Introduction
Endometrial health is the foundation of pregnancy. Endometrial injuries may lead to recurrent miscarriages, infertility, amenorrhea, abnormal placentation, dysmenorrhoea and abnormal uterine bleeding, and therefore are detrimental to the health of affected women (Deans and Abbott, Reference Deans and Abbott2010). Intrauterine adhesion (IUAs) due to endometrial injury is a commonly acquired endometrial disorder (Yu et al., Reference Yu, Wong, Cheong, Xia and Li2008; March, Reference March2011b). The incidence of IUAs has increased in recent years and 90% of IUAs have been associated with damage to the uteri due to intrauterine surgery (Schenker and Margalioth, Reference Schenker and Margalioth1982). Considering the significant function of the uterus in human reproduction, clinical intervention is important to repair endometrial injury and promote the recovery of menstruation, improve the uterine environment, and further to enhance pregnancy rates in infertile patients (Gurtner et al., Reference Gurtner, Werner, Barrandon and Longaker2008; Goulopoulou et al., Reference Goulopoulou, Hannan, Matsumoto and Webb2012).
Various novel therapies for IUA have been developed over the past several decades. In general, transcervical resection of adhesions (TCRA) has been the conventional and standard method of stripping adhesions and restoring the shape of the uterine cavity following development of hysteroscopy technology (Yu et al., Reference Yu, Wong, Cheong, Xia and Li2008). However, two difficult problems, postoperative endometrial restoration and prevention of recurrent IUAs, remain unsolved in clinical practice (Deans and Abbott, Reference Deans and Abbott2010). Oestrogen, an important female sex hormone, has an important function in the regulation and development of the female reproductive system and formation of secondary sex characteristics. Although Johary et al. (Reference Johary, Xue, Zhu, Xu and Velu2014) has reported that postoperative oestrogen therapy may be used to prevent recurrent adhesions, IUA recurrence rate was 62.5% in patients with severe IUAs. Furthermore, the poor efficiency of oestrogen with some adverse effects impelled us to develop novel therapeutic methods for IUAs (Urman et al., Reference Urman, Mercan, Alatas, Balaban, Isiklar and Nuhoglu2000).
Stem cells with their tremendous therapeutic potential can differentiate into specialized cell type(s). Cell replacement strategies have been suggested and examined in some models of endometrium pathology across decades of investigation in animal models (Azizi et al., Reference Azizi, Aghebati-Maleki, Nouri, Marofi, Negargar and Yousefi2018). To date, stem cell therapy has had a good perspective for clinical transplantation and tissue regeneration. Therefore, the use of multipotent or unipotent stem cells may represent a more achievable strategy for the repair of uterine injury.
Several studies have reported that bone marrow mesenchymal stem cells (MSCs), as a promising therapeutic approach, can promote the repair and regeneration of the injured uterus (Ding et al., Reference Ding, Li, Sun, Su, Lin, Péault, Song, Yang, Dai and Hu2014; Zhou et al., Reference Zhou, Yue, Murphy, Peyer and Morrison2014). However, disadvantages of MSCs in the treatment of IUA, such as low efficiency in promoting repair and regeneration of endometrium, have been recognized. Moreover, other restriction factors of bone marrow stem cells (BMSCs) for clinical therapy still need more consideration, for instance methods of isolation and purification for MSCs and the age-dependent differentiation potency (Abumaree et al., Reference Abumaree, Al Jumah, Kalionis, Jawdat, Al Khaldi, Al Talabani and Knawy2013).
ADSCs with their multi-potential differentiation have potential clinical application (Zuk et al., Reference Zuk, Zhu, Mizuno, Huang, Futrell, Katz, Bentham, Lorenz and Hedrick2001; Gomillion and Burg, Reference Gomillion and Burg2006; Cheung et al., Reference Cheung, Han, Marecak, Watkins, Amsden and Flynn2014). Adipose tissue is relatively abundant in humans, and ADSCs can be easily isolated with less donor-site morbidity from subcutaneous adipose tissue such as in the thigh, abdomen and arm (Tobita et al., Reference Tobita, Orbay and Mizuno2011; Mizuno, Reference Mizuno2013). Previous studies have shown that the number of stem cells obtained from adipose tissue was far more than that from equal amounts of bone marrow (Zhang et al., Reference Zhang, Khan, Delling and Tobiasch2012). ASCs have also a higher proliferation capacity and slower senescence than BMSCs (Shekkeris et al., Reference Shekkeris, Jaiswal and Khan2012). Trophic factors secreted by ADSCs can further enforce therapeutic and regenerative outcomes (Melief et al., Reference Melief, Zwaginga, Fibbe and Roelofs2013). There is increasing study of and focus on ADSCs clinical treatments including wound healing, skin rejuvenation and tissue regeneration (Nae et al., Reference Nae, Bordeianu, Stăncioiu and Antohi2013; Kokai et al., Reference Kokai, Marra and Rubin2014). For safety considerations, due to their immunomodulatory and immunosuppressive effects, ASCs have been clinically tested and used in treatments for inflammatory and autoimmune disorders such as multiple sclerosis, graft-versus-host disease and Crohn’s disease (Frölich et al., Reference Frölich, Hagen and Kleinsasser2014; Kokai et al., Reference Kokai, Marra and Rubin2014). ADSCs are currently recognized as the most promising stem cells in regenerative medicine. Here, in a rat model, we aimed to isolate and transplant ADSCs into rat uterus and evaluate the therapeutic effects of ADSCs on regeneration.
