Introduction
Oocytes matured in vivo are enclosed with cumulus cells (CCs). Removal of CCs before in vitro maturation (IVM) has been reported to be detrimental to oocyte maturation in mice (Schroeder & Eppig, Reference Schroeder and Eppig1984), rats (Vanderhyden & Armstrong, Reference Vanderhyden and Armstrong1989), cattle (Chian et al., Reference Chian, Niwa and Sirard1994; Zhang et al., Reference Zhang, Jiang, Wozniak, Yang and Godke1995), bovine (Fatehi et al., Reference Fatehi, Zeinstra, Kooij, Colenbrander and Bevers2002, Reference Fatehi, Roelen, Colenbrander, Schoevers, Gadella, Beverst and van denHurk2005) and pigs (Wongsrikeao et al., Reference Wongsrikeao, Kaneshige, Ooki, Taniguchi, Agung, Nii and Otoi2005). Co-culture with cumulus–oocyte complexes (COCs) or CCs has been found to partially restore the developmental potential of oocytes denuded of CCs (DOs), suggesting that the conventional in vitro maturation method is inappropriate for in vitro maturation of DOs. It is well known that the physiological role of gap junctions between oocytes and CCs is particularly important for normal cytoplasmic maturation of oocytes because they play essential nurturing roles in the regulation of oocyte metabolism, such as in these of energy sources (Eppig et al., Reference Eppig, Pendola, Wigglesworth and Pendola2005) and the metabolism of maturation-promoting factor (MPF) (Miao et al., Reference Miao, Liu, Qiao, Miao, Luo and Tan2005). Studies have demonstrated that denuded human oocytes exhibit accelerated meiotic resumption in vitro, a deficiency in the ability of the cytoplasm to maintain M-phase characteristics while meiosis is progressing, a propensity to activate spontaneously after M-phase arrest, and a lack of coordination between nuclear and cytoplasmic maturation (Combelles et al., Reference Combelles, Cekleniak, Racowsky and Albertini2002). Synthesis of cytoplasmic glutathione by CCs, which plays a crucial role in oocyte maturation and subsequent embryonic development (Maedomari et al., Reference Maedomari, Kikuchi, Ozawa, Noguchi, Kaneko, Ohnuma, Nakai, Shino, Nagai and Kashiwazaki2007). This effect will be absent if CCs are denuded, resulting in inadequate cytoplasmic maturation of oocytes after in vitro maturation, which leads to abnormality in the cell number and Oct-4 expression (Chang et al., Reference Chang, Liu, Zhang, Grifo and Krey2005), or increases DNA fragmentation in oocytes (Wongsrikeao et al., Reference Wongsrikeao, Otoi, Murakami, Karja, Budiyanto, Murakami, Nii and Suzuki2004) during the transition from morula to blastocyst. When oocytes are not supported by CCs, it is easy for the oocytes to adhere to the bottom of the dish, and deformation of oocytes may occur, causing mechanical displacement of organelles in oocytes and injury. Additionally, adherence of oocytes to the bottom of the dish may affect material exchange between oocytes and medium. Therefore, elucidation of the function and mechanisms of CCs and establishment of an effective new method for in vitro maturation of DOs has great of significances for further improvement of oocyte quality in the future.
It has been reported that three-dimensional in vivo growth model has been established using collagen-based demineralized bone matrix (DBM) (Ma et al., Reference Ma, Lin, Miao, Liu, Wang and Dai2007) and extracellular matrix (Combelles et al., Reference Combelles, Fissore, Albertini and Racowsky2005; Vanhoutte et al., Reference Vanhoutte, Nogueira and De Sutter2009) to simulate the ovarian status in vivo. However, these mediators do not provide interdependent common substrates for oocytes growing in vivo. The aims of this study were to establish an optimal in vitro maturation method for DOs using buffalo ovarian tissues and CCs as mediator to simulate the ovarian status in vivo, so as to provide a model to investigate the mechanisms of buffalo oocyte maturation and a technical support for improving human oocyte quality in assisted reproductive technology.
Materials and methods
Collection of oocytes
Buffalo ovaries were collected from a local abattoir. Ovaries were excised within 20 to 30 min of slaughter and transported to the laboratory within 4 h in a thermos containing phosphate-buffered saline (PBS) at 35–37°C. Buffalo COCs were recovered by aspiration of buffalo follicles (diameter 2–6 mm) using a 10-ml disposable syringe with an 18-gauge needle. The COCs were collected under a stereomicroscope and the surrounding CCs were removed by manual pipetting to prepare DOs for subsequent in vitro maturation (IVM) experiments.
In vitro maturation of buffalo DOs
The maturation medium for buffalo DOs was TCM-199 (Gibco BRL, Paisley, Scotland, UK) supplemented with 26.2 mM NaHCO3, 5 mM HEPES, 5% estrous cow serum (OCS, self preparation), 2% bovine follicular fluid (BFF, collected without regard to the stage of the reproductive cycle) and 0.1 μg/ml FSH (follicle stimulating hormone). The buffalo DOs were randomly divided into four groups and placed in a glass culture dish containing 1.5 ml maturation medium for IVM using following four culture systems respectively. The culture density was approximate 25 DOs per dish and culture condition was 38.5°C and 5% CO2 in air in a humidified atmosphere.
Direct culture in maturation medium (M1, control group)
The DOs were cultured directly in the maturation medium (Fig. 1).

