Introduction
It has been known for many decades that the development of fish embryos is influenced by temperature. Within the tolerance levels, low temperature slows down and high temperature accelerates development. Chilling of fish embryos has gathered attention, as it is a way to arrest development and to store embryos at a specific differentiation stage for extended time periods. With the rapid growth of marine aquaculture, several attempts to cryopreserve other types of fish embryos have been carried out in recent years, most notably for species that have great commercial value, such as Paralichthys olivaceus (Zhang et al., Reference Zhang, Liu and Rawson2003; Chen & Tian, Reference Chen and Tian2005), Scophthalmus maximus (Cabrita et al., Reference Cabrita, Robles, Chereguini, Wallace and Herráez2003; Robles et al., Reference Robles, Cabrita, Real, Álvarez and Herráez2003), Sparus aurata (Beirao et al., Reference Beirao, Robles, Herraez, Sarasquete, Dinis and Cabrita2006; Cabrita et al., Reference Cabrita, Robles, Wallace, Sarasquete and Herráez2006) and Pagrus major (Ding et al., Reference Ding, Xiao and Li2007). Several studies have been performed on the chilled storage of fish embryos: in the vendace (Coregonus albula), hatching of embryos can be delayed for several weeks by cooling to 1–2°C (Luczynski, Reference Luczynski1984). When conditions for hatching are optimal, the incubation temperature is raised, and mass hatching of normally developed larvae occurs within 2–3 days (Luczynski, Reference Luczynski1984). In the brown trout (Salmo trutta), embryo development can be extended for up to 4 months at 1.4ºC (Maddock, Reference Maddock1974).
The cooling technique consists of submitting cryoprotected fish embryos at sub-zero temperatures followed by short storage periods, resulting in a decrease in enzymatic and cellular activity (Cloud et al., Reference Cloud, Armstrong, Wheeler, Kucera, Thorgaard, Tiersch and Mazik2000; Ahammad et al., Reference Ahammad, Bhattacharyya and Jana2003). This biotechnological method allows several practical applications such as: transportation of embryos obtained from remote areas (Ahammad et al., Reference Ahammad, Bhattacharyya and Jana2003); synchronizing the development of embryos collected from different spawning events (Lahnsteiner, Reference Lahnsteiner2008); and optimises the use of hatchery facilities (Streit Jr et al., Reference Streit, Digmayer, Ribeiro, Sirol, Moraes and Galo2007).
These efforts, however, have yielded relatively low hatching and larval survival rates. Although efficient cryopreservation techniques for fish embryos are still lacking, chilling techniques are already being used. Recent studies have shown that Piaractus mesopotamicus embryos can be stored at low temperatures without significantly decreasing the hatching rates (Streit Jr. et al., Reference Streit, Digmayer, Ribeiro, Sirol, Moraes and Galo2007; Lopes et al., Reference Lopes, Romagosa, Streit, Ribeiro and Digmayer2011, Reference Lopes, Streit, Fornari, de Oliveira, Ribeiro and Romagosa2013).
Recent studies have evaluated both chilling protocols and cryoprotectant toxicity in Prochilus lineatus (Costa et al., Reference Costa, Velardi, Senhorini, Veríssimo-Silveira and Silveira2012; Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Costa, Velarde, Senhorini and Veríssimo-Silveira2012). However, literature on the chilling of freshwater tropical fish species remains scarce.
Pirapitinga (Piaractus brachypomus) is native to the Amazon and the Araguaia–Tocantins basins, and can grow to 80 cm in length and weigh up to 20 kg. This fish has scales and feeds primarily on fruits and aquatic plants, although it will also eat smaller fish. Piaractus brachypomus is a very important commercial species, both as an ornamental fish and for human consumption (Val & Val-Almeida, Reference Val and Val-Almeida1995).
Studies on the characterization and cryopreservation of P. brachypomus sperm have been conducted by Nascimento et al. (Reference Nascimento, Maria, Pessoa, Carvalho and Viveiros2010), but their embryo-chilling protocols are currently unavailable.
The aim of this study was to chill P. brachypomus embryos at –10ºC and at two storage times. Because the post-gastrulation stage is critical for low temperature storage as well as for cryopreservation (Ahammad et al., Reference Ahammad, Bhattacharyya and Jana2002), the embryos were treated in a solution that contained glucose 17.5%, sucrose 17.5% or tap water and the internal cryoprotector methanol 10%.
