Introduction
In mammals, sperm are formed in the seminiferous tubules and undergo complex modifications while transiting in the epididymis and after ejaculation to become able to fertilize the oocyte (Gadella, Reference Gadella2008). Capacitation is one of the major physiological events that affects sperm after ejaculation and involves increases in the fluidity of the sperm membrane, cholesterol efflux, alteration of membrane potential and induction of hyperactivation. All these events are necessary for sperm to acquire the competence to fertilize an egg (Córdoba et al., Reference Córdoba, Santa-Coloma, Beorlegui and Beconi1997; Rodriguez-Martinez et al., Reference Rodriguez-Martinez, Kvist, Ernerudh, Sanz and Calvete2011; Visconti et al., Reference Visconti, Krapf, de la Vega-Beltran, Acevedo and Darszon2011). In many mammalian species such as bovine, sperm are unable to fertilize oocytes immediately after ejaculation and they must undergo capacitation in the female reproductive tract (in vivo) or by capacitating agents (in vitro) to acquire fertilizing capability (Yanagimachi, Reference Yanagimachi, Knobil and Neill1994; Visconti et al., Reference Visconti, Krapf, de la Vega-Beltran, Acevedo and Darszon2011). At this time, several substances are being used to induce bovine sperm capacitation in vitro, such as bicarbonate (Harrison and Gadella, Reference Harrison and Gadella2005), bovine serum albumin (Xia and Ren, Reference Xia and Ren2009), calcium (Visconti et al., Reference Visconti, Krapf, de la Vega-Beltran, Acevedo and Darszon2011), binder of sperm protein 1 (BSP1; Plante et al., Reference Plante, Thérien, Lachance, Leclerc, Fan and Manjunath2014; Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016) and glycosaminoglycans (Parrish et al., Reference Parrish, Susko-Parrish, Handrow, Sims and First1989). Nevertheless, heparin is the most commonly used compound for sperm capacitation in IVF systems for both research and commercial production of bovine embryos (Parrish, Reference Parrish2014). Despite this spread of use of heparin, the mechanism by which it induces capacitation is largely unknown. Heparin is present in the female genital tract, follicular and oviductal fluid and binds to bull spermatozoa as a typical receptor–ligand interaction, thereby promoting capacitation (Ax and Lenz, Reference Ax and Lenz1987). BSP1 is one of the major proteins of bovine seminal plasma (Manjunath et al., Reference Manjunath, Lefebvre, Jois, Fan and Wright2009; Rego et al., Reference Rego, Crisp, Moura, Nouwens, Li, Venus, Corbet, Corbet, Burns, Boe-Hansen and McGowan2014) and binds to sperm membrane at ejaculation. BSPs promote efflux of membrane cholesterol and phospholipids, an essential step for sperm capacitation (Plante et al., Reference Plante, Prud’homme, Fan, Lafleur and Manjunath2016).
Some authors have suggested that heparin and BSP1 act in conjunction to induce sperm capacitation. This hypothesis is supported by the fact that heparin and BSPs have biochemical affinity and interact during chromatography assays (Parrish et al., Reference Parrish, Susko-Parrish, Handrow, Sims and First1989; Manjunath and Thérien, Reference Manjunath and Thérien2002; Parrish, Reference Parrish2014; Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016; our unpublished results). Our previous study showed that addition of BSP1 to the fertilization medium without the presence of heparin induced capacitation and the acrosome reaction of bovine sperm. Also, when compared with heparin, BSP1 alone added to the IVF medium was associated with better in vitro embryo development after fertilization with epididymal sperm (Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016). When we used ejaculated sperm in the same experiment, BSP1 induced sperm capacitation similarly to the effects of heparin, with similar rates of embryo development (Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016). Therefore, the study on the interaction among capacitator agents is of interest because of the important role played by heparin for bovine IVF and all the known effects of BSPs on sperm function (Parrish et al., Reference Parrish, Susko-Parrish, Handrow, Sims and First1989; Thérien et al., Reference Thérien, Bergeron, Bousquet and Manjunath2005; Plante et al., Reference Plante, Prud’homme, Fan, Lafleur and Manjunath2016; Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016). Given this scenario, the aim of the present study was to evaluate the effect of heparin and BSP1, as well as their potential interaction, on sperm capacitation and fertilization rates using epididymal and ejaculated bovine sperm.
