Introduction
Cryopreservation of gametes and embryos has become an important biotechnological component of infertility treatment cycles by assisted reproductive technologies (ART). Embryos are presently successfully cryopreserved at all development stages from the fertilized oocytes to hatched blastocyst. Cryopreservation can be achieved by either controlled rate slow cooling or vitrification (Edgar and Gook, Reference Edgar and Gook2012; Rienzi et al., Reference Rienzi, Gracia, Maggiulli, LaBarbera, Kaser, Ubaldi, Vanderpoel and Racowsky2017). However, each embryonic stage has its own morphological features that need to be taken into account during cryopreservation (Rienzi et al., Reference Rienzi, Gracia, Maggiulli, LaBarbera, Kaser, Ubaldi, Vanderpoel and Racowsky2017). Embryo cryopreservation at the pronuclear stage makes it possible to obtain a large number of embryos to transfer for a subsequent cryocycle treatment, but it is quite difficult at this stage to predict after thawing the quality of embryo, or development and kinetic potential during progression to more advanced stages. In addition, the effectiveness of embryo cryopreservation at the zygote stage depends on the phase of the cell cycle, as the microtubules of the mitotic spindle are highly susceptible to cryopreservation factors (Balakier et al., Reference Balakier, MacLusky and Casper1993).
Up to days 2 and 3 of development, the embryos consist of 2–8 blastomeres. However, there is a possibility of either lysis of individual blastomeres or increased degree of fragmentation that does not allow their consideration at this stage as optimum for freezing.
Day 5 embryos as a rule reach the blastocyst stage and their concurrent morphology enables the evaluation of the prospects of implantation and further intrauterine development rates of the embryo (Van den Abbeel et al., Reference Van den Abbeel, Balaban, Ziebe, Lundinm, Cuesta, Klein, Helmgaard and Arce2013; Irani et al., Reference Irani, Reichman, Robles, Melnick, Davis, Zaninovic, Xu and Rosenwaks2017). However, the features of this embryo stage, namely the existence of a large fluid filled cavity (blastocoel) in the blastocyst cavity, may require a longer exposure time with cryoprotective agents (CPA), therefore increasing the risk of potential CPA toxic effects for the embryo. In addition, it has been suggested that the presence of a large water content in the embryo cavity increases the risk of ice crystal damage during freeze-thawing. In this regard, blastocoel collapsing procedures were performed to remove excess internal water, and required additional embryo manipulations (Kovačič et al., Reference Kovačič, Taborin and Vlaisavljević2018).
There was therefore an opportunity to separately consider the day 4 development stage of the human embryo, i.e. the morula. At this stage of in vivo pre-implantation development, during natural conception, embryos enter the uterine cavity. In addition, there is an increase in the number of blastomeres and a decrease in their individual cell volumes as a result of embryo cell division. Segregation of blastomeres occurs towards either the centre or the periphery of the cell mass, and tight intercellular junctions are made; these events characterize collectively the development of the morula stage (Fabozzi et al., Reference Fabozzi, Alteri and Rega2016). The structural features of this embryo development stage allowed increased permeation of the blastomeres by cryoprotective solutions and reduced exposure times, therefore reducing the cytotoxic effect of cryoprotectants. At the same time, the individual morphological characteristics of the morulae contributed to their further development and implantation rates. Here, large numbers of fragmentations reduced the size of the intracellular mass and trophectoderm cells (Ivec et al., Reference Ivec, Kovačič and Vlaisavljević2011). In addition, embryos with incomplete compaction had reduced blastocyst formation rate and implantation potential (Ebner et al., Reference Ebner, Moser, Shebl, Sommergruber, Gaiswinkler and Tews2009).
Materials and Methods
In this retrospective study we compared the survival rate of incomplete compacted morulae. The study included 224 morulae cryopreservation cycles performed from January 2015 to September 2017.
