Introduction
Hypocuprosis is the predominant deficiency that globally affects grazing cattle Ramirez et al., Reference Ramirez, Mattioli, Tittarelli, Giuliodori and Yano1998). National Animal Health Monitoring Service categorized 40.6% of United States beef cattle as copper deficient (Dargatz et al., Reference Dargatz, Garry and Clark1999). Ramirez and colleagues (Reference Ramirez, Mattioli, Tittarelli, Giuliodori and Yano1998) reported similar values in Salado River Basin (Argentina), an area of 55793 km2 with 6.5 × 106 beef cattle (Dillon, Reference Dillon1992). Copper deficiency is evidenced by different clinical signs such as pale coat; anemia; spontaneous fractures; poor capillary integrity; myocardial degeneration; hypomyelinization; poor reproductive performance; reduce resistance to infectious disease; diarrhea, and generalized ill-health causing severe economic losses (Tessman et al., Reference Tessman, Lakritz, Tyler, Casteel, Williams and Dew2001).
The mammalian cumulus–oocyte complex (COC) and its extracellular matrix are involved in several reproductive processes, including oocyte pick-up by the oviduct, and subsequent fertilization (Ball et al., Reference Ball, Liebfried, Lenz, Ax, Bavister and First1983; Sirard & Lambert Reference Sirard and Lambert1985; Talbot et al., Reference Talbot, Shur and Myles2003). Communication between oocyte and CC occurs via paracrine by functional gap junctions that allow the passage of cAMP, ions and purines; and participate in oocyte meiotic regulation (Sutton et al., Reference Sutton, Gilchrist and Thompson2003). It is known that the presence of CC is not necessary for nuclear maturation, but plays an important role on oocyte cytoplasmic maturation evidenced by enhancing blastocyst rates (Lonergan & Fair, Reference Lonergan and Fair2008; Anchordoquy et al., Reference Anchordoquy, Anchordoquy, Sirini, Picco, Peral-García and Furnus2014).
The imbalance between reactive oxygen species (ROS) production and the action of the antioxidant cell system produce DNA damage and cause cell apoptosis. Apoptosis and DNA damage are important parameters that are useful indicators of cellular health. It has been demonstrated in bovine that high rates of apoptotic follicular cells affect oocyte competence for subsequent embryo development (Zadák et al., Reference Zadák, Hyspler, Tichá, Hronek, Fikrová, Rathouská, Hrnciariková and Stetina2009). Copper (Cu) plays an important role in mammalian cells as a cofactor of metabolic enzymes involved in respiration, radical detoxification, iron metabolism, and other physiological processes (Petris et al., Reference Petris, Strausak and Mercer2000; Steveson et al., Reference Steveson, Ciccotosto, Ma, Mueller, Mains and Eipper2003; Lutsenko et al. Reference Lutsenko, Barnes, Bartee and Dmitriev2007). Moreover, Cu is a transition metal, for this reason their homeostasis must be regulated to prevent excessive production of ROS damaging cellular mechanisms (Lovejoy & Guillemin, Reference Lovejoy and Guillemin2014). In addition, Cu deficiency increases oxidative stress and oxidative damage (Kambe et al., Reference Kambe, Weaver and Andrews2008). In rat embryos, Cu deficiency caused malformations and reduced superoxide dismutase enzyme (SOD) activity (Hawk et al., Reference Hawk, Lanoue, Keen, Kwik-Uribe, Rucker and Uriu-Adams2003). In previous studies, we have demonstrated in heifers that Cu concentrations in plasma and follicular fluid are similar (Picco et al., Reference Picco, Rosa, Anchordoquy, Anchordoquy, Seoane, Mattioli and Furnus2012). Moreover, the addition of Cu to IVM medium increases the GSH content in bovine oocytes in both oocytes and CC (Picco et al., Reference Picco, Rosa, Anchordoquy, Anchordoquy, Seoane, Mattioli and Furnus2012). The aim of this study was to investigate the influence of Cu on the health of COC during in vitro maturation (IVM). For this purpose, experiments were designed to evaluate the effect of different Cu concentrations added to the IVM medium on apoptosis, DNA integrity of CC, and oocyte viability. Also, the role of CC in the transport of Cu during IVM was evaluated on oocyte developmental capacity.