Materials and methods
Effects of BSP1 and/or heparin on sperm capacitation
Pools from frozen–thawed ejaculated (n = 4) and epididymal (n = 6) bull sperm were thawed at 37°C for 30 s and separated through Percoll gradients (45–90%), as described before (Parrish et al., Reference Parrish, Krogenaes and Susko-Parrish1995; Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016). Briefly, Percoll solution (90%; 400 μl) was placed in two 1-ml Eppendorf tubes, and 400 μl of 45% Percoll was layered smoothly over that. An aliquot of 100 μl of pooled frozen–thawed semen was layered on top of the gradients and then tubes were centrifuged for 10 min at 900 g. The pellets were re-suspended in the same amount of sperm-TALP medium and centrifuged for 5 min at 600 g. The supernatant was removed and incubated in accordance with the following treatments. Control group: Fert-TALP medium without heparin; heparin group: Fert-TALP with heparin (10 UI/ml); BSP1 group: Fert-TALP medium with BSP1 (10 µg/ml for ejaculated sperm; 40 µg/ml for epididymal sperm); HEP + BSP1 group: Fert-TALP medium with heparin (5 UI/ml) and BSP1 (5 µg/ml for ejaculated sperm; 20 µg/ml for epididymal sperm).
The percentage of capacitated spermatozoa was determined by the method of chlortetracycline fluorescence (CTC), as described by Fraser (Reference Fraser1995) and validated in our previous work (Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016). Briefly, CTC was added to the medium containing the capacitated sperm and the control sample. Glutaraldehyde was then added to the mixture to a final concentration of 0.1% (w/v). Sperm capacitation rates were estimated by evaluating the CTC pattern, identifying intact capacitated sperm, the ones with a fluorescence-free band in the post-acrosomal region; and acrosome-reacted sperm, and the ones with a soft fluorescence over the whole spermatozoon except for the thin bright punctate fluorescent band in the equatorial segment. Each sample was evaluated at 0, 15, 30 and 60 min after treatment. Slides were examined at ×400 magnification using excitation at 410 nm on an epifluorescence microscope. The percentages of live and dead sperm for each treatment were determined by counting 100–200 sperm per slide.
In vitro embryo production
Bovine ovaries were obtained at a local abattoir and kept at 32°C during transport to the laboratory. Cumulus–oocyte complexes (COCs) were aspirated with a disposable syringe from follicles with 3–8 mm diameter and washed in Tissue Culture Medium 199 (TCM-199; Sigma-Aldrich, USA), as reported before (Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Wei, Moreira, Caccia, Fernandez Taranco and Bó2012). In summary, oocytes were transferred to micro-drops containing 100 µl maturation medium (10–20 COCs per drop) and matured for approximately 24 h at 38.8°C in an atmosphere of saturated humidity and 5% CO2 in air. The maturation medium consisted of TCM-199 supplemented with 10% (v/v) fetal bovine serum, 0.2 mM sodium pyruvate, 25 mg/ml porcine follicle stimulating hormone (FSH) (Follitropin-V; Bioniche, Canada) and penicillin/streptomycin (10 µl/ml; Schering, USA). After in vitro maturation, COCs were assigned randomly to the different experimental groups.
After each straw of frozen semen (ejaculated or epididymal) was thawed for 1 min at 37°C, sperm were prepared for fertilization using Percoll discontinuous gradient (45% and 90%), as described before (Parrish et al., Reference Parrish, Krogenaes and Susko-Parrish1995). Fertilization medium consisted of Fert-TALP medium supplement with the different capacitator factors and COCs were fertilized in 50 µl droplets (8–20 COC/droplet) covered with mineral oil. A 3 µl aliquot of 1 × 106 sperm/ml was used to fertilize the COCs. For culture synthetic oviductal fluid (SOF) medium supplemented with amino acids, 1.5 mM d-glucose and 0.4% of BSA was used. After 18–20 h, COCs were pipetted to remove the cumulus cells and excess sperm cells, washed once in SOF and transferred into 50 µl droplets of SOF medium under mineral oil in a controlled atmosphere (5% CO2, 5% O2 and 90 % N2) at 38.8°C. Zygotes were cultured in vitro in SOF medium for 7 days at 38.8°C, using a humidified incubator with 5% CO2 and 5% O2 in air. Cleavage and blastocyst rates were evaluated on days 2 and 7 after fertilization, respectively (Robertson and Nelson, Reference Robertson and Nelson2007). Oocyte maturation and embryo production were performed under the same conditions for epididymal and ejaculated sperm.