Figure 1 Direct culture of buffalo denuded oocytes in the maturation medium.
Co-culture with cumulus cell monolayers (M2)
The surrounding CCs of COCs matured in vitro were removed by gentle pipetting and harvested from the maturation medium by centrifugation (5 min at 300 g). After two washes in embryo culture medium (CM, modified TCM199 + 5% OCS), CCs were placed onto culture in a 30 mm glass culture dish at a density of 1–2 × 106/ml. The cumulus cell monolayers were formed (Fig. 2) after culture of 48–72 h in an incubator with 5% CO2, and then buffalo DOs were introduced to the culture dish with maturation medium.

Figure 2 Cumulus cell monolayers.
Co-culture embedded in cumulus cell clumps (M3)
Fully expanded and cotton-like cumulus cell clumps with relatively high viscosity were collected. Then buffalo DOs were placed in the middle of the cumulus cell clumps and suspended in the maturation medium (Fig. 3).

Figure 3 Co-culture embedded in the expanded cumulus cell clumps.
Co-culture embedded in ovarian tissue (M4)
Boat-shaped thin slices of ovarian tissue were collected during recovery of oocytes. After washing with maturation medium, buffalo DOs were placed into the boat-like cavity of the slices of ovarian tissue for IVM using an ultra-thin pipette (Fig. 4).

Figure 4 Co-culture embedded in the ovarian tissues.
Evaluation of nuclear maturation
After IVM for 24 h, oocytes with the first polar body (PB1) were considered to become nuclear maturation.
Evaluation of cytoplasmic maturation
Parthenogenetic activation of oocytes was performed according to the method that had been previously described (Lu et al., Reference Lu, Shi, Wei, Yang and Wei2005). Briefly, after 24 h IVM, the surrounding CCs of oocytes were removed by pipetting in 0.1% hyaluronidase, and the DOs were washed three times with maturation medium. Then, DOs were activated with 5 μM ionomycin for 5 min and followed by culture in 2 mM 6-dimethyl-aminopurine (6-DMAP) for 3–4 h. After washing three times with CM, DOs were transferred to the monolayer of granulosa cells for culture in vitro.
Detection of gap junctions between oocytes and cumulus cells
Gap junctions between oocytes and CCs were detected using the procedure that has been previously described (Isobe et al., Reference Isobe, Maeda and Terada1998; Isobe and Terada, Reference Isobe and Terada2001). Briefly, after 24 h IVM, DOs adhered with relatively more CCs were selected and 4 pl of 10% (w/v) Lucifer yellow CH (Sigma, St. Louis, MO) was injected into the oocyte cytoplasm via a micromanipulator. The gap junctions between oocytes and CCs were confirmed to be reconstructed if Lucifer yellow dye was observed in the surrounding adherent cumulus cells under the confocal microscopy (LSM 510 Meta, Zeiss) within 10–15 min after injection.
Statistical analysis
All data were analysed using the chi-squared (χ2) test. Probability values p < 0.05 were considered to be significant.
Results
Morphology of buffalo DOs matured in vitro for 24 h with the four methods
The DOs clustered together after 24 h IVM with M1 and M2. The cytoplasm was dark, and some oocytes showed severe deformation. The cytoplasm of oocytes matured in vitro with M3 was homogeneous, pale and translucent, and some of oocytes were surrounded or partially surrounded with adherent CCs and released the PB1 (Fig. 5). While the cytoplasm of oocytes matured in vitro with M4 was relatively dark, and adherent filamentous substances were observed in the zona pellucida.