Materials and methods
Reagents and media
All reagents for media preparation and comet assay were purchased from Sigma Chemical Co. (St. Louis, MO, USA), whereas FSH was purchased from Bioniche (Belleville, Ontario, Canada). The maturation medium was bicarbonate-buffered TCM-199 supplemented with 5% (v/v) FCS, 0.2 mM sodium pyruvate, 1 mM glutamine, 10 mg/ml LH (NIHoLH-S1), 1 mg/ml FSH, 1 mg/ml 17β-estradiol, and 50 mg/ml kanamycin (Furnus et al., Reference Furnus, de Matos and Moses1998). Copper content in IVM medium supplemented with fetal calf serum is 0.05 µg/ml Cu. Cooper concentration was measured using a double-beam flame atomic absorption spectrophotometer (GBC 902) through an internal quality control. Standard copper sulphate water solution was purchased from Merck (Tokyo, Japan). The fertilization medium consisted of TALP supplemented with 6 mg/ml BSA-fatty acid free, 20 mM penicillamine, 10 mM hypotaurine, and 10 mg/ml heparin sulphate. The composition of the TALP medium was described previously by Parrish et al. (Reference Parrish, Susko-Parrish, Leibfried-Rutledge, Critser, Eyestone and First1986). The culture medium for embryo development consisted of modified synthetic oviduct fluid (SOFm), composed of SOF (Tervit et al., Reference Tervit, Whittingham and Rowson1972) supplemented with 1 mM glutamine, 2% (v/v) BME-essential amino acids, 1% (v/v) MEM-nonessential amino acids, and 4 mg/ml fatty acid free BSA (274–276 mOsm/kg) (Gardner et al., Reference Gardner, Lane, Spitzer and Batt1994).
Oocytes
Bovine ovaries were obtained from an abattoir and transported to the laboratory in sterile NaCl solution (9 g/L) with antibiotics (streptomycin and penicillin) at 37 °C within 3 h after slaughter. Ovaries were pooled, regardless of stage of the estrus cycle of the donor. Cumulus–oocyte complexes were aspirated from 3 to 8 mm follicles, using an 18-G needle connected to a sterile syringe. Only cumulus-intact complexes with evenly granulated cytoplasm were selected, using a low-power stereomicroscope (×20–30 magnification) for IVM. Replicates (four to six) were performed on different days, with a separate batch of COC for each day.
In vitro maturation (IVM)
Cumulus–oocyte complexes were washed twice in TCM-199 buffered with 15 mM HEPES, and twice in IVM medium. Groups of 10 COC were transferred into 50 μl of IVM medium under mineral oil (Squibb, Princeton, NJ) pre-equilibrated in a CO2 incubator. The incubations were performed at 39°C in an atmosphere of 5% CO2 in air with saturated humidity for 24 h. In a preliminary experiment, the presence of a PB + MII plate was evaluated in sampled oocytes from COC matured with different Cu concentrations and IVM medium alone with Hoechst 33342 after 24 h of in vitro maturation.
Culture of cumulus cells
Cumulus cells monolayers were prepared by pipetting COC with a narrow-bore pipette. The oocytes were discarded and cumulus mass were vigorously pipetted to allow separation. CC were counted in a hemocytometer chamber, and aliquots of the cell suspension (0.5 ml, 1 × 106 cells/ml in IVM medium) were placed in four-well plate under mineral oil, at 39ºC in an atmosphere of 5% CO2 in air with saturated humidity. The medium was changed every 48 h until 70–80% confluence, which was normally attained within 4–5 days. The percentage of live cells, evaluated by vital stain with trypan blue, was over 80% at the beginning of the culture.