Fertilization rates of frozen–thawed ejaculated sperm after treatment with BSP1 and/or heparin
Matured COCs (n = 1079) were allocated randomly into treatments and fertilized with frozen–thawed ejaculated sperm from four different bulls from a commercial artificial insemination centre. For fertilization, COCs were incubated with ejaculated sperm into the different treatments groups. Treatments were defined as: Control group: Fert-TALP medium without heparin; Heparin group: Fert-TALP with heparin (10 UI/ml); BSP1 group: Fert-TALP medium with BSP1 (10 µg/ml); HEP + BSP1 group: Fert-TALP medium with heparin (5 UI/ml) and BSP1 (5 µg/ml) for 18 h. For each in vitro fertilization routine, conducted weekly, we used the pool of four different bulls for all treatments.
Fertilization rates of epididymal frozen–thawed sperm after treatment with BSP1 and/or heparin
Matured COCs (n = 1014) were allocated randomly into treatment groups and fertilized with a pool of frozen–thawed epididymal sperm from six different bulls. Cauda epididymal sperm used in this experiment were collected and frozen as described before (Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016). For fertilization, COCs were incubated with epididymal sperm into the different four treatments groups. Treatments were defined as: Control group: Fert-TALP medium without heparin; Heparin group: Fert-TALP with heparin (10 UI/ml); BSP1 group: Fert-TALP medium with BSP1 (10 µg/ml) and HEP + BSP1 group: Fert-TALP medium with heparin (5 UI/ml) and BSP1 (5 µg/ml) for 18 h.
Statistical analysis
Sperm capacitation rates were compared among all treatments by Fisher’s exact test. Data related to cleavage and blastocyst rates were transformed by square root and then analyzed by analysis of variance (ANOVA), considering bulls and BSP1 and/or heparin concentrations as the main effects as well their interactions. The protected least significant difference (LSD) test was used for subsequent multiple comparisons when ANOVA revealed statistically significant differences (P < 0.05). All data were analyzed using the Info-Stat software (UNC, Argentina).
Results
Capacitation was greater when ejaculated sperm were treated for 60 min with heparin (32 ± 1.5%) in comparison with the other treatments (Control: 6.3 ± 0.6%; HEP + BSP1: 22.3 ± 1.4%; BSP1: 19.3 ± 2.9%). Furthermore, capacitation of ejaculated sperm happened faster during the first 15 min of incubation with heparin compared with HEP + BSP1 or with BSP1 only (P < 0.03; Fig. 1). Nevertheless, cleavage rates and blastocyst formation were similar after ejaculated sperm were incubated with heparin, Hep + BSP1 or BSP1 (P > 0.05; Table 1).

Figure 1. Sperm capacitation rates (means ± standard error of the mean (SEM)) of frozen–thawed bovine ejaculated sperm in vitro capacitated into different treatments, evaluated during a period from 0 to 60 min. Control: Fert-TALP medium without heparin; HEP: Fert-TALP with heparin (10 UI/ml); BSP1: Fert-TALP medium with BSP1 (10 µg/ml); HEP + BSP1: Fert-TALP medium with heparin (5 UI/ml) and BSP1 (5 µg/ml).
Table 1. Cleavage and blastocyst rates (means ± standard error of the mean (SEM)) after in vitro fertilization with bovine ejaculated sperm treated with heparin and/or BSP1

Control: Fert-TALP medium without heparin; Heparin: Fert-TALP with heparin (10 UI/ml); BSP1: Fert-TALP medium with BSP1 (10 µg/ml); Hep + BSP1: Fert-TALP medium with heparin (5 UI/ml) and BSP1 (5 µg/ml).