Materials and methods
Embryo collection
This experiment was conducted between December 2012 and April 2013 at the Aquaculture Research Center (Centro de Pesquisas em Aquicultura–CPAq) of DNOCS in Pentecoste, Ceará, which is located at 03º45′0″’S and 039º10′24″W and is 82 km from the state capital Fortaleza.
Fish were maintained in rearing ponds with a surface area of 350 m2. Twenty males and 16 females of P. brachypomum with an average age of >3 years were tagged with chips to avoid data loss.
Piaractus brachypomum embryos were collected from a pool of eggs that originated from eight best breeding pairs. Females were induced with two intramuscular injections of 0.5 and 5.0 mg kg crude carp pituitary extract (cPE; Danúbio Aquacultura, Blumenau, Santa Catarina, Brazil), within a 12-h interval. Males were induced with a single dose of cPE at 1.0 mg kg−1. The gametes were released after 280 h-degree, and the recently fertilized eggs were transferred to 18-l conical incubators in a flow-through system. Embryo development was monitored until larval hatching, and the water temperature was maintained at 28±1°C.
Viable eggs were divided into control and treatment groups for each of the four storage times. Each experimental unit consisted of 100 viable embryos. There were three replicates for the control group and six replicates for each treatment group. The treatment groups were divided into two completely randomized blocks: the first block was based on embryonic stage, and the second was based on the chilling time.
Control group
The control group was used to determine the influence of the egg-selection process on the hatching rate for different ontogenetic stages in the absence of chilling. The control group was composed of four subgroups, each representing one of the following embryonic stages (Lopes et al., Reference Lopes, Romagosa, Streit, Ribeiro and Digmayer2011): control 1: blastoderm – 1.2 h post fertilization (hpf) (~64 cells); control 2: epiboly – 5 hpf (25% epiboly); control 3: blastopore closure – 8 hpf (90% epiboly); and control 4: appearance of optic vesicle – 13 hpf. Control embryos were incubated without cryoprotectant in 12 3-l incubators in a flow-through system until larval hatching (approximately 18 hpf).
Embryos used for the chilling experiments