Apoptosis by annexin V staining assay
In the early stages of apoptosis, changes occur at the cell surface (Andree et al., Reference Andree, Reutelingsperger, Hauptmann, Hemker, Hermens and Willems1990; Fadok et al., Reference Fadok, Voelker, Campbell, Cohen, Bratton and Henson1992; Creutz, Reference Creutz1992). One of these plasma membrane alterations is the translocation of phosphatidylserine (PS) from the inner part of the plasma membrane to the outer layer, by which PS becomes exposed at the external surface of the cell (Vermes et al., Reference Vermes, Haanen, Steffens-Nakken and Reutelingsperger1995). The analysis of PS on the outer leaflet of apoptotic cell membranes is performed by using annexin-V–fluorescein and propidium iodide (PI) for the differentiation from necrotic cells. Annexin V is a Ca2+-dependent phospholipid-binding protein with high affinity for PS. This protein can hence be used as a sensitive probe for PS exposure upon the outer leaflet of the cell membrane and is therefore suited to detect apoptotic cells (Koopman et al., Reference Koopman, Reutelingsperger, Kuijten, Keehnen, Pals and van Oers1994; Homburg et al., Reference Homburg, de Haas, von dem Borne, Verhoeven, Reutelingsperger and Roos1995; Verhoven et al., Reference Verhoven, Schlegel and Williamson1995; Vermes et al., Reference Vermes, Haanen, Steffens-Nakken and Reutelingsperger1995) in cell populations. Since necrotic cells also expose PS according to the loss of membrane integrity, apoptotic cells have to be differentiated from these necrotic cells. The simultaneous application of a DNA stain which is used for dye exclusion tests allows the discrimination of necrotic cells from the annexin V positively stained cell. Annexin-V-Fluos binds in a Ca2+-dependent manner to negatively charged phospholipid surfaces and shows high specificity to phosphatidylserine. Therefore, it stains apoptotic as well as necrotic cells. Propidium iodide stains DNA of leaky necrotic cells only. Early apoptosis was evaluated by membrane redistribution of PS with the annexin-V-Fluos staining kit (Roche, Cat. #11-858-777-001). Briefly, the end of IVM oocytes were stripped of surrounding CC as described above, washed twice with PBS and centrifuged at 200 g for 5 min. Then the pellet was resuspended in 100 μl of annexin-V–Fluos labelling solution (annexin V + fluorescein, HEPES buffer and propidium iodide), and incubated in the dark for 10 to 15 min at 15–25ºC. In total, 200 cells were analyzed under a fluorescence microscope per group (Control and different Cu concentrations).
Comet assay
The comet assay allows measuring DNA strand breaks in eukaryotic cells. Cells embedded in agarose on a microscope slide are lysed with detergent and high salt to form nucleoids containing supercoiled loops of DNA linked to the nuclear matrix. Electrophoresis results in structures resembling comets. When measured by fluorescence microscopy, the intensity of the comet tail relative to the head reflects the number of DNA breaks (Collins, Reference Collins2004). DNA strand breaks occur as a late event of apoptosis but also in cell death processes other than apoptosis
The DNA damage was evaluated by comet assay. The comet assay is a simple, sensitive and rapid technique by which visual evidence of DNA damage in eukaryotic cells may be measured. The comet assay is based on quantification of DNA damaged fragments migrating out of the cell nucleus during electrophoresis. Broken DNA migrates farther in the electric field resembling a ‘comet’ with a fluorescent head and a tail which increases as damage increases. At the end of IVM, oocytes were stripped of surrounding CC by repeated pipetting with a narrow-bore glass pipette in TCM-199 buffered with HEPES. Then, CC were washed three times in calcium- and magnesium-free PBS containing 1 mg/ml PVP. Complete cell disruption was achieved by repeated aspiration using a narrow-bore pipette. Samples were then mixed with low melting point agarose. Single-cell gel electrophoresis was performed using the alkaline version previously described (Singh et al., Reference Singh, McCoy, Tice and Schneider1988) with modifications (Tice and Strauss Reference Tice and Strauss1995). Briefly, slides were covered with 180 μl of 0.5% normal agarose (Carlsbad, Carlsbad, CA, USA). Then, 75 μl of 0.5% low melting point agarose (Carlsbad) was mixed with cells and layered onto the slides, which were immediately covered with cover slips. After agarose solidification at 4°C for 10 min, cover slips were removed and slides were immersed overnight at 4°C in fresh lyses solution. Slides were equilibrated in alkaline solution for 20 min. Electrophoresis was done for 30 min at 25 V and 300 mA (1.25 V/cm). Thereafter, slides were neutralized by washing (5 min each) three times with Tris buffer (pH 7.5), and then with distilled water. Slides were stained with 1/1000 SYBR Green I solution (Molecular Probes, Eugene, OR, USA) (Olive et al., Reference Olive, Durand, Jackson, Le Riche, Luo, Ma, McLaren, Aquino-Parsons, Thomson and Trotter1999). Scoring was made at ×400 magnification using a fluorescence microscope (Olympus BX40 equipped with a 515–560 nm excitation filter) connected through a Sony 3 CCD-IRIS Color Video Camera, and saved using Image Pro Plus software. Two hundred randomly selected nuclei from each treatment were classified into two groups: with (comets with tail) or without DNA damage (comets without tail) and then analyzed for Olive Tail moment (OTM) and percentage of DNA in the head (%DNAH) (Olive et al., Reference Olive, Banáth and Durand1990; Bocker et al., Reference Bocker, Bauch, Muller and Streffer1997).