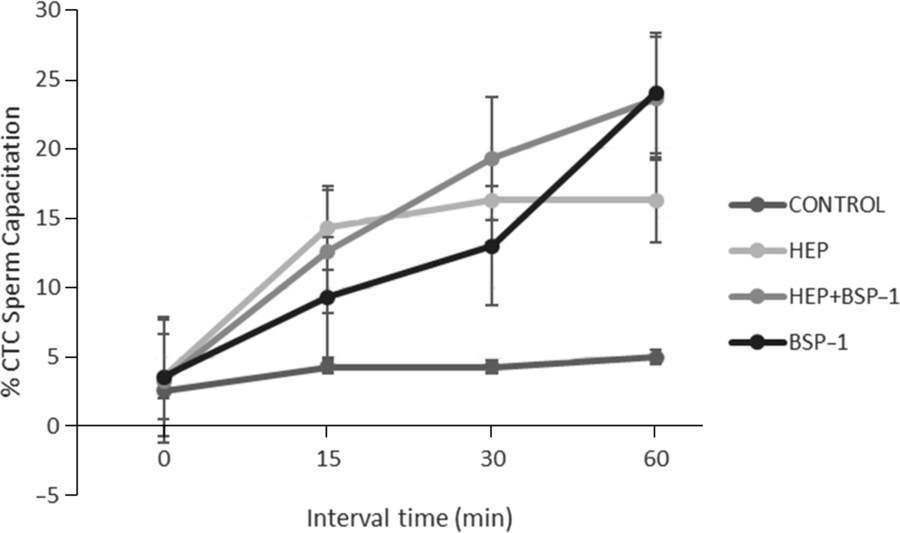
Capacitation rates of epididymal sperm gradually increased over time (15–60 min) after incubations with BSP1, reaching the highest rates at 60 min. Capacitation rates after treatment with heparin had sharp increases in the first 15 min but, after 60 min, those rates were significantly lower (P < 0.05) compared with the ones detected with BSP1 treatment (Fig. 2). Also, the combination of BSP1 with heparin did not increase (P > 0.05) capacitation rates of epididymal sperm beyond that detected with BSP1 alone at 60 min of incubation (BSP1: 24 ± 1.1%; Hep + BSP1: 23.6 ± 1.7%). When using epididymal sperm, only BSP1 significantly enhanced cleavage rates above the other groups (Table 2). However, effects of heparin + BSP1 on cleavage rates were equivalent to those achieved with either heparin or BSP1 alone (P > 0.05). The percentage of blastocyst formation was greater after BSP1 (29.7 ± 2.4) or BSP1 + Hep (30.4 ± 2.1) treatment in comparison with those obtained with heparin alone (19.4 ± 2.2%) and with the control treatment (13.8 ± 1.3%; Table 2). The combination of BSP1 with heparin had similar results compared with BSP1 by itself in blastocyst formation when IVF was carried out with frozen–thawed epididymal sperm.

Figure 2. Sperm capacitation rates (means ± standard error of the mean (SEM)) of frozen–thawed bovine epididymal sperm in vitro capacitated into different treatments, evaluated during a period from 0 to 60 min. Control: Fert-TALP medium without heparin; HEP: Fert-TALP with heparin (10 UI/ml); BSP1: Fert-TALP medium with BSP1 (40 µg/ml); HEP + BSP1: Fert-TALP medium with heparin (5 UI/ml) and BSP1 (20 µg/ml).
Table 2. Cleavage and blastocyst rates (means ± standard error of the mean (SEM)) after in vitro fertilization with bovine epididymal sperm treated with BSP1 and/or heparin

Control: Fert-TALP medium without heparin; Heparin: Fert-TALP with heparin (10 UI/ml); BSP1: Fert-TALP medium with BSP1 (10 µg/ml); Hep + BSP1: Fert-TALP medium with heparin (5 UI/ml) and BSP1 (5 µg/ml).