Materials and Methods
Animal and endometrial injury model
Here, 10–11-week-old adult female Sprague-Dawley rats (weight: 180–200 g, grade: specific pathogen free (SPF)) from the SLAC Laboratory Animal Co., Ltd (Shanghai, China) were used to establish a rat model for IUAs. Four or five rats per cage with free access to water and food were housed in a room with controlled light (14 h/10 h light/dark cycle), temperature (23–25°C), and humidity (40–60%). All animal experiments were approved and conducted under the strict control of the Biological Research Ethics Committee of the Chinese Academy of Sciences.
Briefly, rats were exposure to general anaesthesia after administration of 80% chloral hydrate by intraperitoneal injection, then the abdominal wall was opened with a vertical incision and the uteri were exposed. After disinfection with iodophors solution, 5 ml 95% alcohol were injected into uterine horns and then reset.
ADSCs isolation and culture
Rats were sacrificed to obtain intraperitoneal adipose tissue. Details of the experimental process is described in Supporting Information Materials and Methods S1.
Differentiation properties of human ADSCs
The ability of ADSCs to differentiate through adipogenesis, osteogenesis and chondrogenesis was visualized by Oil Red O staining, Alizarin red staining and Alcian blue staining. Details of experimental process are described in Materials and Methods S1.
Transfecting and tracing of transplanted ADSCs
Following the experiment protocol described by Tiscornia et al. (Reference Tiscornia, Singer and Verma2006), ADSCs were infected with a green fluorescent protein (GFP) reporter using a lentivirus vector. Briefly, lentivirus-containing supernatants were harvested at 48 and 72 h respectively after lentivirus packed plasmid co-transfection. ADSCs were infected with the lentivirus supernatants when cells reached 70% confluency. At 2 days after transfection, GFP expression was evaluated using a fluorescence microscope, and the infection efficiency was calculated. Equal numbers of ZsGreen–hADSCs were injected directly into the uteri, and the distribution of ADSCs surrounding the wound bed were detected using immunofluorescence analysis at different time points (24 h, day 3, day 7, day 14), respectively.
Groups and treatment
In total, 40 ICR female rats with endometrial injury were used in the study. Similarly, 40 AS rats were randomly divided into four groups: Group 1: normal control (10 rats); Group 2: sham operated group (10 rats were merely operated on making a low abdominal midline incision); Group 3: ADSC treated group (10 rats, injected 1 × 106 ADSCs cells into uterine horns); and Group 4: PBS treated group (10 rats). Briefly, scar tissue in the uterus was destroyed with sterile needles to make a fresh injured surface; 1 ml of ADSC suspension (5 × 106 cells) was injected into the uterine cavity in Group 3 rats. Group 4 rats were injected with 1 ml PBS.
HE staining
At the appointed time, rats were sacrificed and bilateral uterine horns were resected. Tissues were fixed in 4% phosphate-buffered paraformaldehyde at the appointed time and then were embedded into paraffin, 4–6 μm sections were cut for haematoxylin–eosin staining. Rat uterine histomorphology was observed using an Olympus BX43 inverted phase contrast microscope. The number of glands and endometrial thickness were recorded in detail. At least five fields in each image were selected randomly for counting.
Masson trichrome staining
The degree of endometrial fibrosis was detected by Masson trichrome staining. Details of the experimental process are described in Materials and Methods S1.
Immunofluorescence staining
Immunofluorescence studies were carried out to detect co-localization of GFP-positive ADSCs. Details of the experimental process are described in Materials and Methods S1.
Western blotting
Proteins were extracted from cells or tissues and the total protein concentration was determined using the bicinchoninic acid (BCA) assay. Total protein was separated on a 10–15% SDS-PAGE gel, and then transferred to a polyvinylidene difluoride (PVDF) membrane. The PVDF membranes were blocked in 5% non-fat skimmed milk, and then incubated with primary antibodies (Abcam) at 4°C overnight: rabbit polyclonal to anti-oestrogen receptor α (anti-ERα) (1:1000), rabbit polyclonal anti-oestrogen receptor β (anti-ERβ) (1:1000), rabbit monoclonal progesterone receptor (PR) (1:2000). Membranes were washed three or four times with PBS and then incubated with the secondary antibody; immunoreactivity was visualized using enhanced chemiluminescence. Levels of protein expression were normalized against β-actin. The density of the bands was analyzed using ImageJ software.