The treatment groups consisted of viable embryos collected at various embryonic stages (1.2, 5, 8 and 13 hpf). Each group was placed in a Vacutainer® tube that contained a solution of 17.5% glucose or 17.5% sucrose (Fornari et al., Reference Fornari, Ribeiro, Streit, Vargas, Barrero and Moraes2011) or tap water and the cryoprotectant (10%) methanol. Then, tubes were transferred to a polystyrene box that contained ice under constant temperature control, chilled at a rate of approximately 1ºC per min (Lopes et al. Reference Lopes, Streit, Fornari, de Oliveira, Ribeiro and Romagosa2013) from 20°C down to –10ºC for 6 or 10 h. The tubes were then removed from the chilling device, incubated for 5 min in room temperature water and transferred to 3-l incubators. Those embryos were placed in an incubator with continuous water flow until embryonic development was complete (Fig. 1).
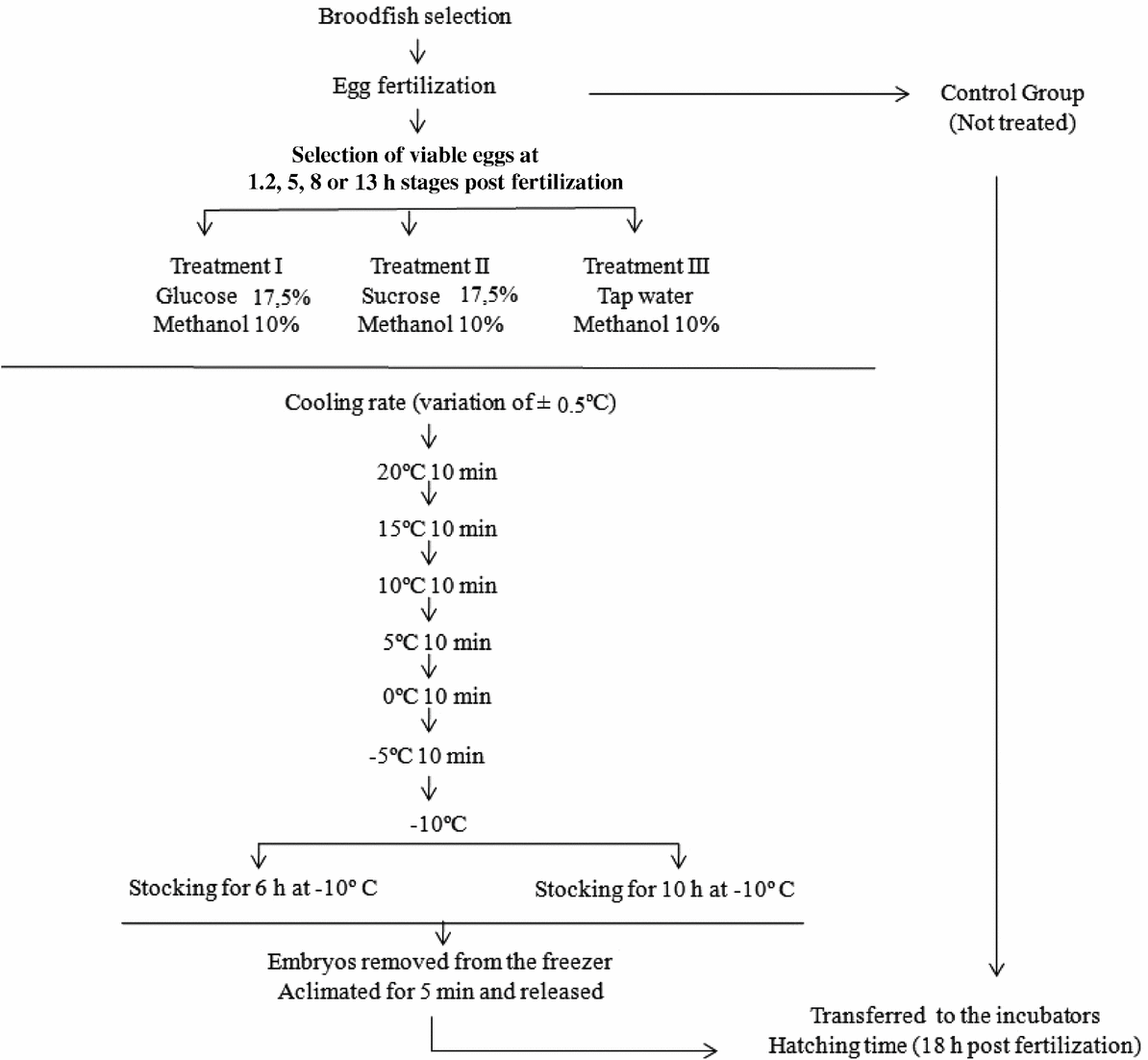
Figure 1 A flowchart showing the P. brachypomus embryo chilling procedure for four embryonic stages and four storage times. Adapted from Lopes et al. (Reference Lopes, Romagosa, Streit, Ribeiro and Digmayer2011).
Evaluation of larval survival rate
After embryonic development was complete (approximately 18 hpf), both control and treated embryos were removed from the incubators to estimate the number of live larvae that had completed development. The total numbers of live larvae that had developed from the treated embryos per hour of chilling were determined for each ontogenetic stage. The effects of the treatments for each chilling time were also determined for each ontogenetic stage.
The authors assert all procedures that contributed to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The authors assert all procedures that contributed to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.
Values are expressed as mean ± standard deviation (SD). When data did not fit the normal distribution, an arcsin transformation was performed. Data were tested for significant differences using the analysis of variance (ANOVA) statistical test, followed by the honest significant difference (HSD) Tukey test, when applicable. The level of significance for all statistical tests was set at 0.05. All statistical analyses were performed using the R software package.
Results
Control study
The percentages of living larvae that developed from the control embryos selected at various ontogenetic stages are shown in Table 1.
Table 1 The percentages of live control P. brachypomum larvae (showing complete embryonic development) that developed from embryos chosen at 1.2, 5, 8 and 13 h post fertilization (hpf) respectively

Each mean±standard error of the mean value represents three replicates consisting of 100 embryos each.
The effects of chilling time on embryonic development
The larval survival rate for embryos chilled in a sucrose–methanol solution was significantly higher (P≤0.05) than the rate for embryos treated with glucose–methanol or water–methanol solutions (Fig. 2).
