Introduction
Discus (Symphysodon aequifasciatus) are fish that are found in the Amazon basin and that belong to the family of cichlids and Symphysodon gender (Fishbase, 2013). Their bodies have a discoidal shape (Wattley, Reference Wattley1991) that is covered in scales and can be found in a wide range of colors (Santos et al., Reference Santos, Ferreira and Zuanon2006).
This species has high market value, its varied colors and complex captive breeding attract the attention of many aquarists, making this species one of the most important examples in the ornamental fish trade in Brazil.
The lack of information on the early life of this fish results in reduced zootechnical performance and in high mortality, often affecting the development of the offspring and thus the economic sustainability of aquaculture activity. A greater knowledge of embryos and larvae is very important because this would help in the location of spawning areas and in the study of growth of the species in its natural environment (Reynalte-Tataje et al., Reference Reynalte-Tataje, Zaniboni-Filho and Muelbert2001).
Ontogenetic studies have provided information about embryo physiology that is important for conservation of the ichthyofauna and in the improvement of cultivation techniques (Godinho et al., Reference Godinho, Santos, Sato, Godinho and Godinho2003). Moreover, a description of the embryo stages in the teleost has contributed to the accurate evaluation of their development (Senhorine, Reference Senhorine1993).
Initial and larval ontogeny differs among the species that have been studied previously, this research has highlighted the necessity for independent studies for each species. These studies have provided morphological and physiological information that has been extremely important in the goal to maximize survival in the early period of life and in the transition to feeding fish (Nakatani et al., 2001).
This study aimed to describe the embryonic and larval development of the discus fish (S. aequifasciatus) and determine the time required (hours post fertilization) until the appearance of embryonic and larval structures.
Material and methods
The eggs used were derived from a natural spawning from Symphysodon aequifasciatus. The breeding couple made up the roster of fish from the aquaculture sector of the Laboratório de Zootecnia e Nutrição Animal (LZNA) and from the Centro de Ciências e Tecnologias Agropecuárias (CCTA) of Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF), Campos dos Goytacazes, Rio de Janeiro, Brazil.
The couple was kept in an aquarium with a volumetric capacity of 60 l. The aquarium was equipped with an aeration system that consisted of a constant and regular air compressor and porous stone. A mechanical filter was responsible for the capture of solid particles dispersed in water. The aquarium received a substrate for spawn made in a PVC pipe of 30 cm in length.
The couple was observed sporadically until they initiated the characteristic pre-spawning behavior, after which they were observed constantly until the end of spawning, which occurred naturally. The substrate that was already adhered to the eggs was removed from the aquarium, then a representative sample of 10% of the eggs was observed under an optical microscope at ×25 or ×10 magnification, thus identifying the first stage embryo and the characteristics of shape and color.
After the first evaluation, the eggs were distributed into floating sifters that were placed in an experimental polystyrene aquarium with a volumetric capacity of 40 l. The tank contained four sifters that received 15 eggs each. This treatment consisted of maintaining the temperature of the water in the aquarium at 28°C.
Oxygenation of the experimental aquarium was maintained by an aeration and porous stone system. To maintain the stability of the temperature, the tank was equipped with an automatic thermostat.
The temperature of 28°C was chosen for the experiment because this temperature is close to the natural conditions for discus, and as higher temperatures typically require greater spending on energy for water heating, as well as low temperatures for cooling. Frequent observations were made of the appearance of the embryos, from fertilization until the time at which oriented swimming occurred. The interval between observations varied according to the ontogenic stage, for example 30 min intervals during the incubation period (0–24h), every 2 h at 24–48 h, and longer intervals as the onset and development of structures became less evident.
At each observation time point, a sample from one-third of the total number of eggs was removed from the screen at random and placed on a glass slide for evaluation under an optical microscope (×25 or ×100 magnification). Images were recorded using a Sony DSC W220 digital camera attached to the microscope. At the end of the evaluation, the eggs were returned to the sifter.
The embryonic development stages were considered to be complete from the time at which 50% of the eggs present in the sample reached the same stage. The values of temperature, pH and dissolved oxygen were measured with the aid of a digital thermometer, pH meter and a digital oximeter, respectively.
The classification of embryonic stages was based on morphological characteristics, in accordance with the methodology used for the dojo (Misgurnus anguillicaudatus) (Fujimoto et al., 2004).
Results
Physico-chemical parameters of water
The mean values observed for the physico-chemical parameters of the experimental aquarium water where the eggs were kept were 6.48 ± 0.072 for pH; 27.9±0.0376°C for temperature and 8.8 mg/l±0.1745 for dissolved oxygen. The values are within the recommended ranges for the species imaged.