Flow cytometry
For cell surface staining, cells were centrifuged and collected after washing with cold PBS. Collected single cells were fixed, permeabilized and stained with PE-labelled anti-CD19 mAb, anti-CD34 mAb, anti-CD45 mAb or APC-labelled anti-CD105, anti-CD73, anti-CD90 antibodies (BD Biosciences). Cells were washed several time and subjected to fluorescence activated cell sorting (FACS) analysis using Cell Quest software (BD Biosciences).
Analysis of gene expression by quantitative real-time PCR
Briefly, total RNAs were extracted from tissue using TRIzol reagent (Invitrogen, USA). RNA reverse transcription was performed using the Prime-Script™ RT Reagent Kit according to the manufacturer’s instructions (TaKaRa, Japan). Relative mRNA expression was detected by quantitative real-time PCR (qRT-PCR). Expression of housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal loading control. The following primer pairs were used:

ELISA
Total protein was extracted from the blood samples from rats in the experimental groups and used to determine serum concentrations for ERα (cat. no. KGE014), ERβ (cat. no. MB100B) and PR (cat. no. DY3214). ELISAs were performed following the manufacturer’s protocol (R&D Systems, Inc., Minneapolis, MN, USA).
Fertility testing
To investigate fertility after transplantation of ADSCs or injection of normal saline, injured female rats treated with ADSCs for 4 weeks were bred with male rats (female:male = 5:2) for 1 week and then isolated from the males. Female rats were observed every day for the following 2 weeks and all rats were found to be pregnant without delivery. Pregnancy rate and the fetal number of each uterine horn were recorded and assessed.
5-Ethynyl-2′-deoxyuridine (EdU)-labelling assay
ADSCs were harvested after 50 μM EdU treatment. The ability of cells to proliferate was evaluated using the Cell-Light™ EdU Apollo Kit (RiboBio Co., Ltd, China) according to the manufacturer’s protocols. ADSCs were fixed in 4% paraformaldehyde for 30 min at 4°C then permeated with 0.5% TritonX-100. ADSCs were stained using a fluorescently labelled dye and fluorescence images were captured using an Olympus inverted fluorescence microscope.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). Statistical comparisons between two groups were performed using the unpaired Student’s t-test. Multiple groups were analyzed by one-way analysis of variance (ANOVA) to determine statistical significance. The data in Table 1 were analyzed using chi-squared test. All in vitro experiments were repeated at least three times and analyzed. A P-value < 0.05 was considered to be statistically significant. Spss19.0 software was used to carry out statistical analysis with two-tailed tests.
Table 1. Comparison of reproductive outcomes among different groups

Results
Characterization and differentiation of ADSCs
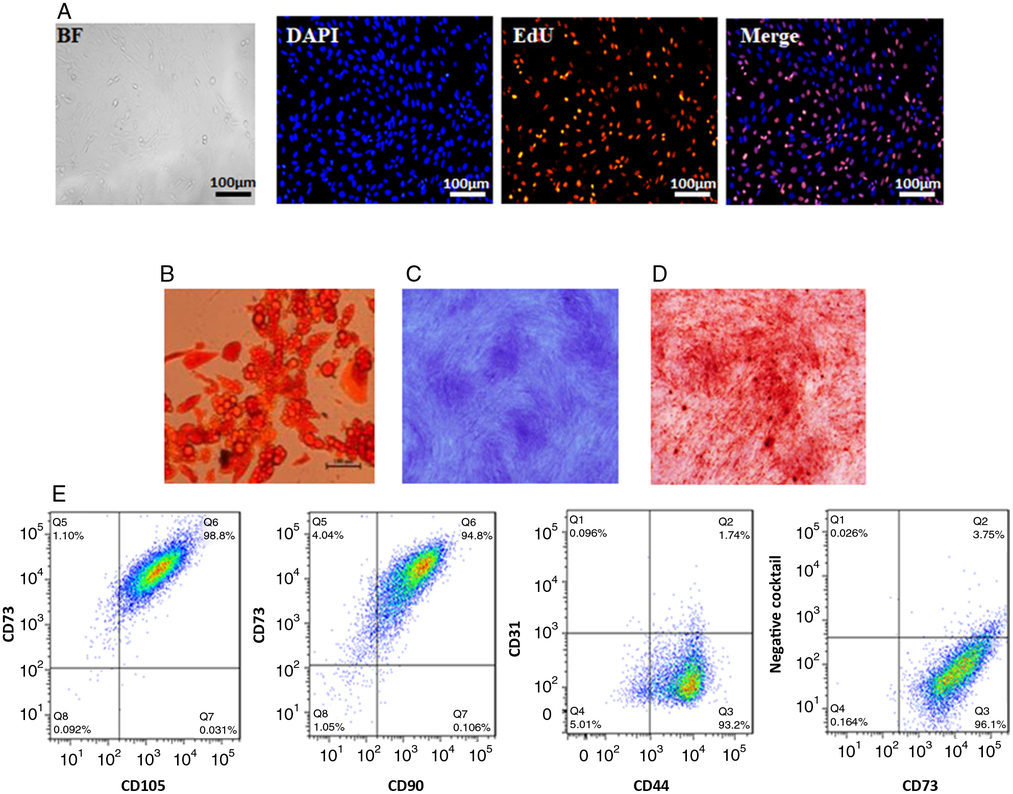
ADSCs with typical fibroblastic morphology had strong proliferative ability after the third passage. (Fig. 1 A) (Zuk et al., Reference Zuk, Zhu, Ashjian, De Ugarte, Huang, Mizuno, Alfonso, Fraser, Bentham and Hedrick2002; Liu and Chen, Reference Liu and Chen2018). Adipogenic differentiation of ADSCs was examined using Oil Red O staining after culture in adipose differentiation medium for 7 days. The result showed that the number of stained nodules representing lipid accumulation increased in a time-dependent manner (Fig. 1 B). In addition, ADSCs could also differentiate into chondrocytes and osteogenic cells as seen by Alcian blue staining and Alizarin red staining (Fig. 1 C, D). Data from flow cytometry analysis showed that ADSCs expressed high levels of stem cell markers CD105, CD73, CD44 and CD90, but did not express haematopoietic lineage markers CD11b/c, CD31, CD34, and CD45) (Fig. 1 E). These results confirmed that ADSCs had the ability of differentiation.