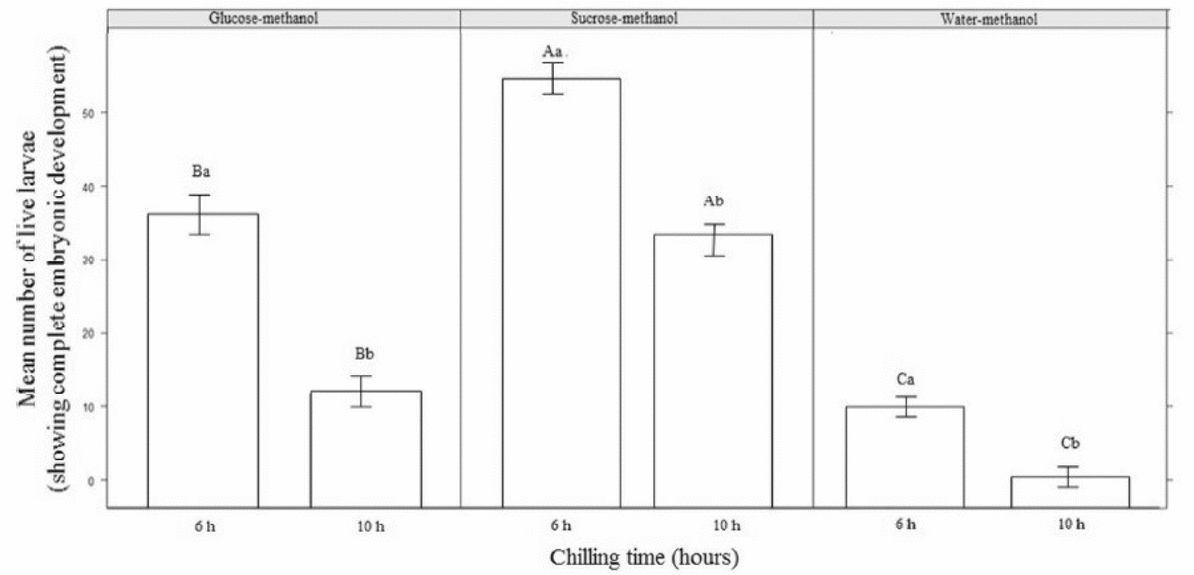
Figure 2 The mean numbers of live P. brachypomus larvae (showing complete embryonic development) following embryonic treatment with different cryoprotectant solutions and chilling for two storage times. Uppercase letters represent differences between treatments for the same hours and lowercase letters represent differences within the treatment.
In embryos incubated in sucrose–methanol, the number of live larvae decreased significantly (P≤0.05) as the storage time increased, with the highest mean observed for embryos chilled for 6 h. Larval survival was significantly different (P>0.05) between embryos chilled in glucose–methanol compared with water–methanol for the 6- and 10-h storage times. However, embryos chilled in water–methanol had a significantly lower survival rate (P≤0.05) compared with embryos chilled in the other cryoprotectants when the embryos were chilled for 10 h.
The effects of chilling treatment at different ontogenetic stages
There was no survival of embryos at blastoderm (~1.2 h post fertilization – hpf) or epiboly (~5 hpf) stages in any treatment group, only embryos at the blastopore closure stage (~8 hpf) with appearance of the optic vesicle (~13hpf) were alive at the end of the experiment.
The embryos treated with the sucrose–methanol solution had statistically higher (P≤0.05) larval survival rates compared with embryos that underwent the other treatments. Embryos that were chilled at stage 13 hpf with sucrose–methanol had statistically higher (P≤0.05) larval survival rates compared with embryos in the same treatment group at stage 8 hpf (Fig 3).

Figure 3 The mean numbers of live P. brachypomus larvae (showing complete embryonic development) that developed from embryos treated with sucrose–methanol, glucose–methanol or water–methanol solutions at various ontogenetic stages. Uppercase letters represent differences between treatments for the same stages and lowercase letters represent differences within the treatment. hpf, hours post fertilization
Significant differences (P>0.05) were observed in the larval survival rates between the glucose–methanol and water–methanol treatment groups. Embryos treated with the glucose–methanol had statistically higher (P≤0.05) larval survival rates compared with those embryos treated with water–methanol. Furthermore, no significant differences (P> 0.05) were observed between the 8-hpf and 13-hpf stages and treated with glucose–methanol or between the 8-hpf and 13-hpf stages treated with water–methanol (Fig 3).
The combined effects of treatment, storage time and developmental stage
It was observed that the mean numbers of completely developed live larvae decreased as the storage time at –10ºC increased (Fig. 2). For embryos treated at the appearance-of-optic-vesicle stage (~13hpf), live larvae were observed after up to 10 h of chilling at –10ºC, with significantly (P≤0.05) higher larval survival rates for embryos treated with the sucrose–methanol solution. However, few live larvae were observed after 10 h of chilling for glucose–methanol or water–methanol.