Cumulus expansion
After IVM, cumulus expansion was measured in each COC using a computerized image-digitizing system with Image ProPlus® 3.1 which allows measurement of irregular areas. The system units were transformed to μm2 by calibration with a Maklert chamber. For comparison, each COC area was measured before IVM.
Oocyte viability
After maturation oocyte viability was evaluated. For this purpose, oocytes were incubated for 10 min at 37ºC in PBS medium with 2.5 μg/l fluorescein diacetate fluorochrome and 2.5 g/L Trypan Blue. Then, oocytes were washed in PBS and observed in a Nikon Optiphot epifluorescence microscope with a 409 fluor objective (Nikon) equipped with a 330–490 nm excitation filter and 420–520 nm emission filter at ×100 magnification. Live oocytes are visible in green fluorescence, whereas dead oocytes show a characteristic blue staining under white light (Hoppe & Bavister Reference Hoppe and Bavister1984).
In vitro fertilization (IVF)
Oocytes were washed twice in HEPES-TALP supplemented with 3 mg/ml bovine serum albumin-fatty acid free (BSA-FAF) and placed into 50 μl drops of IVF medium under mineral oil. In all experiments, frozen semen from the same bull was used. Three straws, each containing 40 × 106 spermatozoa, were thawed in a 37°C water bath. Spermatozoa were washed in a discontinuous Percoll gradient prepared by depositing 2 ml of 90% Percoll under 2 ml of 45% Percoll in a 15-ml centrifuge tube. Semen samples were deposited on the top of the Percoll gradient and centrifuged for 20 min at 500 g. The pellet was removed and resuspended in 300 μl of HEPES-TALP solution and centrifuged at 300 g for 10 min. After removal of the supernatant, spermatozoa were resuspended in IVF medium, counted in a hemocytometer chamber, and further diluted. The final sperm concentration in IVF was 2 × 106 sperm/ml. Incubations were conducted at 39°C in 5% CO2 in air with saturated humidity for 24 h.
In vitro culture (IVC)
After IVF, presumptive zygotes were washed twice in HEPES-SOF, and then cultured in SOFm. Embryo culture was carried out in 40 μl drops of medium under mineral oil (10 presumptive zygotes per drop) at 39°C in an atmosphere of 7% O2, 5% CO2, and 88% N2 with saturated humidity. All embryos were cultured in the absence of glucose during the first 24 h, and further cultured for 7 days in the presence of 1.5 mM glucose. The medium was changed every 48 h, and the embryos were incubated for 8 days (Day 0 = day of fertilization). At the end of incubations, the embryos were evaluated for the morphological stages of development with an inverted microscope (Diaphot, Nikon, Tokyo, Japan).
Experimental design
Copper and apoptosis of cumulus cells
In Experiment 1, the addition of 0, 20, 40 and 60 µg/dl Cu to IVM medium were evaluated on CC apoptosis (Section 2.5) after 24 h of in vitro maturation as described above. For this purpose, 800 COC were matured in four replicates (separate batch of ovaries for each day), with 200 COC distributed in groups of 50 COC per experimental group.
Copper and DNA integrity of cumulus cells
In Experiment 2, the effect of Cu added during IVM on DNA damage of CC was evaluated by comet assay. For this purpose, COCs were matured with 0 µg/dl Cu (Control), 20 µg/dl Cu, 40 µg/dl Cu and 60 µg/ml Cu (Picco et al., Reference Picco, Rosa, Anchordoquy, Anchordoquy, Seoane, Mattioli and Furnus2012). The COC were matured for 24 h as described above and DNA damage was evaluated (see the section on comet assay). For this purpose, 800 COC in four replicates from different days (200 COC per replicate, 50 COC per group) were matured in vitro with various concentrations of Cu. Each batch of 50 COC was processed for preparing slides to analyze at least 250 single cells per treatment for the comet assay.