Ovarian stimulation
Human gametes and pre-implantation embryos were obtained from infertility treatment cycles using in vitro fertilization (IVF). The mean age of women taking part in the intracytoplasmic sperm injection (ICSI) cycles was 30.3 ± 4.2 years. Stimulation was performed in a standard manner using a luteal long protocol of gonadotropin-releasing hormone (GnRH) agonist (Decapeptyl, Ferring, Malmo, Sweden) or GnRH antagonist (Cetrotide, Serono, Geneva, Switzerland), with daily administration of recombinant follicle-stimulating hormone (FSH) (GONAL-f, Serono). Oocytes were retrieved by transvaginal aspiration under ultrasound guidance in 35 h after injection of recombinant human chorionic gonadotropin (Ovidrel, Serono, Switzerland).
IVF and ICSI
The retrieved oocytes were exposed briefly to 80 IU/ml hyaluronidase (Sydney IVF Hyaluronidase, Cook Medical, Spencer, IA, USA), and mechanically separated from their surrounding cumulus cells by aspiration through a glass pipette. All oocytes at the MII stage were selected for micromanipulation.
The ICSI procedure was based on an established protocol (Palermo et al., Reference Palermo, Joris, Devroey and Van Steirteghem1992). Fertilization was assessed at 16–20 h after insemination by the presence of two pronuclei and two polar bodies. Normally, fertilized oocytes were transferred into 0.8 ml Global® total culture medium (Life Global, USA).
Grading of morulae compaction
Blastomere compaction of embryos to day 4 was evaluated and graded. Embryo images were obtained using an Olympus IX-71 microscope (Olympus, Japan) and processed using the Axio Vision program. The shapes of the circular areas of the compacted or uncompacted embryo areas were approximately spheres or ellipsoids. Calculating their relative volume determined the degree of blastomere compaction:
therefore,
![]() ${\rm BCD} = {{{1 \over 6}\pi {D_1}^3} \over {{1 \over 6}\pi {D_2}^3}} \times 100 = {{{D_1}^3} \over {{D_2}^3}} \times 100(\% )$
${\rm BCD} = {{{1 \over 6}\pi {D_1}^3} \over {{1 \over 6}\pi {D_2}^3}} \times 100 = {{{D_1}^3} \over {{D_2}^3}} \times 100(\% )$
where V1 = the volume of the embryo compacted area; D1 = the diameter of the embryo compacted area; V2 = the volume of whole embryo blastomeres; and D1 = the diameter of whole embryo blastomeres.
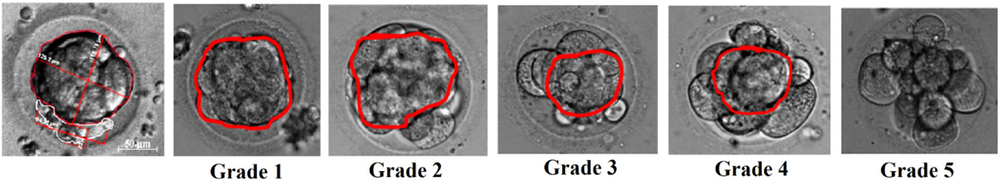
According to the BCD calculations, all morulae (n = 224) could be divided into five groups: grade 1: full compaction (BCD is 100%, n = 37), grades 2–4: compaction occupies not the whole embryo (BCDs were about 75, 50, 25%; n = 52, 55, 51 respectively); and grade 5: embryo at the morula stage of development with no compaction (n = 29) (Fig. 1).
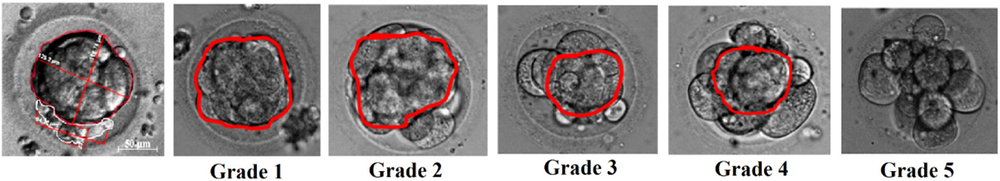
Figure 1. Blastomere compaction degree grading. Grade 1: full compaction; grades 2–4: compaction occupies 75, 50 or 25% of embryo volume, respectively; and grade 5: non-compacted morula.
Each morula was analyzed independently by two embryologists. They made images of morula alone, calculated the BCD index and then compared the results: 97% (224/218) of results matched.