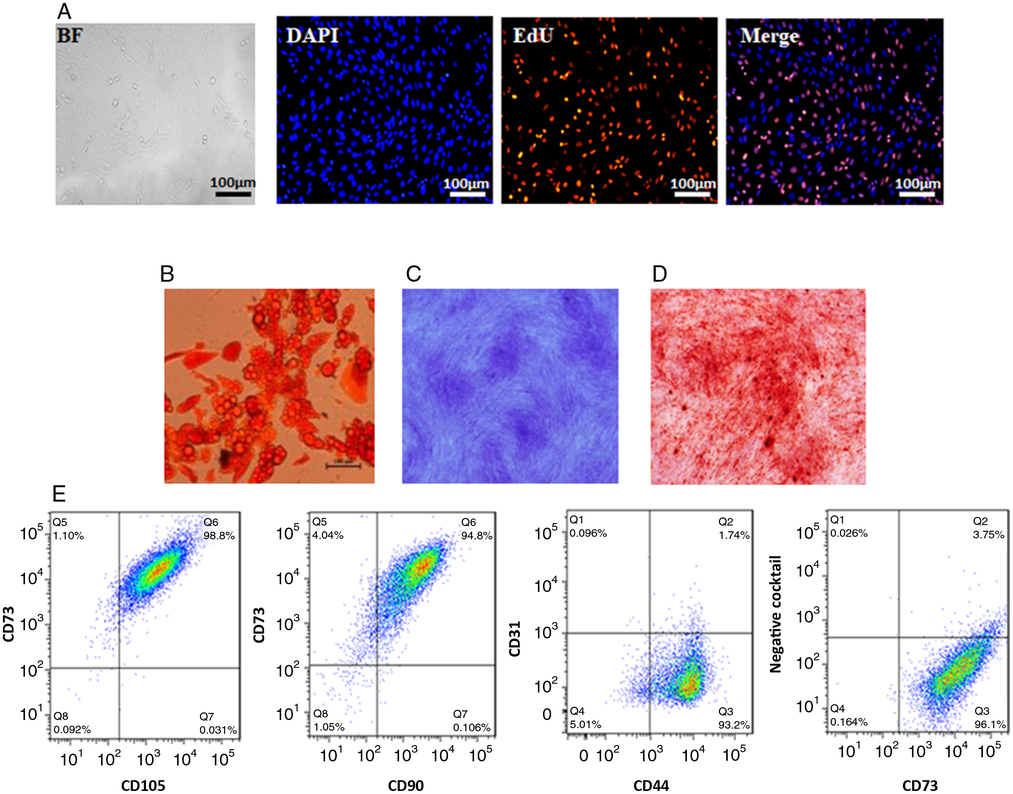
Figure 1. Characterization of GFP BMSCs. (A) Morphology of ADSCs and EdU staining. (B) Oil Red staining of ADSCs after adipogenic induction. (C) Alcian blue staining of ADSCs after chondrogenic induction. (D) Alizarin red staining of ADSCs after osteogenic induction. (E) Representative results of flow cytometry analysis of ADSCs indicating abundant expression of CD73, CD105, CD90, CD44 and the absence of CD31,CD34, CD11b/c, and CD45. Negative cocktail: anti-PE-conjugated anti-CD31, anti-CD34, anti-CD11b/c, anti-and CD45 mix.