Eggs characteristics
Discus eggs are considered to be ‘polilecitos’ as they have a large amount of yolk and as telolecitos because the yolk is concentrated at the vegetal pole, and the cytoplasm with its organelles is at the animal pole.
The unfertilized and non-viable eggs were seen to be off-white in color to the eye, and the chorion touch sensitive when compared with the fertilized eggs.
Viable eggs had an oval shape and were yellow in color (Fig. 1 A), after spawning they remained adhered to the substrate, however they had no visible attachment structure, therefore attachments was by adhesives only.

Figure 1 (A) Recently fertilized egg with only one blastomere. (B) 16 Blastomeres. (C) Gastrula 60%. (D) Gastrula 90%. (E) Differentiation of the embryonic axis. (F) Optical primordium.
Embryonic development
To describe embryo development, five periods were chosen that were divided into cleavage, blastula, gastrula, organogenesis, and hatching stages. This method has also been adopted for the description of embryo development in other teleosts (Reid & Holdway, Reference Reid and Holdway1995; Humphrey et al., Reference Humphrey, Klumpp and Pearson2003; Fujimoto et al., 2004).
Cleavage period
Cleavage can be defined as the period in which the blastomeres divide sequentially, doubling their numbers of cells in each division and until the blastodisc has 64 blastomeres (Fujimoto et al., 2004).
When the eggs were collected, the presence of only one blastomere was observed (Fig. 1 A), at 3.0 hpf after cleavage, and the fourth mitotic division resulted in 16 blastomeres (Fig. 1 B).
At the end of the cleavage phase at 4 hpf, the presence of 64 blastomeres was observed, composing the blastodisc, with overlapping layers and reduced in size compared with the initial stages of cleavage.
Blastula period
The blastula period, in accordance with the description by Fujimoto et al. (2004), began when the seventh cleavage occurred, at which time 128 blastomeres form the blastodisc and develop until 1024 blastomeres are formed.
The beginning of this phase was observed at 5 hpf; after another 0.5 hpf a further cell division resulted in a blastodisc with approximately 256 cells.
During the blastula period when the blastodisc had more than 2000 cells, it was still possible to observe an acute angle between the syncytial blade and the yolk sac, which gave the egg an elliptical aspect.
This phase is characterized by a remarkable continuity between the limits of the blastoderm and the yolk sac. In discus eggs, this phase continued from 9.5 hpf until 13 hpf. The eggs became slightly more rounded, but did not acquire a spherical shape.
Gastrula period
The most obvious characteristic of the gastrula stage is the epibole, which occurs gradually with the progress of the blastoderm on the yolk sac, until it is completely covered; at which time, closure of the blastopore is evident.
At the start of the gastrula period, at 15.5 hpf, 10% of the vesicles was covered. At 17 hpf, 20% of the yolk was covered; at 18 hpf, the blastoderm covered 30% of the yolk sac. Half of the yolk sac was covered by the blastoderm after 21.5 hpf.
The development of the gastrula reached 60% at 24 hpf (Fig. 1 C), and 80% at 26 hpf. At 27.5 hpf, the gastrula covered up to 90% of the yolk (Fig. 1 D). However at 28.5 hpf, when the gastrula was 90% covered, differentiation of the embryonic axis was observed, thus defining where the embryo would locate (Fig. 1 E). The axis differed from the rest of the embryo by appearing as an amber line that settled along the yolk, in the perivitelline space.
The closed blastopore was seen at 31.5 hpf, when the edges of the blastoderm merged, completely covering the yolk.
Organogenesis period
During organogenesis formation and differentiation of tissues and organs occur. In S. aequifasciatus embryos this period began during the gastrula stage, when the differentiating embryonic axis was observed.
Differentiation of head and tail
At 31.5 hpf, the blastopore was closed and, in the embryo axis, the cephalic and caudal region were differentiated. The anterior portion of the embryonic axis was more prominent, making the distinction between the two extremities clear. At this point, seven pairs of somites were counted and the optic primordium was visible (Fig. 1 F). At 14 hpf, the presence of 6–7 somites crude pairs was observed in the embryo (Fig. 2 A).
