trounson1998; Vajta et al., Reference Vajta, Lewis, Trounson, Purup, Maddox-Hyttel, Schmidt, Pedersen, Greve and Callesen2003; Pedersen et al., Reference Pedersen, Schmidt, Sangild, Strøbech, Vajta, Callesen and Greve2005; Tecirlioglu et al., Reference Tecirlioglu, Cooney, Lewis, Korfiatis, Hodgson, Ruddock, Vajta, Downie, Trounson, Holland and French2005; Misica-Turner et al., Reference Misica-Turner, Oback, Eichenlaub, Wells and Oback2007). In addition, cytoplasm volume has been shown not only to play a central role in mediating early development, but also to affect the length of cell cycles in embryos (Liu & Keefe, Reference Liu and Keefe2000). The amount of cytoplasm in reconstructed embryos might be one reason for the difference in the timing of blastocyst formation (Feng & Gordon, Reference Feng and Gordon1997). With increasing cytoplasm volume, the embryos in the current study started to cleave earlier, while formation of the morula and/or blastocyst occurred later. Furthermore, it has also been reported that when the cytoplasm volume was increased by fusing cytoplast, the large cytoplasm volume tended to allow or promote more cell divisions to occur; the extra ooplasmic components may boost development beyond 8–16-cell stage, resulting in increased developmental ability of the reconstructed embryo and increased total cell number (Ribeiro et al., Reference Ribeiro, Gerger, Ohlweiler, Ortigari, Mezzalira, Forell, Bertolini, Rodriques, Ambrósio, Miglino, Mezzalira and Bertolini2009).
After measuring the hemicytoplasts to be used for HMC in the current study, a significant difference was observed on the morphology of reconstructed embryos with 75% versus 50% cytoplasm volume. The first experiment confirmed that the developmental ability of reconstructed embryos was affected by the final cytoplasm volume, but not the volume used for the first fusion during HMC. Hence, the current study is the first to confirm by time-lapse that reconstructed embryos having reduced cytoplasm volume need more time to cleave and less time to form the morula and/or blastocyst. That might be the reason why higher cell numbers are found in embryos with increased cytoplasm volume. However, although the increased cytoplasm volume in the reconstructed embryos could improve developmental ability, making embryos even larger to achieve better development is not a viable option because of the limited oocyte resources and the total efficiency related to the whole procedure.
Timing of division of the different cell cycles in the embryos with reduced cytoplasm volume could be another reason accounting for the limited developmental ability with fewer cell numbers. A certain cytoplasm volume is required to support the development of the embryos, such as the mitochondria DNA number (El Shourbagy et al., Reference El Shourbagy, Spikings, Freitas and St John2006). As reported previously, the reduction of cytoplasm in oocytes leads to decreases in centrosomes, microtubules and some regulatory factors in the cell, or some relative protein synthesis is impaired (Cui et al., Reference Cui, Huang and Sun2005). In addition, the preimplantation development gene in mouse, human and bovine embryos has a remarkable regulatory function on the timing of embryo development (Warner et al., Reference Warner, Cao and Exley1998; Cao et al., Reference Cao, Brenner, Alikani, Cohen and Warner1999; Fair et al., Reference Fair, Gutierrez-Adan, Murphy, Rizos, Martin, Boland and Callesen2004). The decreasing amount of cytoplasm in the cloned embryos reduced not only embryo survival and development to the compact morula and/or blastocyst stage, but also led to lower numbers of cells observed in morulae and blastocysts (Saito & Niemann, Reference Saito and Niemann1991; Westhusin et al., Reference Westhusin, Collas, Marek, Sullivan, Stepp, Pryor and Barnes1996). It would be likely that oocytes with very low cytoplasm volumes would simply not have enough cytoplasmic content, such as mtDNA copy numbers, maternal RNAs and proteins (Memili & First, Reference Memili and First2000) to produce the number of divisions and dilutions required to sustain development as shown in human oocytes (Van Blerkom et al., Reference Van Blerkom, Sinclair and Davis1998; St John, Reference St John2002). In contrast, previous studies have reported that the cytoplasm volume could play a central role in mediating both development and cell cycles in embryos (Liu & Keefe, Reference Liu and Keefe2000). The timing of blastocyst formation is probably affected by an unavoidable inconsistency in the amount of cytoplasm volume in reconstructed embryos (Feng & Gordon, Reference Feng and Gordon1997). Therefore, a reduced volume of cytoplasm in the reconstructed embryos means that they will need more time to cleave because they have less energy available to form the first cell cycle. Even though embryos develop into blastocysts, the total cell numbers are lower because of the limited cell divisions, and therefore the inner cell mass in the embryos is not of sufficient size to support full term development (St John, Reference St John2002).
To modify the nucleo-cytoplasmic ratio, most studies have focused on changing the cytoplasm volume, but no study has focused on the difference of the nucleus, i.e. the nucleus from donor cells or from the oocyte itself. The influence of modified nucleo-cytoplasmic ratio on competence of early embryonic development was first investigated in metaphase II (MII) oocytes (Westhusin et al., Reference Westhusin, Collas, Marek, Sullivan, Stepp, Pryor and Barnes1996; Bordignon & Smith, Reference Bordignon and Smith1998; Cui et al., Reference Cui, Huang and Sun2005). The current study observed both HMC and PA embryos by time-lapse to investigate their developmental kinetics, and the decreased development potential of the reconstructed embryos was observed when the cytoplasm volume was reduced.
In the current study, the developmental ability was improved by increasing the cytoplasm volume, but an effect on the development kinetics was also demonstrated. The current results therefore support observations that the developmental potential of nuclear transfer embryos can be increased by increasing the amount of cytoplasm in the reconstructed embryos. It is concluded that the final cytoplasm volume is an important factor for the development of cloned embryos, and developmental kinetics varies with different cytoplasm volume which might be one important reason for the effect on the developmental competence.
Acknowledgements
The authors would like to thank A. Pedersen, J. Adamsen, R. Kristensen and A.K. Nielsen for invaluable technical assistance.
The study was supported by grants from the ‘Pigs & Health’ project (Danish Advanced Technology Foundation no. 013–2006–2), the ‘DAGMAR’ project (the Danish National Research Infrastructures Programme no. 09–065333) and ‘the Fundamental Research Funds for the Central Universities’ with the No. of KYZ201115 in China.