ADSCs treatment improves physiologic recovery
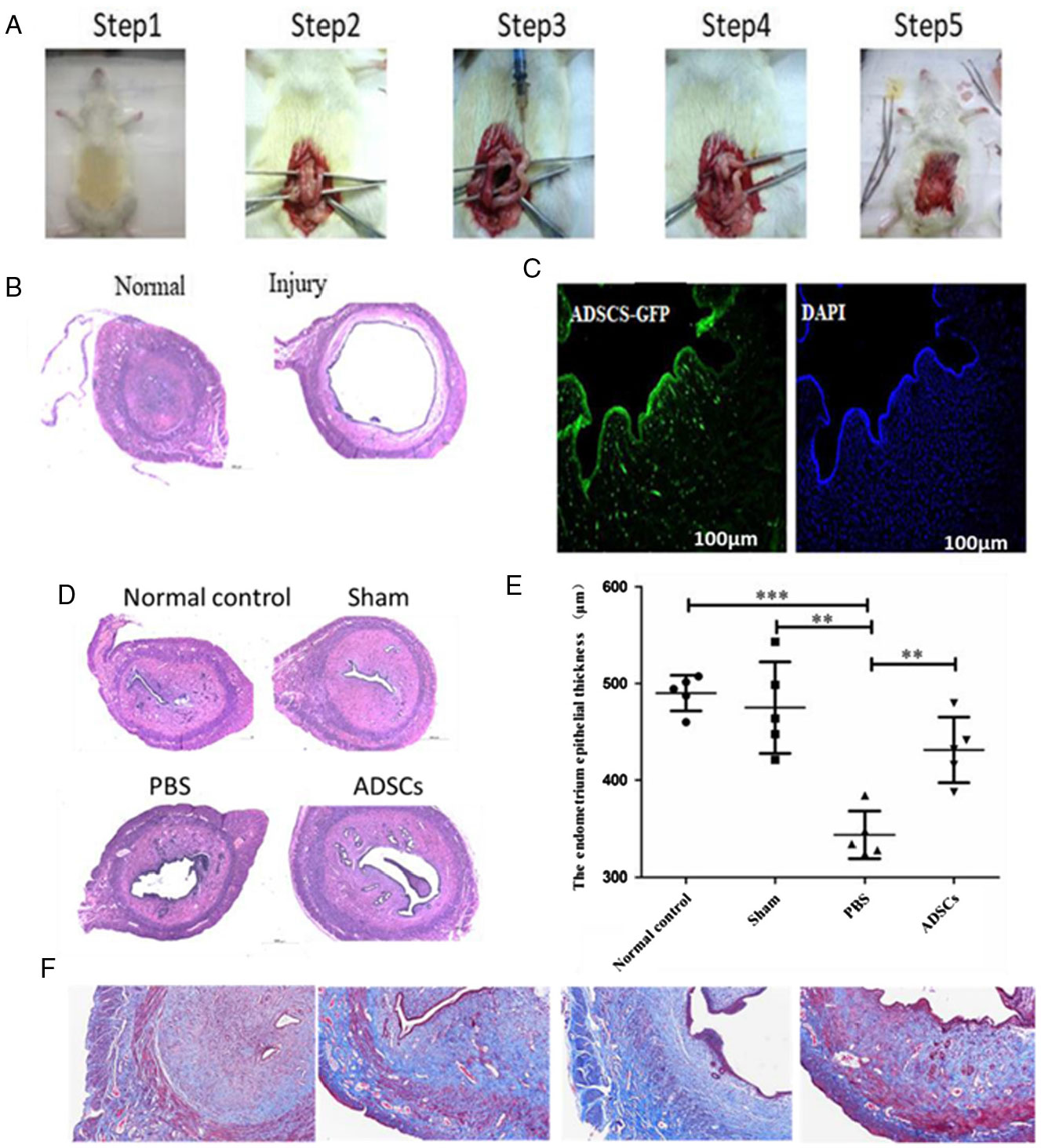
A model of endometrial injury was established by injecting ethanol (95%) into rat uteri (Fig. 2 A, B). ADSCs were injected into the injury site of endometrial cavity. Four weeks later, there were several ADSCs surrounding the wound edges and fusing with host cells. Interestingly, clusters of cells labelled with green fluorescence were primarily distributed in the basal layer of the regenerated endometrium (Fig. 2 C). On day 30 after endometrial injury, haematoxylin–eosin and Masson staining demonstrated that the uterine cavity of the control group was covered by columnar epithelium and oval glands. In the model group, the uterine cavity was covered with flat columnar epithelial cells or stratified flat epithelial cells, and glands were rare. The numbers of endometrial glands in the ADSCs groups were increased, and the newly formed glands were round or oval compared with the PBS treatment group. No significant difference in the numbers of glands was detected between the sham group and the normal control group (Fig. 2 D, F). In addition, the endometrial epithelium in the normal control group (487.15 ± 17.3 μm) was thicker than in the PBS treatment group (347.2 ± 22.9 μm) (P < 0.001). Interestingly, thickness of endometrial epithelium was increased after ADSC treatment (ADSC group: 442 ± 23.5 μm; PBS group: 347.2 ± 22.9 μm) (P < 0.01) (Fig. 2 E).