Cryopreservation and thawing protocols
Cryopreservation was achieved by vitrification. The vitrification procedure was performed using the Cryotop method (Kuwayama et al., Reference Kuwayama, Vajta, Kato and Leibo2005). Morulae were equilibrated in 7.5% (v/v) ethylene glycol (EG) + 7.5% dimethyl sulfoxide (DMSO) in Global total medium at 20°C for 15 min. Then morulae were placed for 1 min into vitrification solution, consisting of 15% ethylene glycol + 15% DMSO + 0.5 M sucrose in the same medium as the equilibration solution. Next, embryos were immediately placed on a Cryotop strip and plunged into liquid nitrogen. For warming, the Cryotop was removed from liquid nitrogen and instantly placed in 1.0 M sucrose in Global total medium at 37°C for 1 min and then placed in 0.5 M sucrose in Global total medium for 3 min; after that embryos were placed the same medium for further culture.
After warming, the survival rate and developmental kinetics of the embryos were evaluated by daily observations of grade 1 morulae.
Biopsy of excluded fragments in embryos with incomplete compaction
Excluded fragments were removed using an Olympus inverted microscope equipped with Hoffman system and Narishiga hydraulic micromanipulators (Narishiga, Japan), and a 1.48 mm wavelength diode laser (OCTAX Laser Shot™ System, Germany) in a computer-controlled contact mode. This instrument was used for zona pellucida drilling (12–13), with one blunted pipette of about 12–17 μm, and excluded fragments were removed. Embryos were washed with human tubal fluid (HTF) medium containing 10% synthetic serum substitute (SSS) and incubated at 37°C with 5% CO2 in air until the time of transfer (Fig. 2). Removal of excluded fragments was carried out for grade 2 (n = 18), grade 3 (n = 19) and grade 4 (n = 18) morulae.

Figure 2. Excluded fragments removal procedure. Embryo development to blastocyst stage, magnification ×600 (n = 55).
Data analysis
Statistical analysis of the data was carried out using Past 3.0 software. Quantitative data distribution was evaluated using the Shapiro–Wilk test. Results are presented as the mean ± standard deviation. For comparison of two independent samples, a nonparametric Mann–Whitney test was used; for paired comparisons, the Wilcoxon signed rank test was applied. Differences were considered significant at P-values < 0.05.
Results
The survival rate of embryos after the incubation stage in vitrification solutions for grades 1 and 2 was 100%, and 87 ± 7.4%, 68 ± 5.9% and 53 ± 6.7% for grades 3–5 respectively. Different osmotic reactions were noted for compacted and non-compacted morulae areas at the incubation stage. The compacted area was a response to change in overall osmotic pressure, in contrast with the regions not involved into embryo compaction. After embryo vitrification and warming, the survival rate of grade 1 embryos was 98 ± 6.2%, for grade 2–5 were 85 ± 4.1%, 38.2 ± 4.4%, 50 ± 5.8% and 22.2 ± 4.4%, respectively (Fig. 3).

Figure 3. Survival rate of morulae with different grades of compaction after vitrification. A statistically significant difference was observed between vitrified morulae and biopsied morulae with the same grade of compaction, marked with an asterisk (*), and between vitrified grade 1 morulae and the study group (#); P < 0.05.
Blastocyst formation rate (BFR) of morulae correlated with survival rate after freeze-warming (r = 0.94). The cleavage rate of embryos to the blastocyst stage was 92.8 ± 7.9%, 71.3 ± 8.2%, 44.7 ± 9.9% and 12.4 ± 4.9% for grades 1–4 respectively (Fig. 4). There were no blastocysts in groups with grade 5 morulae either fresh or vitrified.

Figure 4. Blastocyst formation rate of morulae with different grades of compaction after vitrification. A statistically significant difference was observed between fresh morulae and vitrified morulae or biopsied morulae with the same grade of compaction, marked with an asterisk (*) and between grade 1 fresh morulae and the study group (#); P < 0.05.
Damaged necrotic areas of the embryo can affect their further development rate and viability due to the release of toxic metabolites or inhibitory cell molecular signalling pathways (Van den Abbeel et al., Reference Van den Abbeel, Camus, Van Waesberghe, Devroey and Van Steirteghem1997).