Figure 5 The oocyte after co-culture embedded in the expanded cumulus cell clumps for 24 h.
Nuclear maturation of DOs matured in vitro with the four methods
As shown in Table 1, more oocytes extruded the PB1 when they were cultured in vitro for 24 h with M3 (56.89%) in comparison with M1 (45.14%) and M4 (40.48%; p < 0.05), indicating that M3 was more suitable for IVM of DOs.
Table 1 Nuclear maturation of buffalo denuded oocytes cultured in vitro for 24 h using the four methods

a,bWithin columns, values with different superscripts are significantly different (p < 0.05).
Parthenogenetic development of DOs matured in vitro with the four methods
As illustrated in Table 2, the cleavage rate of oocytes matured in vitro with M3 after parthenogenetic activation (64.07%) was higher that of oocytes matured in vitro with M4 (45.24%, p < 0.05), and the blastocyst yield of oocytes matured in vitro with M3 (25.75%) was also higher than that of oocytes matured in vitro with M1 (15.97%) and M4 (13.49%, p < 0.05). However, there was not different in the hatching rate of blastocysts among the four groups (p > 0.05).
Table 2 Parthenogenetic development of buffalo denuded oocytes matured in vitro using the four methods

a,bWithin columns, values with different superscripts are significantly different (p < 0.05).
Reconstruction of gap junctions between oocytes and cumulus cells during IVM
When DOs were cultured with M1, M2 and M4 for 24 h, no adherent CCs were observed around the oocytes. However, more CCs adhered to the zona pellucida of oocytes when DOs were cultured with M3. Injection of Lucifer yellow into the cytoplasm of oocytes matured in vitro with M3 indicated that the gap junctions between CCs and oocytes was reconstructed as Lucifer yellow was observed in the surrounding cumulus cells under the confocal microscopy (Fig. 6).
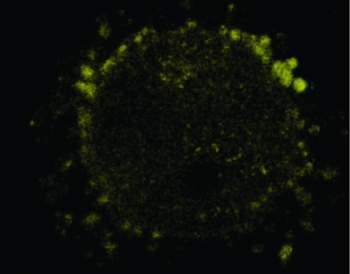
Figure 6 Lucifer yellow was observed in the cumulus cells surrounding oocytes cultured using M3.
Discussion
It is well known that oocytes need to undergo cytoplasmic maturation as well as nuclear maturation to become able to support successful fertilization and embryo development (Mermillod et al., Reference Mermillod, Oussaid and Cognie1999; Trounson et al., Reference Trounson, Anderiesz and Jones2001). Oocytes acquire their developmental competence (cytoplasmic maturity) after a long series of preparatory processes that involve transcription and subsequent translation of transcripts during the meiotic prophase, in which the cytoplasmic organelles proliferate greatly, RNA levels double, a large number of proteins are synthesized, and energy-rich substrates are accumulated continuously. In the antral ovarian follicle, CCs surround the oocyte, while mural granulosa cells form the follicular wall. The CCs communicate with each other and with the oocyte by means of gap junctions (Cha & Chian, Reference Cha and Chian1998; Mermillod et al., Reference Mermillod, Oussaid and Cognie1999; Hardy et al., Reference Hardy, Wright, Franks and Winston2000). Further studies have demonstrated that cytoplasmic maturation of oocytes requires optimal intracellular cAMP concentrations and meiotic arrest (Vaccari et al., Reference Vaccari, Horner, Mehlmann and Conti2008; Norris et al., Reference Norris, Ratzan, Freudzon, Mehlmann, Krall, Movsesian, Wang, Ke, Nikolaev and Jaffe2009). Direct communication between oocytes and CCs is very important for maintaining meiotic arrest. This can only be accomplished by the cumulus–oocyte complex, not DOs. In addition, CCs may promote cytoplasmic maturation of oocytes (Li et al., Reference Li, Norman, Armstrong and Gilchrist2000). Therefore, effective communication by gap junction between oocytes and CCs during the entire process is necessary for in vitro growth and development of oocytes (Canipari, Reference Canipari2000; Thomas and Vanderhyden, Reference Thomas and Vanderhyden2006; Orisaka et al., Reference Orisaka, Tajima, Tsang and Kotsuji2009). In view of this requirement, researchers have used collagen-based DBM (Ma et al., Reference Ma, Lin, Miao, Liu, Wang and Dai2007) and extracellular matrix (Combelles et al., Reference Combelles, Fissore, Albertini and Racowsky2005; Vanhoutte et al., Reference Vanhoutte, Nogueira and De Sutter2009) to establish a three-dimensional structural model successfully for IVM of DOs. However, these mediators are not substrates for intercommunication and interaction with oocytes in vivo. Therefore, we used ovarian tissue and CCs as the mediators to simulate the in vivo ovarian three-dimensional environment and create a growth pattern of oocytes similar to that of the cumulus–oocyte complex. The three-dimensional structure and morphology of oocytes may be kept intact within a certain period of culture time. In this way, it will be expected that gap junctions between oocytes and CCs may be reconstructed and a new model for study of in vitro oocyte maturation will be established.
In this study, we found that DOs adhered to the bottom of culture dish wall and some oocytes showed severe deformation when they were cultured directly. The possible reasons for this may be that oocytes contact with the bottom of the dish and deform gradually due to gravity when they have lost the three-dimensional support, which will cause mechanical displacement of intracellular organelles and injury. This problem was resolved by employment of cumulus cell blocks and ovarian tissue blocks as support mediators for oocytes during culture in the present study.