Copper and cumulus expansion
In Experiment 3, the influence of 0, 20, 40, and 60 µg/dl Cu added to IVM medium was evaluated on cumulus expansion. The COC were matured individually for 24 h as described above, and cumulus area was measured both before (T 0) and after IVM (T 24) (described in the section on Cumulus expansion). For this purpose, 240 COC were matured in four replicates (separate batch of ovaries for each day), 60 COC in each experimental group.
Copper and oocyte viability
In Experiment 4, the addition of 0, 20, 40 and 60 µg/dl Cu to IVM medium were evaluated on oocyte viability (see the section on oocyte viability) after 24 h of in vitro maturation as described above.
Effect of CC and Cu on cleavage and development
In Experiment 5, oocytes were matured in vitro during 24 h with or without 60 µg/ml Cu in three oocyte categories: (i) intact cumulus–oocyte complex COC; (ii) denuded oocytes with cumulus cell monolayer (DO + CC); and (iii) denuded oocytes (DO). Denuded oocytes (DO) were obtained by pipetting COC with a narrow-bore pipette when was required for the experimental design. Cleavage rates were recorded 48 h after insemination. Data reported for development to the blastocyst stage included embryos that progressed to the expanded.
Statistical analysis
Completely randomized block designs were used and statistical models included the random effects of block (n = 4–5 depending on experiment) and the fixed effect of treatment (0 vs. 20 vs. 40 vs. 60 μg/dl Cu in Experiments 1 to 4; and 0 vs. 60 μg/dl Cu in Experiment 5). Continuous response variables such as area of cumulus,% DNAH and Olive Tail moment were analyzed with linear models by using the MIXED procedure of SAS (SAS Institute, Cary, NC, USA). Cumulus area before IVM (T0) was used as covariate in the analysis of cumulus expansion. Apoptosis (%), oocyte viability, cleavage rates and blastocyst rates were analyzed by logistic regression using GENMOD procedure (SAS Institute, Cary, NC, USA). Cleavage and blastocysts percentages were analyzed by a randomized block design with a 2×3 factorial arrangement. The statistical models included the random effects of block (n= 4) and the fixed effects of Cu concentration (0 vs. 0.6 μg/ml Cu), oocyte category (COC vs. DO + CC vs. DO) and their second order interaction. Data for OTM, DNAH, and cumulus expansion were expressed as Least Squares Means (LSM) ± SEM. Apoptosis, oocyte viability, cleavage and blastocyst rates were expressed as percentage. Statistical significance was set at P < 0.05, and at P < 0.10 for interactions.
Results
Copper and apoptosis of cumulus cells
In Experiment 1, the percentage of apoptotic cells after IVM was higher in CC matured without Cu than in CC matured at any Cu concentrations (Early apoptosis: 12.9%; 11.6%; 11.9% and 11.2% for COCs exposed to 0, 20, 40, and 60 µg/dl Cu, respectively; P < 0.05). Statistical differences were not found between 20, 40 and 60 µg/dl Cu (Fig. 1). Early apoptosis was lower in CC matured with Cu supplementation during IVM.

Figure 1 Percentages of early apoptosis in cumulus cells obtained from COC matured with different Cu concentrations. Results are presented as percentages (%). Bars with different letters differ statistically (P < 0.05). Bovine COCs were incubated in IVM medium alone (0 μg/dl Cu) or supplemented with 20 μg/dl, 40 μg/dl and 60 μg/dl Cu (800 COC were matured in four replicates, 200 COC per treatment).
Copper and DNA integrity of cumulus cells
In Experiment 2, Olive Tail moment (OTM) was significantly higher in CC from COC matured without Cu (0 µg/dl Cu, P < 0.01) respect to cells treated with Cu (OTM: 3.07 ± 0.6; 2.12 ± 1.06; 1.01 ± 0.56; 1.12 ± 0.43 for COC exposed to 0, 20, 40, and 60 µg/dl Cu, respectively). The percentages of DNA in the head (%DNAH) were 93.4 ± 0.76; 96.81 ± 1.16; 97.61 ± 0.8 and 97.22 ± 0.88 for 0, 20, 40, and 60 µg/dl Cu, respectively. The percentages of DNAH were statistically different in all Cu concentrations respect to 0 µg/dl Cu (P < 0.01) (Fig. 2). The OTM values and DNAH percentages indicated that Cu supplementation during IVM diminished DNA damage in CC.