For embryos treated at the blastopore-closure stage (8 hpf) and the appearance-of-optic-vesicle stage (~13hpf), the larval survival rate for the sucrose–methanol group was significantly different (P≤0.05) from those of the other treatment groups after 6 and 10 h of chilling. Larval survival rates decreased as the storage times increased (Fig. 4). The mean of live larvae decreased significantly (P>0.05) in the glucose–methanol treatment group when at the 10 h storage time compared with 6 h of storage.
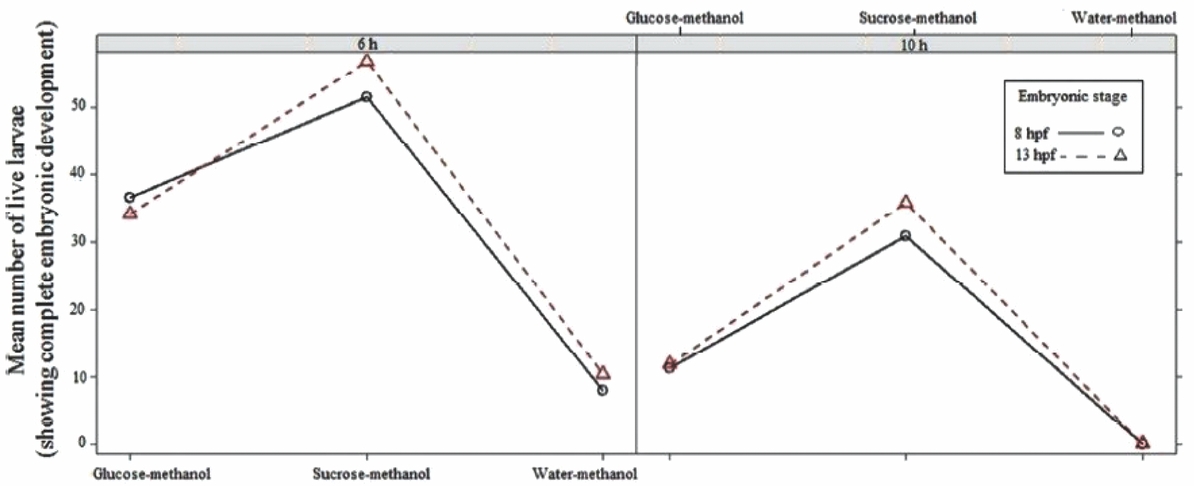
Figure 4 The mean numbers of live P. brachypomus larvae (that showed complete embryonic developed) that developed from embryos treated with cryoprotectant solutions at various ontogenetic stages for various chilling times (6 or 10 h).
For embryos treated at the appearance-of-optic-vesicle stage (13 hpf), the sucrose–methanol treatment group had significant higher number of live larvae after 6 or 10 h of chilling compared with the other treatments (P≤0.05); survival rates decreased for the two storage times (Fig. 4). At the 8-hpf and 13-hpf stages, no statistically significant differences were observed (P>0.05) in the larval survival rates of embryos treated with water–methanol for any of the two chilling times.
Discussion
There was no survival of chilled embryos at initial ontogenic stages (1.2 hpf and 5 hpf) after storage at –10°C. According to Zhang et al. (Reference Zhang, Liu and Rawson2003), the ontogenetic process can fail, thereby rendering cryopreservation unviable. Similarly, Zhang & Rawson (Reference Zhang and Rawson1995) reported that this failure occurred in the initial stages of Danio rerio embryos. The P. brachypomus fish used in the present study may have a greater susceptibility to cooling, due to its high lipid concentrations, which may have affected cryoprotectant action. Furthermore, initial stages of embryonic development are more susceptible to the toxic effects of cryoprotectants (Bart, Reference Bart, Tiersch and Mazik2000), as regulatory metabolic pathways are not fully developed (Lahnsteiner, Reference Lahnsteiner2008). In general, cold sensitivity is related to mechanisms for repairing cells and tissues (Dinnyés et al., Reference Dinnyés, Urbányi, Baranyai and Magyary1998) therefore, the more complex the cells, the less likely they are to react in the same way when exposed to cold.