Some studies have shown that removal of CCs is deleterious to the maturation of oocytes, while co-culture with cumulus cell monolayers significantly promotes cytoplasmic maturation of oocytes. The possible reasons for these findings are that CCs may regulate MPF activity, maturation and cleavage process (Ge et al., Reference Ge, Sui, Lan, Liu, Wang and Tan2008). However, the results of our study showed that the proportion of oocytes extruded with the PB1, cleaved and developing to blastocysts after parthenogenetic activation were not significantly improved when buffalo DOs were co-cultured with granulosa cell monolayers for 24 h. Hashimoto et al. (Reference Hashimoto, Saeki, Nagao, Minami, Yamada and Utsumi1998) reported that the medium supplemented with CCs could improve the developmental competence of bovine cumulus–oocyte complex, but had no effects on DOs. We speculate that some factors secreted by CCs may act through gap junctions to promote the maturation of oocytes, co-culture with cumulus cell monolayers does not result in the reconstruction of gap junctions between cumulus cells and oocytes.
In the ovarian tissue, the oocyte is surrounded by CCs, granulosa cells, follicle membrane and ovarian tissues, which communicate with each other and with the oocyte to regulate the maturation of oocytes by means of gap junctions (Conti, Reference Conti2010). However, in the present study, when DOs were cultured by embedded in ovarian tissue in vitro, the release rate of the PB1 and the blastocyst rate after parthenogenetic activation were significantly lower than in the control group, indicating that embedded co-culture in ovarian tissue blocks causes inhibition of oocyte maturation rather than promotion. We speculate that ovarian tissue blocks secrete some factors that inhibit oocyte maturation. Within the body, these factors may first enter the parietal granulosa cells and CCs through the follicular membrane and undergo modifications that they may be processed, and transformed into factors that can promote oocyte maturation. These factors act together with factors secreted by parietal granulosa cells and CCs, thereby regulate oocyte maturation. However, these important intermediary steps are missing when oocytes are directly cultured with ovarian tissue, and then the ovarian factors display inhibitory action on oocyte maturation.
Adequate maturation of the oocyte cytoplasm is necessary to ensure normal fertilization and subsequent development of the oocytes. In the present study, DOs were cultured by embedding in the expanded cumulus cell clumps and found that the release rate of the PB1 was significantly higher than in direct culture, indicating that expanded cumulus cell clumps are beneficial to the nuclear maturation of DOs. Furthermore, parthenogenetic activation revealed that the cleavage rate and blastocyst rate of oocytes were also significantly higher than directly cultured oocytes, suggesting that the expanded cumulus cell clump is also beneficial to the cytoplasmic maturation of DOs. Some studies have indicated that CCs may regulate the pH equilibrium in oocytes through a variety of pathways (Fitzharris & Baltz, Reference Fitzharris and Baltz2006), and may antagonize oxidative stress during fertilization and protect oocytes (Fatehi et al., Reference Fatehi, Roelen, Colenbrander, Schoevers, Gadella, Beverst and van denHurk2005), induce communication between oocytes and the extrafollicular environment or reaction between oocytes and medium, or remove those components in the medium that inhibit embryo development (Fatehi et al., Reference Fatehi, Zeinstra, Kooij, Colenbrander and Bevers2002). In addition, it is possible that gap junctions are reconstructed between CCs and oocytes, and thereby maturation-inducing soluble factors secreted by the cumulus cells are transported efficiently to oocytes through these junctions, which will promote oocyte maturation and substrate accumulation for subsequent development. In this study, injection of Lucifer yellow CH into the oocytes co-cultured by embedding in the expanded cumulus cell clumps displayed that Lucifer yellow CH was observed in CCs adherent to oocytes, indicating that gap junctions were reconstructed between cumulus cells and oocytes. Then, maturation-inducing soluble factors secreted by the CCs can be transported efficiently to oocytes through these junctions, and promote oocyte maturation and substance accumulation for subsequent development.
In conclusion, co-culture of DOs embedded in cumulus cell clumps can improve their nuclear and cytoplasmic maturation, possibly through the gap junctions between oocytes and CCs reconstructed in vitro. This new model will be useful for studying the mechanisms of oocyte maturation, and fully utilizing the immature oocytes in intracytoplasmic sperm injection (ICSI) in human-assisted reproductive technology.
Acknowledgements
This work was funded by the China High Technology Development Program (2011AA100607, 2007AA100505), China Transgenic Project (2011ZX08007-003) and Guangxi Science Foundation (2010GXNSFF013002).