Figure 2 DNA damage in cumulus cells obtained from COC matured with different Cu concentrations. Values are expressed as LSM ± standard error of the mean (SEM). Bars with different letters differ (P < 0.01). Bovine COCs were incubated in IVM medium alone (0 μg/dl Cu) or with 20 μg/dl Cu, 40 μg/dl Cu and 60 μg/dl Cu.
Copper and cumulus expansion
In Experiment 3, cumulus expansion area did not show significantly differences in COC matured with 0, 20, 40, and 60 µg/dl Cu concentrations after IVM [484,097 ± 63,756 µm² (n: 60); 561,336 ± 63,756 µm² (n: 60); 465,794 ± 63,756 µm² (n: 60)¸ 592,316 ± 63,765 µm² (n:60), respectively]. Cumulus cells number per COC either before or after IVM were similar at any Cu concentration (P ≥ 0.05). Copper addition during IVM of bovine COCs did not modify, neither extracellular matrix mucification nor cumulus cells number, after 24 h of IVM.
Copper and oocyte viability
In Experiment 4, oocyte viability was not significantly different among COC treated with 0, 20, 40, and 60 µg/dl Cu during IVM (88.8%, 93.7%, 95.7% and 88% for 0, 20, 40, and 60 µg/dl Cu, respectively; (P ≥ 0.05). Oocyte viability was similar with or without Cu addition after 24 h of maturation.
Effect of cumulus cells and Cu on cleavage and development
In total, 1143 oocytes were in vitro matured with or without Cu supplementation to IVM medium. Cleavage and blastocyst rates were recorded after IVM in three oocyte categories: intact cumulus oocyte complexes (COC), denuded oocytes with CC monolayer (DO + CC) and denuded oocytes (DO). Cleavage rates were significantly higher in COC and DO + CC matured with or without Cu than in DO (P < 0.01; Table 1). In addition, blastocyst rates were significantly higher in COC than in DO + CC (P < 0.01) and DO (P < 0.01; Table 1). In all oocyte categories (COC, DO + CC and DO) blastocyst rates were higher when 60 µg/dl Cu was added to IVM medium compared to medium alone (P < 0.01, Table 1). Copper addition during bovine oocyte in vitro maturation improved subsequent embryo development until blastocyst stage regardless of cumulus cells presence.
Table 1 Effect of cumulus cells and cooper on developmental capacity of bovine oocytes matured in vitro
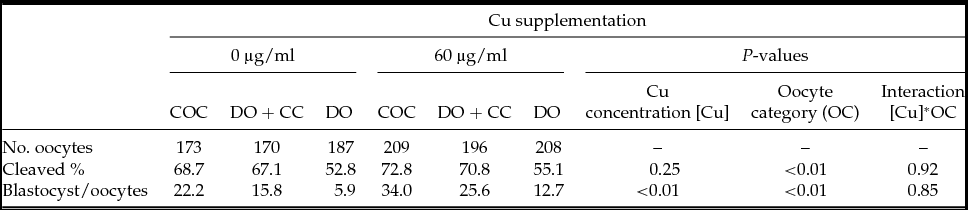
COC = cumulus–oocyte complex.
DO + CC = denuded oocytes cultured with cumulus cell monolayer.
DO = denuded oocytes.
Cleavage rates were recorded 48 h after insemination. Data reported for development to the blastocyst stage included those embryos that progressed to the expanded or hatched blastocyst stages after 8 days in culture. All values for cleavage and blastocyst rates are expressed as percentages.
Cleavage and blastocysts percentages were analyzed by a randomized block design with a 2×3 factorial arrangement. The statistical models included the random effects of block (n = 4) and the fixed effects of Cu concentration (0 vs. 0.6 μg/ml Cu), oocyte category (COC vs. DO + CC vs. DO) and their second order interaction.
Discussion
The results of the present study indicate that Cu supplementation to IVM medium: (i) decreased DNA damage and apoptosis in cumulus cells; (ii) did not modify oocyte viability and cumulus expansion; and (iii) improved subsequent embryo development up to blastocyst stage regardless of cumulus cells presence during in vitro maturation.