We suggested that removal of uncompacted fragments in grades 2–4 morulae by biopsy prior to cryopreservation would reduce the negative effect of necrotic factors and thereby increase their survival and BFRs. We did not biopsy the grade 5 morulae because of absence of compaction.
Removal of blastomeres and excluded fragments of incomplete compacted morula before embryo cryopreservation led to an increase in their post-thawing survival rate up to 93.1 ± 4.1% and 75 ± 8.8% and BFR up to 85.2 ± 10.4% and 59.4 ± 5.2%, P < 0.05 in grades 2 and 3 respectively (Figs. 3 and 4). There was an increase in BFR for grade 4 morulae after using this technique and before cryopreservation in contrast with the effect on their survival rate.
Discussion
The complexity of predicting embryo development potential at the cleavage stage and emergence of epigenetic risks during prolonged in vitro cultivation of pre-implantation embryos, gave the advantage to transfer the embryos to the uterine cavity at the morula stage (Chason et al., Reference Chason, Csokmay, Segars, DeCherney and Armant2011). At this stage of natural development in vivo, the embryo enters the uterine cavity and interacts with its microenvironment. In addition, transcription of the embryonic phenotype begins on day 3 of development and actively progresses to day 4 (Kanka, Reference Kanka2003). The development potential of embryos at the 2–8 blastomere stage is difficult to estimate visually. Prognostic signs of implantation and pregnancy onset after transfer of early embryos to the uterine cavity could be correlated with their morphological characteristics and development kinetics, which in turn often corresponded to their chromosomal status (Magli et al., Reference Magli, Gianaroli, Ferraretti, Lappi, Ruberti and Farfalli2007). Although embryos at the morula stage have been transferred since 1994 (Goto et al., Reference Goto, Kanzaki, Nakayama, Takabatake, Himeno, Mori and Noda1994; Huisman et al., Reference Huisman, Alberda, Leerentveld, Verhoeff and Zeilmaker1994), an objective morphological evaluation for selection of day 4 embryos has not been developed, and the characteristics of day 1–3 embryos cannot be applied due to the complexities of blastomere number counting, and estimation of their size and morphology (Rienzi et al., Reference Rienzi, Ubaldi, Iacobelli, Romano, Minasi, Ferrero, Sapienza, Baroni and Greco2005a). High outcome predictability after IVF use a combined score for zygote and embryo morphology and growth rates that have been reported previously (De Placido et al., Reference De Placido, Wilding, Strina, Alviggi, Alviggi, Mollo, Varicchio, Tolino, Schiattarella and Dale2002). Assessment criteria of embryos at the blastocyst stage (such as morphological evaluation of trophectoderm and intracellular cell mass) are rarely used (Gardner and Schoolcraft, Reference Gardner and Schoolcraft1999).
Evaluation of morulae by morphology and degree of compaction, which correlate with the level of implantation and development potential rates, has been used (Ebner et al., Reference Ebner, Moser, Shebl, Sommergruber, Gaiswinkler and Tews2009). A high degree of morula fragmentation reduced the implantation rate and, correspondingly, the pregnancy rate, while the presence of small amounts of fragmentation had no obvious adverse effect (Alikani et al., Reference Alikani, Cohen, Tomkin, Garris, Mack and Scott1999). Two distinctly different types of fragmentation were identified by Van Blerkom et al. (Reference Van Blerkom, Davis and Alexander2001), namely final fragmentation, representing stable, persistent fragments, clearly separated from blastomeres, and pseudo-fragmentation, which was characterized by appearance during, or shortly after, cell division, but not found at later stages of development (Van Blerkom et al., Reference Van Blerkom, Davis and Alexander2001).
Compaction onset is an important prognostic criterion for BFR and the potential for implantation. Some authors have stated that embryos exhibiting compaction on day 3 of development have a high probability for implantation (Le Cruguel et al., Reference Le Cruguel, Ferré-L’Hôtellier, Morinière, Lemerle, Reynier, Descamps and May-Panloup2013). Early day 3 compaction may be a useful additional criterion for embryo transfer. Others authors have indicated that embryos that do not reach the compaction stage until day 4, but show mitotic activity to day 3, are comparable for BFR with embryos in which compaction occurred on day 4. However, a decrease in the BFR of embryos that remained at the same stage on days 3 and 4 was noted (Fabozzi et al., Reference Fabozzi, Alteri and Rega2016). In addition, implantation rate after transfer of day 4 compacted embryos was reported to be higher when compared with embryos that exhibited early compaction (Tao et al., Reference Tao, Tamis, Fink, Williams, Nelson-White and Craig2002; Feil et al., Reference Feil, Henshaw and Lane2008).