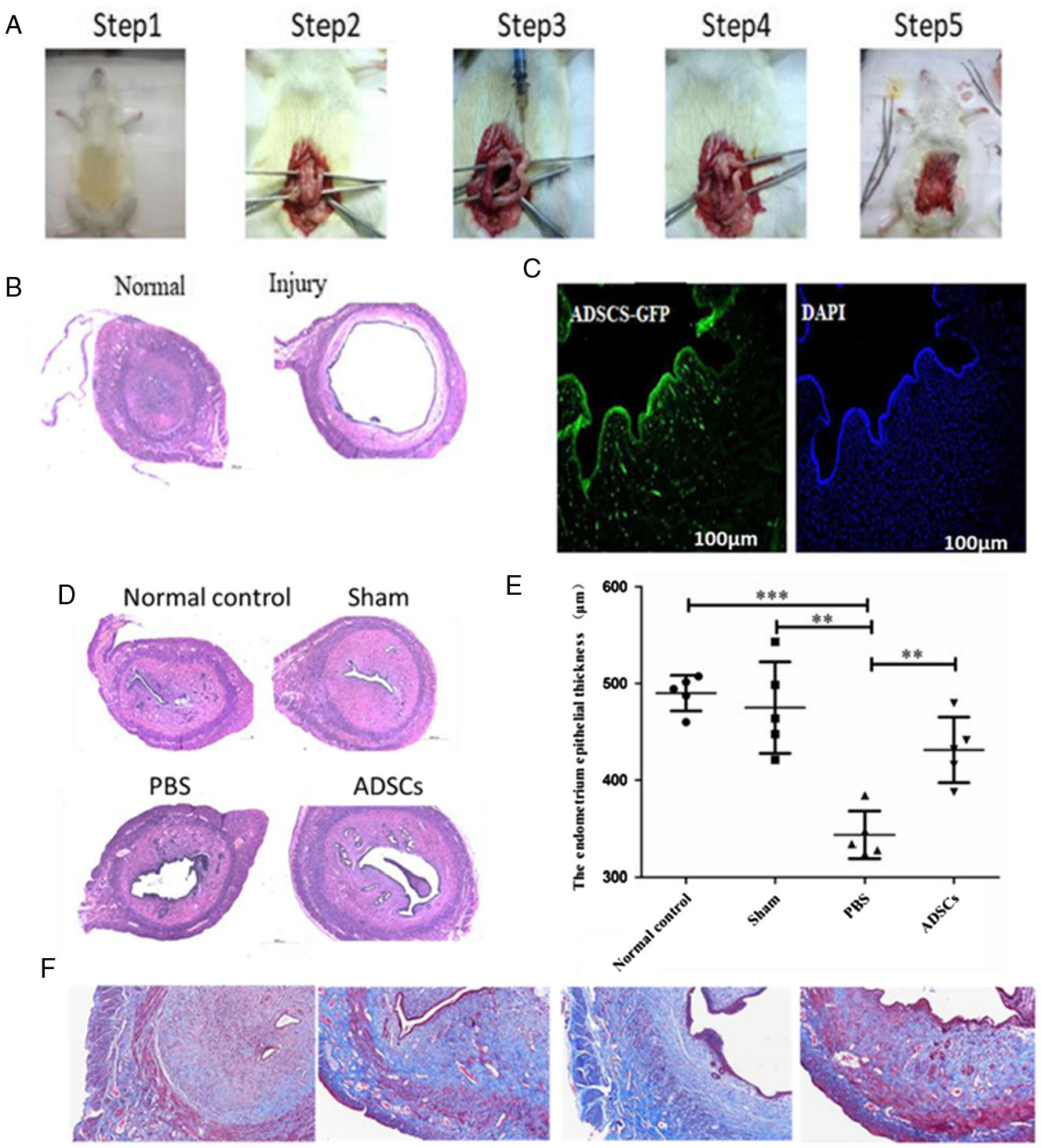
Figure 2. GFP-labelled ADSCs are recruited to the endometrium in response to injury. (A) The establishment process of endometrial injury model. (B) Morphological observations of injured endometrium in the four groups were examined by haematoxylin/eosin (H&E) staining. (C) Immunofluorescence staining was carried out to detect the localization of GFP-positive ADSCs. (D) Morphological observations of injured endometrium in the four groups was examined by H&E staining. (E) The thickness of endometrium epithelium. (F) Masson staining was carried out to detect microvessel density and hepatic fibrosis degree. ***P < 0.001, **P < 0.01.