The present study demonstrated that addition of Cu during IVM improves DNA integrity of CC after in vitro maturation. The composition of follicular fluid and IVM media affect the oocyte developmental competence (Wrenzycki & Stinshoff, Reference Wrenzycki and Stinshoff2013). The antioxidant role of Cu may be an important mechanism in preventing oxidative damage in CC. Copper acts as an electron transfer intermediate in redox reactions and plays an important supporting role in scavenging excess ROS and preventing oxidative damage to critical cellular components (Rayssiguier et al., Reference Rayssiguier, Gueux, Bussiere and Mazur1993; Chen et al., Reference Chen, Saari and Kang1994; Sukalski et al., Reference Sukalski, LaBerge and Johnson1997; Johnson & Thomas, Reference Johnson and Thomas1999; Pan & Loo, Reference Pan and Loo2000). Low ROS concentrations in vitro promote developmental competence of oocytes and influence subsequent embryo development (Blondin et al., Reference Blondin, Coenen and Sirard1997; Combelles et al., Reference Combelles, Gupta and Agarwal2009) whereas, high ROS concentrations alter the normal cellular function by inducing oxidative damage of intracellular components and inducing apoptosis (Guerin et al., Reference Guerin, El Mouatassim and Menezo2001). Many antioxidant supplementation protocols have been tested, however a balance of oxidant and antioxidant molecules are required in the follicular fluid for in vivo maturation, consequently the addition of antioxidants is not good enough to combat ROS damage (Guerin et al., Reference Guerin, El Mouatassim and Menezo2001). Copper deficiency produces a failure of Cu enzymes, many of which contribute to the antioxidant defense system [e.g. copper/zinc superoxide dismutase (Cu/Zn-SOD) and ceruloplasmin] (Pan & Loo, Reference Pan and Loo2000; Picco et al., Reference Picco, Abba, Mattioli, Fazzio, Rosa, De Luca and Dulout2004). In our study, DNA integrity in CC was significantly improved when Cu was added to IVM medium at any concentrations evaluated. It is important to contemplate that DNA damage might be either the end product of apoptosis phenomenon or one of the factors for its progress (Wang, Reference Wang2001; Rana, Reference Rana2008).
Apoptosis, also known as programmed cell death is a crucial process involving intrinsic and highly conserved molecular components (Miller & Marx, Reference Miller and Marx1998). Main events include Bcl-2 family proteins modulation (Sadoul, Reference Sadoul1998), release of mitochondrial factors, caspases activation, and DNA fragmentation (Rossi et al., Reference Rossi, Marchese, De Martino, Piccirilli, Rotilio and Ciriolo2001a, b). Copper deficiency most likely leads to perturbations in inter- and intracellular milieu, leading to activation of apoptosis-associated genes and endonucleases activity (Rao et al., Reference Rao, Yeldandi, Subbarao and Reddy1993). It has been demonstrated that low Cu concentrations induces apoptosis in pancreatic acini cells (Rao et al., Reference Rao, Yeldandi, Subbarao and Reddy1993), heart cells (Balevska et al., Reference Balevska, Russanov and Kassabova1981; Kang et al., Reference Kang, Zhou, Wu, Wang, Saari and Klein2000; Gybina & Prohaska, Reference Gybina and Prohaska2003) and neuronal cells (Rossi et al., Reference Rossi, Marchese, Lombardo, Rotilio and Ciriolo2001b) by depleting antioxidant defense, impairing mitochondrial function and decreasing Bcl-2 content (Rossi et al., Reference Rossi, Marchese, Lombardo, Rotilio and Ciriolo2001b; Gybina & Prohaska, Reference Gybina and Prohaska2003). In agreement with those studies, we have demonstrated that supplementation of Cu in the maturation medium decreased apoptotic rates in CC. It is well known that the degree of apoptosis in CC correlates with the developmental competence of bovine enclosed oocytes (Ikeda et al., Reference Ikeda, Imai and Yamada2003). Conversely, oocyte viability was not modified by Cu supplementation to IVM medium. These results are in agreement with that reported by Kang et al. (Reference Kang, Xiao, Ren, Zhang, Le, Trentalange, Gupta, Lin and Bondarenko2014) who demonstrated that insufficient Cu level in the culture medium does not alter culture viability profiles of Chinese hamster ovary cells (CHO).