As the main trend in multiple pregnancy prevention and its related risks is the transfer of one selected embryo, and with the aim to increase cumulative pregnancy rate, cryopreservation procedures should be used. The proposal of prognostic criteria to evaluate embryo cryoresistance is also important.
In this study, we evaluated the survival and BFR of day 4 embryos with different compaction degrees of blastomeres after cryopreservation. The osmotic reaction during exposure to cryoprotectant solutions was also evaluated. Blastomeres not included in compaction have been shown to be aneuploid (Lagalla et al., Reference Lagalla, Tarozzi, Sciajno, Wells, Di Santo, Nadalini, Distratis and Borini2017). Moreover, morula stage embryos, cryopreserved by slow cooling, that had fragmentation rates >20%, had significantly lower post-thaw survival and transferable rates compared with morulae that exhibited good quality and full compaction (Tao et al., Reference Tao, Craig, Johnson, Williams, Lewis, White and Buehler2004). Cryopreservation factors can lead to additional lesions in uncompacted blastomeres: increased osmotic pressure on the membrane cells during CPA exposure and wash out; toxic effects of CPAs; or ice re-crystallization at the thawing stage (Men et al., Reference Men, Agca, Mullen, Critser and Critser2005; Mazur and Paredes, Reference Mazur and Paredes2016). Even more, the toxic effect of high CPA concentrations at embryo vitrification can lead to the release of necrotic factors from weak non-compacted morulae cells. These factors can adversely affect the compacted area of the embryo, reducing survival rate and future development.
Microsurgical removal of degenerated blastomeres of embryos was first carried out by Alikani et al. (Reference Alikani, Olivennes and Cohen1993). This method was applied to human embryos, cryopreserved using slow cooling rates (Rienzi et al., Reference Rienzi, Ubaldi and Lacobelli2005b; Elliott et al., Reference Elliott, Colturato, Taylor, Wright, Kort and Nagy2007; Liu et al., Reference Liu, Luo, Huang, Wang, Zhao, Yue and Zheng2007). The removal of non-compacted morulae cells and fragmentations was similar to blastomere biopsy for PGD, which does not affect the survival rate of embryos after vitrification–warming (Zhang et al., Reference Zhang, Trokoudes and Pavlides2009).
Removal of cytoplasmic fragments in fresh embryos led to an improvement in their quality and developmental ability (Alikani et al., Reference Alikani, Olivennes and Cohen1993). This finding correlated with our results, which showed that the prior cryopreservation removal of fragments not included in compaction of day 4 embryo increased their survival rate and BFR, if embryo compaction was 50% or higher (grades 2 and 3). Therefore, this procedure allowed improved outcomes for freezing grades 2 and 3 morulae with incomplete compaction, thereby increasing the chances of pregnancy. At the same time, estimation of proposed morula quality enabled prediction of survival rate for embryos after freeze-thawing and expediency of carrying out biopsy of non-compacted areas. These data should be taken into account when counselling patients who are undergoing fertility treatment using assisted reproductive technology and cryopreservation methods.
Acknowledgements
The authors are grateful to ART-Clinic of Human Reproduction, Kharkiv and its staff for enabling them to perform this research.
Financial support
The work was carried out within the framework of the programme ‘Support for priority scientific research directions development’ of the National Academy of Science of Ukraine.
Conflicts of interest
The authors have no conflict of interest with regard to these results.
Ethical standards
All the manipulations of gametes and embryos were performed according to the report of the Steering Committee On Bioethics (CDBI) on The Protection of the Human Embryo In Vitro CDBI-CO-GT3 (Strasbourg, 19 June 2003) with informed patient consent and the decision of the Committee in Bioethics of the Institute for Problems of Cryobiology and Cryomedicine of the NAS of the Ukraine.