ADSC differentiation in vivo
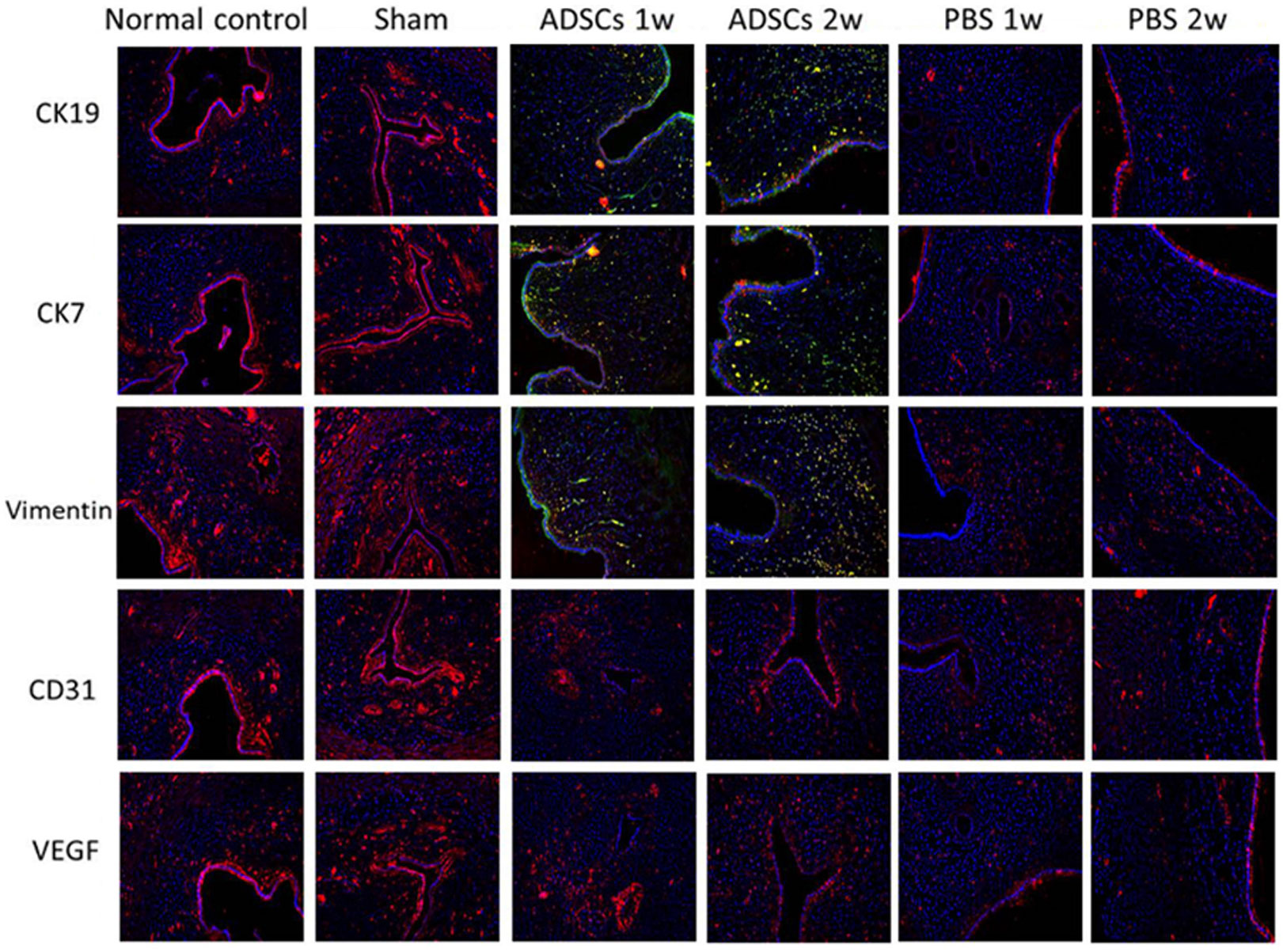
Based on further data from immunofluorescence staining, some ADSCs may have directly differentiated into CK19/CK7-positive endometrial stromal cells and the expression levels of CD31 and vimentin, a mesenchymal cell marker, were also higher in ADSC treatment group than that in the PBS treatment group (Fig. 3). The higher expression of vascular endothelial growth factor (VEGF) after ADSC treatment indicated that ADSC treatment could promote angiogenesis (Fig. 3). These representative images illustrate differentiation of ADSC cells during development in the uterine microenvironment.

Figure 3. Immunofluorescence staining was used to assess the differentiation of ADSCs in vivo. Scale bar = 50 μm. ADSCs 1w, One week after ADSCs injection; ADSCs 2w, Two weeks after ADSCs injection; PBS 1w, One week after PBS injection; PBS 2w, Two weeks after PBS injection.