In this study, the best results with respect to the number of completely developed live larvae were observed after shorter storage times and treatment at progressively later embryonic stages; this finding suggested that storage time has a strong influence on the hatching success of chilled P. brachypomus embryos. Intermediary embryonic stages of P. brachypomus (8 and 13 hpf) were more resistant both to injuries caused by cold and to toxicity caused by cryoprotectants. Differential sensitivity to chilling at different developmental stages has been reported for the embryos of many fish species, including rainbow trout (Maddock, Reference Maddock1974; Haga, Reference Haga1982), fathead minnows (Begovac & Wallace, Reference Begovac and Wallace1989), carp (Jaoul & Roubaud, Reference Jaoul and Roubaud1982; Roubaud et al., Reference Roubaud, Chaillou and Sjafei1985), zebrafish (Zhang & Rawson, Reference Zhang and Rawson1995; Hagedorn et al., Reference Hagedorn, Kleinhans, Freitas, Liu, Hsu, Wildt and Rall1997) and goldfish (Liu et al., Reference Liu, Chou and Lin1993). The majority of these studies shows that post-gastrulation embryos are less sensitive to cold-induced damage. This result is consistent with our findings and, indeed, the best results were observed when P. brachypomus embryos were chilled at an even more advanced developmental stage, namely after the appearance of the optic vesicle (13 hpf). This phenomenon may be due to the fact that early-stage embryos are more susceptible to cytotoxicity (Bart, Reference Bart, Tiersch and Mazik2000), as their metabolic regulatory pathways are not well developed at these stages, making them unable to compensate for the toxicity of the cryoprotectants (Lahnsteiner, Reference Lahnsteiner2008).
Of the tested cryoprotectants, a combination of sucrose and methanol resulted in higher larval survival rates compared with combinations of glucose and methanol or tap water and methanol. In recent studies on Piaractus mesopotamicus and Prochilodus lineatus embryos, good results have been obtained using combinations of 17.5% sucrose and methanol prior to embryo storage at negative temperatures (Fornari et al., Reference Fornari, Ribeiro, Streit, Vargas, Barrero and Moraes2011; Lopes et al., Reference Lopes, Romagosa, Streit, Ribeiro and Digmayer2011, Reference Lopes, Streit, Fornari, de Oliveira, Ribeiro and Romagosa2013; Costa et al., Reference Costa, Velardi, Senhorini, Veríssimo-Silveira and Silveira2012; Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Costa, Velarde, Senhorini and Veríssimo-Silveira2012).
When testing the cryoprotective effects of methanol, glycerol, dimethyl sulphoxide (DMSO), methylglycol, sucrose and sea salt, Robertson et al. (Reference Robertson, Lawrence, Neill, Arnold and McCarthy1988) found that methanol and DMSO had the lowest toxicity levels towards red drum (Sciaenops ocellatus) embryos. Furthermore, the toxicity of specific cryoprotectants may be affected by the embryonic stage at the time of treatment. Indeed, Dinnyés et al. (Reference Dinnyés, Urbányi, Baranyai and Magyary1998) showed in carp (C. carpio) that DMSO was less toxic than methanol during the morula and segmentation stages, whereas methanol was less toxic than DMSO after the initiation of the heart beat.
A study by Harvey & Ashwood-Smith (Reference Harvey and Ashwood-Smith1982) reported that methanol penetrates cells significantly faster than others cryoprotectants (DMSO or glycerol) in salmonid embryos. The superior penetrative ability of methanol is primarily due to its low molecular weight, which is one of the most desirable characteristics for an embryonic cryoprotectant. These findings are consistent with those of Zhang et al. (Reference Zhang, Rawson and Morris1993), who observed that methanol rapidly penetrates the chorion of zebrafish embryos. Additionally, Zhang et al. (Reference Zhang, Rawson and Morris1993), observed in zebrafish that hatching rates were higher in embryos treated with methanol compared with embryos treated with DMSO or methylglycol.
Therefore, in conclusion, P. brachypomus embryos at the blastopore-closure (8-hpf) or appearance-of-optic-vesicle (13-hpf) stages performed the best for use with chilling protocols and that these embryos can be maintained and with better results in a 17.5% sucrose–10% methanol solution for up to 6 h at –10°C. The best results for live larvae were obtained by storage of 13-hpf embryos for 6 h. This report is the first on chilling of P. brachypomus embryos at temperatures below freezing. Despite these promising results, many variables remain to be addressed in future studies, including the factors that affect chilling damage, embryonic permeability and osmoregulation, as well as the effectiveness of other extracellular cryoprotectants.
Acknowledgements
We thank DNOCS for providing the broodstock and Fundação Cearense de Apoio à Pesquisa (FUNCAP) for the economic support.
Conflicts of interest
The authors declare no conflicts of interest.