Within the follicle, granulosa cells can be divided into two functional groups: the mural granulosa cells around the antrum and the CC (Edson et al., Reference Edson, Nagaraja and Matzuk2009). During oocyte maturation, optimal expansion of cumulus mass appears to be essential for embryo development up to blastocyst stage (Furnus et al., Reference Furnus, de Matos and Moses1998). In the present study, the addition of Cu to IVM medium did not affect cumulus expansion.
Oocyte and CC maintain a close relationship revealed by nutrients transport trough gap junctions, and an ideal microenvironment to guarantee further developmental competence (Eppig, Reference Eppig1991; Pangas & Matzuk, Reference Pangas and Matzuk2005; Gilchrist et al., Reference Gilchrist, Lane and Thompson2008). These cell-to-cell communications allow metabolites, ions and amino acids transport through oocytes and CC (Eppig, Reference Eppig1982; Larsen & Wert, Reference Larsen and Wert1988; Larsen, Reference Larsen, Sperelakis and Cole1989). Premature interruption of cumulus–oocyte gap junctions communication produces the absence of specific signals that affect oocyte developmental capacity (Modina et al., Reference Modina, Luciano, Vassena, Baraldi-Scesi, Lauria and Gandolfi2001; Gilchrist et al., Reference Gilchrist, Ritter and Armstrong2004; Lodde et al., Reference Lodde, Modina, Galbusera, Franciosi and Luciano2007). Besides, CC produce antioxidant substances to protect the oocyte from ROS damage. In brief, intercellular communications that coupled CC with the oocyte were not indispensable for nuclear maturation, however they play an important role in cytoplasmic maturation and subsequent embryo development (Chian et al., Reference Chian, Niwa and Sirard1994; Kim et al., Reference Kim, Minami, Yamada and Utsumi1996).
In our study, cleavage rates were similar in the presence or absence of Cu during IVM, however cleavage rates were diminished when oocytes were not present (DO). Conversely, percentages of blastocysts did improve when Cu was present during in vitro maturation regardless of the connection between CC and the oocyte. However, the best performance of blastocyst production was obtained when Cu was added during in vitro maturation of intact COC. This might be indicate that Cu influences and enhances cytoplasm quality evidenced by an improvement in blastocyst rates. Furthermore, denuded oocytes co-cultured with CC monolayers during IVM restored partially the developmental potential of denuded oocytes matured alone (Zhang et al., Reference Zhang, Jiang, Wozniak, Yang and Godke1995; de Matos et al., Reference de Matos, Furnus and Moses1997; Anchordoquy et al., Reference Anchordoquy, Anchordoquy, Sirini, Picco, Peral-García and Furnus2014). Indeed, blastocysts rates were higher when denuded oocytes were matured with Cu. This can be explained by the fact that CC secrete soluble factors play an important role in the acquisition of bovine denuded oocyte developmental competence (Luciano et al., Reference Luciano, Lodde, Beretta, Colleoni, Lauria and Modina2005).
In our study, Cu improved blastocyst rates regardless of cumulus cells presence during in vitro maturation suggesting that oocyte might take up Cu from maturation medium. Copper is an essential cofactor of numerous enzymes but also it can be toxic, generating ROS (Arnesano et al., Reference Arnesano, Banci, Bertini, Mangani and Thompsett2003). In mammalian cells, Cu concentration is regulated by cellular transport systems (Bull & Cox, Reference Bull and Cox1994; Kuo et al., Reference Kuo, Zhou, Cosco and Gitschier2001). Copper can be transported by different mechanisms including the divalent metal transporter (DMT1) (Zheng & Monnot, Reference Zheng and Monnot2012), copper transporter (CTR) (Kim et al., Reference Kim, Wu and Lee2013), and ATP7A and ATP7B Cu transporting ATPases (Kim et al., Reference Kim, Turski, Nose, Casad, Rockman and Thiele2010). In conclusion, Cu increased blastocyst rates regardless of CC presence during IVM, highlighting the importance of this mineral in oocyte metabolism.
Acknowledgements
This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Tecnológicas (PIP 022 – CONICET); Ministerio de Ciencia, Tecnología e Innovación Productiva de la Nación Argentina. We are grateful to Centro de Inseminación Artificial La Elisa S.A. (CIALE) for making available bovine frozen semen; and the staff of Frigorífico Gorina S.A. for providing the bovine ovaries.