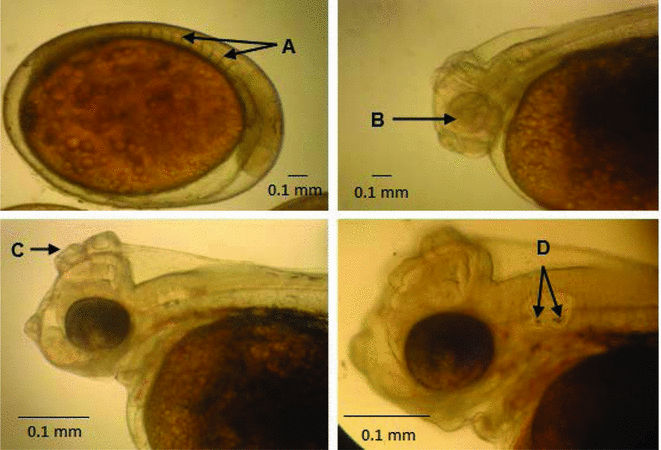
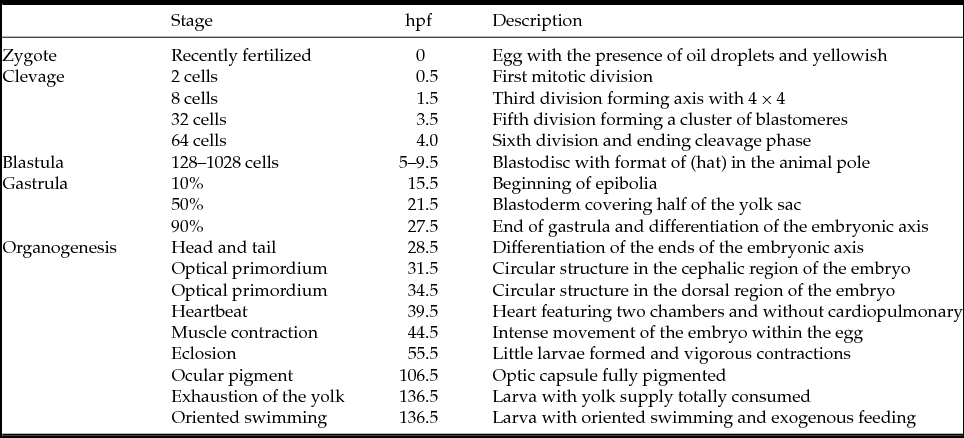
Figure 2 (A) Rudimentary somites. (B) Without optic vesicle pigment. (C) Structure of attachment. (D) Otolith present in the optic vesicle.
Ocular development
The first ocular structure to be seen at 31.5 hpf, was a pair of early optics, which appeared as two rounded vesicles, resulting from an evagination in the cephalic region of the embryo axis (Fig. 1 F). From this structure, the embryo eye developed. At 34.5 hpf, in S. aequifasciatus embryos, greater development of the ocular apparatus was noted, which became well defined and more evident.
Inside the optical primordium, at 33 hpf, a new circular structure called the optic vesicle developed. The discus of the embryos exhibited hatching until the time that some development of the visual system occurred, by the time of hatching no other sign of development was noticed. At birth, the larva has no pigmentation in the optic vesicle and was also not mobile.
Somites
The somites are rudimentary structures that result in the formation of vertebrae, ribs and axial musculature. In S. aequifasciatus embryos, somites were seen for the first time as about seven pairs, at 31.5 hpf.
In the course of development, somites increased in number and had taken on a different shape, similar to a ‘V’. At 33 hpf, 10 pairs were observed, and at approximately 39.5 hpf 19 pairs were present. The ‘V’ formation in the somites was seen initially at 41.5 hpf, all somites took on this shape after 48 hpf.
Embryonic pigmentation
The first signs of embryonic pigmentation occurred at 39.5 hpf as small melanophores located in the yolk. Subsequently, more melanophores appeared, but these did not spread throughout the embryo and were, until the time of hatching, concentrated in the dorsal portion of the yolk and the ventral base of the embryo (Fig. 3 A).

Figure 3 (A) Larvae after hatching. (B) During oriented larva swimming.
Circulatory system
A rudimentary heart can be seen as axial artery dilation-promoting movements similar to peristalsis. In discus fish, at 39.5 hpf, these early cardiac movements occurred at the frequency of 76 beats per min (bpm), however it was not possible to observe any blood circulation.
At 44.5 hpf, it was already possible to observe blood circulation in the embryo and in the yolk, which at that time was still colorless. The initiation of blood pigmentation was only observed at 46.5 hpf, the blood was fully pigmented at 53.0 hpf. Closer to hatching, at 53.0 hpf, a rate of 124 bpm was measured, indicating that the beats were more frequent as time went by.
Optic vesicle
The hearing system of fish is not only related to the capture and transduction of sound, but also has a direct relationship with balance.