Discussion
Binder of sperm proteins are the predominant protein of the bovine seminal plasma, are mainly secreted by the accessory sex glands and bind to sperm at the moment ejaculation occurs. In the present study, we have shown that responses of epididymal and ejaculated sperm to heparin and BSP1 are quite different, as evaluated by capacitation rates as well as by cleavage rates and early embryo development. To our knowledge, this is the first study focused on the evaluation of combined effects of heparin and BSP1 on both epididymal and ejaculated sperm capacitation and fertilization capacity in vitro.
Treatments with either heparin or BSP1 induced in vitro capacitation of bovine sperm (ejaculated or epididymal). However, capacitation rates were significantly affected by the duration of treatment and type of sperm (ejaculated or cauda epididymal sperm) used. As well known, both BSP1 and heparin promote capacitation and acrosome reaction of bovine sperm (Thérien et al., Reference Thérien, Bleau and Manjunath1995; Sankhala et al., Reference Sankhala, Damai, Anbazhagan, Kumar, Bulusu and Swamy2011; Parrish, Reference Parrish2014; Plante et al., Reference Plante, Prud’homme, Fan, Lafleur and Manjunath2016; Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016). In the present experiment, we demonstrated that heparin induced the highest rates of capacitation in frozen–thawed ejaculated sperm, as described by other authors (Parrish et al., Reference Parrish, Susko-Parrish, Winer and First1998, Reference Parrish2014; Visconti and Kopf, Reference Visconti and Kopf1998). In fact, heparin is, at this time, the main agent used to capacitate bovine sperm in vitro. Furthermore, our study demonstrated that BSP1 presented a different performance for capacitation rates throughout the time assayed. During the first minutes of incubation, treatments with BSP1 induced lower capacitation rates in comparison with heparin. In the case of ejaculated sperm, treatments with BSP1 increased the rates over the time, but did not achieve the same rates induced by heparin. Therefore, BSP1 could be used for sperm capacitation but it requires more time to exert its effect when compared with heparin, as also shown by previous studies (Manjunath et al., 2002, Reference Manjunath, Bergeron, Lefebvre and Fan2007; Plante et al., Reference Plante, Thérien and Manjunath2012, Reference Plante, Thérien, Lachance, Leclerc, Fan and Manjunath2014; Therrien et al., Reference Therrien, Manjunath and Lafleur2013; Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016). The reason for this delayed effect of BSP1 is probably related to the time necessary for BSP1 to interact with and remove both cholesterol and phospholipids from sperm membrane (Thérien et al., Reference Thérien, Moreau and Manjunath1999; Manjunath and Thérien, Reference Manjunath and Thérien2002; Manjunath et al., Reference Manjunath, Bergeron, Lefebvre and Fan2007; Therrien et al., Reference Therrien, Manjunath and Lafleur2013). Furthermore, the use of heparin and BSP1 together did not present any synergic effect on the capacitation rates of ejaculated sperm. The precise pathways for BSP-induced sperm capacitation are still unknown, but it is known that BSP promotes removal of cholesterol from the sperm plasma membrane to promote capacitation (Xia and Ren, Reference Xia and Ren2009; Parrish, Reference Parrish2014).
BSP1 had greater effects on capacitation rates of cauda epididymal, allowing further acrosome reaction and normal fertilization. This finding has been confirmed by other authors, who used exogenous BSP1 to trigger capacitating-related events on epididymal sperm (Gadella and Luna, Reference Gadella and Luna2014; Parrish, Reference Parrish2014; Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016). In the present experiment, epididymal sperm treated with BSP1 had an initial delayed capacitation, but differences in comparison with heparin gradually decreased over time, as BSP1 achieved greater effect on sperm capacitation after 60 min of incubation. These observations confirmed that BSP1 needs more time to induce capacitation when compared with heparin, regardless the type of sperm cells (epididymal or ejaculated). Also, we showed that addition of heparin to BSP1 treatment did not cause a significant increase in capacitation rates of epididymal sperm. Therefore, it became certain that BSP1, and not heparin, was the component causing the main effect on capacitation of epididymal sperm. Heparin was associated with lower rates of epididymal sperm capacitation in comparison with treatments with BSP1 because of the type of heparin interaction with the sperm membrane (Parrish, Reference Parrish2014). Therefore, heparin induced higher capacitation rates in ejaculated compared with epididymal sperm probably because heparin induces capacitation by removing BSP-bound molecules present in the ejaculated sperm (Parrish et al., Reference Parrish, Susko-Parrish, Handrow, Sims and First1989; Plante et al., Reference Plante, Prud’homme, Fan, Lafleur and Manjunath2016). Given that bovine epididymal sperm do not have BSP molecules bound to them (Souza et al., Reference Souza, Moura, Monaco and Killian2008), capacitation rates were greater only with treatments including BSP1.
Effects of heparin and BSP1 on cleavage rates after IVF using ejaculated and epididymal sperm were similar to those described previously (Thérien et al., Reference Thérien, Moreau and Manjunath1999; Plante et al., Reference Plante, Prud’homme, Fan, Lafleur and Manjunath2016; Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016). Furthermore, embryo developmental rates produced by epididymal and ejaculated sperm treated with BSP1 were similar or higher compared with heparin, probably due to the capacity of BSP1 to help sperm–oocyte interaction. It is known that BSPs bind to bovine zona pellucida (Liberda et al., Reference Liberda, Ryslavá, Jelínková, Jonáková and Tichá2002) and BSP absence may even inhibit the sperm–oocyte adhesion (Turmo et al., Reference Turmo, Mondéjar, Grullón, Calvete, Manjunath, Coy and Aviles2009). Therefore, it is possible that some of the remaining BSP1 bound to the sperm surface after capacitation also participates in sperm–oocyte adhesion (Manjunath et al., Reference Manjunath, Sairam and Uma1987; Plante et al., Reference Plante, Prud’homme, Fan, Lafleur and Manjunath2016). We have reported before that BSP molecules remain bound to bovine sperm even after the capacitation/acrosome reaction and after contact of sperm with secretions of the oviductal fluid (Souza et al., Reference Souza, Moura, Monaco and Killian2008).
BSP1 and heparin presented similar effects on embryo development when we used ejaculated sperm for IVF, as reported before (Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016). Nevertheless, BSP1 + heparin did not have any synergic effect on embryo development when we used ejaculated sperm for IVF. This result confirmed that these two agents probably had the capacity to induce capacitation through the same pathway, producing the efflux of cholesterol efflux and membrane modifications (Thérien et al., Reference Thérien, Moreau and Manjunath1999; Plante et al., Reference Plante, Prud’homme, Fan, Lafleur and Manjunath2016; Rodríguez-Villamil et al., Reference Rodríguez-Villamil, Marulanda, Martins, Oliveira and Aguiar2016). Therefore, it seems that BSP1 and heparin compete for the same adhesion sites on the sperm membrane, suggesting that there is a competitive relationship among them instead of a synergic effect. When we used IVF systems with epididymal sperm, formation of blastocysts was greater after treatment with BSP1 in comparison with heparin. It appears therefore that heparin needs an interaction with sperm membrane-bound BSP1 to induce capacitation (Gadella and Luna, Reference Gadella and Luna2014; Parrish, Reference Parrish2014). Given that bovine epididymal sperm did not have any BSP1 attached to them, these cells were unable to respond to heparin alone.
In conclusion, the present study confirms that either BSP1 or heparin can be used as a capacitator agent for bovine ejaculated sperm during in vitro fertilization, also associated with proper embryo development. However, BSP1 seems to be more efficient compared with heparin to induce capacitation in epididymal sperm and formation of blastocysts. Such results clearly indicate that ejaculated and epididymal sperm have distinct physiological status, related to the presence, or not, of BSP1. We also report that BSP1 and heparin have no synergic effects on sperm capacitation.
Financial Support
We acknowledge the Conselho Nacional de Desenvolvimento Tecnologico e Científico CNPq for the financial support.
Conflict of Interest
No conflict of Interest.
Ethical Standards
The study was conducted in accordance with the ethical and animal welfare statements approved by the Department of Animal Science of the Federal University of Ceará, Fortaleza, Brazil, on August 30, 2010.
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.