The optic primordium emerged as a rudimentary circular structure at 34.5 hpf in a position posterior to the optic vesicle. At 44.5 hpf two pairs of otolith were observed located within the optic vesicle, the otolith are structures made from calcium carbonate that are responsible for sound transduction in the internal ear (Fig. 2 D).
Muscle contractions
At 44.5 hpf, the discus embryos began to show subtle muscle contractions that became noticeably more vigorous at 46.5 hpf; the intensity increased nearer to the time of hatching. This movement was more pronounced adjacent to the time of hatching and may be related to the disruption of the chorion.
Anchoring structures
After hatching, the discus larvae remained adhered to the substrate, adhesion was conferred by anchoring structures that are released from a viscous substance secreted by glands and enables the attachment of the larvae to the substrate. These structures begin to form at 50.5 hpf in a position just below the eye and in a position at the front of the head at 53.0 hpf.
Eclosion
In the time period that preceded eclosion, the embryos had already released the button caudal, and moved with more frequency and force, facilitating the breaking of the chorion. Eclosions occurred from 55.5 hpf to 58.5 hpf, the larvae exhibited an average of 120 bpm.
After eclosion, the optic vesicles in the larvae were non-pigmented (Fig. 2 B). Even after eclosion, there was no formation of a well defined mouth, only seen at 76.0 hpf.
Larval development
The larvae when hatched contained a large amount of yolk, possibly due to their long growth period that continued even after eclosion (Fig. 3 A). The anus was closed as the digestive system was still undifferentiated. Movement of the mouth could be seen only at 90.5 hpf, although there were still spaced and discrete movements. From 106.5 hpf, it was possible to observe more intense mouth movements that were apparently functional (Fig. 3 B).
Early optic vesicle pigmentation occurred after eclosion of the embryo at 62.5 hpf, at this period a few melanophores were seen in the optic capsule and, at 106.5 hpf, pigmentation was complete and proved functional.
The embryos hatched with the optic vesicle clearly visible and with the presence of two otoliths. As the larvae developed, the optic vesicle underwent a transformation process, with cavities located in their interior. In the period after the start of oriented swimming the larvae presented the optic structures covered by a network of melanophores that prevented visualization.
The anchoring structures observed before hatching continued to develop, and it was noticed that there was migration of the posterior optic vesicle to the region just above it (Fig. 2 C). As the larvae developed it was clear that such structures decreased in size – possibly because the larvae after completion of development do not require adherence to the substrate, due to the start of swimming.
Symphysodon aequifasciatus hatches with the somites arranged in a ‘V’ shape, attached to each other. A few melanophores were found in the upper part of the larvae, while in the bottom part a large amount of this pigment was observed, especially in the region near to the yolk.
The beginning of oriented swimming occurred at 136.5 hpf, in this period the presence of caudal fin rays was observed.
The mouth was open and the larvae showed intense movement, fully pigmented eyes, an open anus and exhausted yolk; the combination of these characteristics led us to believe that, at this time, S. aequifasciatus larvae were able to begin to capture food (Fig. 3 B). The larvae at this period, although having advanced development of organs and tissues, still demonstrated metamorphosis – with the cephalic region greater in height when compared with the rest of the body. A summary of events in the embryonic and larval stages is listed in Table 1.
Table 1 Description of morphological events and hours post fertilization
Discussion
With regard to external characteristic, discus eggs were similar to those of other species that belong to the cichlid family, as observed by Paes et al. (Reference Paes, Makino, Vasquez, Fernandes and Nakaghi2012) for apaiarí eggs (Astronotus ocellatus).
The oval shape and fixing capacity of the substrate have also been recorded for Cichlasoma dimerus eggs (Meijide & Guerrero, Reference Meijide and Guerrero2000). Nakatani et al. (Reference Nakatani, Agostinho, Baumgartner, Bialestzki, Sanches, Makrakis and Pavanelli2001) reported that the fixing characteristics of the eggs to the substrate is a strategy observed in many species of fish. In the cleavage phase, still at the beginning of embryonic development, it was noticed that the blastomeres significantly reduced their size up to the beginning of the gastrula stage. This reduction in size of the blastomeres as they divide has also been cited by Puvaneswari et al. (Reference Puvaneswari, Marimuthu, Karuppasamy and Haniffa2009) in his study of Heteropneustes fossilis. The blastula was considered to be late when compared with other species studied, such as Melanotaenia praecox (Radael et al., Reference Radael, Cardoso, Andrade, Mattos, Motta, Manhães and Vidal2013), Glossolepis incisus (Ferreira, Reference Ferreira2007) and Betta splendens (Duarte et al., Reference Duarte, Vasconcellos, Vidal, Ferreira, Mattos and Branco2012).
During the gastrula stage, differentiation of the embryonic axis was realized; this structure forms the body of the embryo and became evident even before closure of the blastopore when the blastoderm edges are starting organogenesis. Humphrey et al. (Reference Humphrey, Klumpp and Pearson2003) reported that, in Melanotaenia splendida, it was possible to observe differentiation of the embryonic axis in the period in which the gastrula was at 70%.
At the end of epibole, closure of the blastopore was verified when the two edges of the blastoderm met each other. This phase has been described by other authors, such as Puvaneswari et al. (Reference Puvaneswari, Marimuthu, Karuppasamy and Haniffa2009) in Heteropneustes fossilis at 7 hpf, and by Reynalte-Tataje et al. (Reference Reynalte-Tataje, Zaniboni-Filho and Esquivel2004) in Brycon orbignyanus at 6.5 hpf. The closure of the blastopore is an important event and is considered to be the period at which fertilization of the oocyte can be confirmed (Woynarovich & Horváth, Reference Woynarovich and Horváth1983).
After the start of segmentation the characteristic that soon becomes evident is the differentiation of the head and tail. According to Marimuthu & Haniffa (Reference Marimuthu and Haniffa2007), in their study of embryos and larvae of Channa striatus, differentiation of the head and tail occurred at approximately 11 hpf.
Hatching of S. aequifasciatus occurs even before full formation of the embryo, characterized as altricial larvae. According to Nakatani et al. (Reference Nakatani, Agostinho, Baumgartner, Bialestzki, Sanches, Makrakis and Pavanelli2001), most newly hatched neotropical larvae have altricial features – little morphological development plus little swimming motility, small amounts of yolk reserves plus an incomplete digestive tract at the time of exogenous feeding. After hatching the larvae remained attached to the substrate where spawning occurred, such attachment is possible by substances secreted from a gland and released through a structure in the head of the larvae. The characteristic of attachment of larvae to the substrate after hatching may be linked to the strategy of parental care displayed by this species, keeping the larvae under parental care and decreasing the dispersion of larvae into the water column. This feature has also been observed in other species of the same family such as Astronotus ocellatus by Paes et al. (2012) and Cichlasoma dimerus (Meijide & Guerrero, Reference Meijide and Guerrero2000).
The development of the ocular structure was similar to that of other species; Radael et al. (Reference Radael, Cardoso, Andrade, Mattos, Motta, Manhães and Vidal2013) observed that, in embryos of M. praecox, the optic primordium forms at 21.89 hpf. Fujimura & Okada (2007) observed the appearance of the optic primordium in tilapia (Oreochromis niloticus) at 44 hpf. After hatching, full development of the eye structure was not complete in S. aequifasciatus larvae; development and pigmentation was completed only near the end of consumption of yolk reserves and at oriented swimming. This characteristic may be related to oriented swimming and capture of exogenous food.
As to pigmentation of the embryos and the larvae, the melanophores initially appeared as small dots in the yolk sac, and later diffused into the dorsal region of the embryo, dissimilar to events in B. splendens reported by Duarte et al. (2012), in which the melanophores appeared randomly throughout the embryonic axis and diffused into the yolk. Continuity of pigmentation was observed up to the time at which the yolk reserves were exhausted and at oriented swimming when most of the larval body was covered by a network of melanophores. Increased pigmentation at the start of ‘free’ life could be related to the ability to mimic objects within the environment, thereby freeing the larvae from possible attacks by predators (Baras, Reference Baras1999; Hilsdorf et al., Reference Hilsdorf, Azeredo-Espin, Krieger and Krieger2002).
In the auditory system, gradual development from an optical primordium can be seen, however it was not possible to observe clearly the structures that form the embryonic inner ear and later the larvae. Before hatching it was possible to identify the presence of otoliths, which increased in size until the mixotrophic period of larval oriented swimming and yolk exhaustion.
Although these structures are sometimes forgotten in studies of fish ontogeny, the otoliths have a primary role in the transduction of sound and balance (Gauldie & Nelson, Reference Gauldie and Nelson1988), and play an important role in oriented swimming of the larvae and in prey capture.
In conclusion, we can say that S. aequifasciatus embryos have characteristics similar to those of other species of the cichlid family and are altricial (hatched without complete formation of optical, auditory, circulatory and digestive systems). However at the start of larval oriented swimming all systems were apparently functional, as the larvae started to search for exogenous intake of food.
Acknowledgements
We thank CAPES, CNPq e FAPERJ for support of the research.