Expression levels of ERα, ERβ, PR, integrin αV subunit 3 (αVβ3), leukaemia inhibitory factor (LIF) in a rat model of IUA
As presented in Fig. 4 A, for levels of serum ERα/β and PR in the oestrogen, the ADSC group had significantly higher levels than that in the PBS group (P < 0.05). However, there were no significant differences between the normal control group and the sham group. Additionally, the protein levels for ERα, ERβ, PR, αVβ3 and LIF were significantly increased in the ADSC group compared with that in the PBS group at 4 weeks post-treatment (P < 0.05) (Fig. 4 B, C). Consistent with the results from western blotting, 3 days after IUA, mRNA levels of integrin alpha V (ITGAV), integrin beta 3 (ITGB3) and LIF were significantly increased in the ADSC group compared with that in the PBS group (P < 0.05) (Fig. 4 D). No significant difference in expression levels of ITGAV, ITGB3 and LIF were detected between the normal control group and sham group (P > 0.05) (Fig. 4 D).

Figure 4. The expression of ERα, ERβ, PR, αVβ3, LIF in the endometrium. (A) Expression of ERαis increased after ADSCs treatment compared with the PBS group. (B, C) Western blot detected the expression of ERα, ERβ, PR, αVβ3, LIF, ITGAV, ITGB3 in the endometrium. (D) qPCR detected the expression of LIF, ITGAV, ITGB3 in the endometrium. *Denotes that differences are statistically significant between normal/sham groups and the PBS group (P < 0.01), #Denotes that differences are statistically significant between ADSCs and PBS groups (P < 0.05).
ADSC treatment improves pregnancy rate
To investigate whether pregnancy rate was improved after ADSC treatment, the ability to accept an embryo was tested. After 4 weeks of modelling and treatment as described above, adult female rats were mated with the adult male rats (male:female ratio was 1:2) and the pregnancy of the female rats was observed continuously for 1 month. Although the pregnancy rate was 60% for ADSC treatment group, the pregnancy rate was increased compared with 0% in the PBS group (P = 0.005) (Table 1). In addition, the total number of implanted embryos in the ADSC treatment group (average number 3) was significantly increased compared with that in the PBS group (average number 0) (P = 0.020) (Table 1). Therefore, these data showed that ADSC transplant could re-establish maternal uterine functionality and support embryo implantation and fetal development.
Discussion
Intrauterine adhesion also named Asherman's syndrome is a common gynaecological disease affecting menstrual and reproduction as the secondary to endometrial injury (March, Reference March2011a). The uterine cavity adhesion was indicated by fibrosis, sparsely glandular, inactive or cystic dilatation and ischaemic injury. Infertility is a common phenomenon in patients with uterine cavity adhesion. Therefore, it is important to pay more attention to this severe disease.
Petit-Zeman (Reference Petit-Zeman2001) in 1999 first proposed this novel subject and named it tissue engineering. Stem cells are considered to be one of the most promising tools in regenerative medicine. Stem cell therapy, including MSCs, has shown huge potential in many clinical areas (Ramakrishna et al., Reference Ramakrishna, Janardhan and Sudarsanareddy2011). It is easy to separate MSCs from various tissues and MSCs cultured in vitro also have a good proliferative capacity without any change in their biological features, such as low damage to normal cells or tissues and weak immunogenicity. It is therefore a better choice to apply MSCs for regenerative medicine and cell therapy in various tissues such as in the uterus (Campo et al., Reference Campo, Cervello and Simon2017; Azizi et al., Reference Azizi, Aghebati-Maleki, Nouri, Marofi, Negargar and Yousefi2018). To date, much research has focused on MSCs found within the bone marrow stroma. However, increasing evidence has indicated that adipose tissue is a better source of MSCs than bone marrow (Zhao et al., Reference Zhao, Zhang, Wang and Li2015; Bacakova et al., Reference Bacakova, Zarubova, Travnickova, Musilkova, Pajorova, Slepicka, Kasalkova, Svorcik, Kolska, Motarjemi and Molitor2018). Interestingly, because of their multipotentiality, ADSCs should be consider as an alternative to BMSCs (Eto et al., Reference Eto, Suga, Matsumoto, Inoue, Aoi, Kato, Araki and Yoshimura2009). Autologous ADSCs can be isolated in large amounts from adipose tissue from the patient/animal, with minimum donor morbidity (Gadelkarim et al., Reference Gadelkarim, Abushouk, Ghanem, Hamaad, Saad and Abdel-Daim2018). In addition, ADSCs have a greater secretory capacity than BMSCs, such as for interleukin 6 (IL-6) and insulin-like growth factor-1 (IGF-1), which are important for cell therapy and tissue regeneration (Melief et al., Reference Melief, Zwaginga, Fibbe and Roelofs2013). Zhou et al. (Reference Zhou, Chen, Zhang, Min, Yu, He and Jin2013) reported that spinal cord injury can be effectively improved after ADSC treatment, with the improvement attributed to increased secretion of growth factors and immune regulators from ADSCs.
To date, few studies have evaluated the therapeutic effect of ADSCs on endometrial injury. In this study, we successfully established a rat model of endometrial injury. Data from a flow cytometer (FCM) showed that the ADSCs cultured in vitro have higher expression of ADSCs markers, including CD73, CD105, CD90, CD44 (Fig. 1 E). The results from Oil O Red staining, Alcian blue staining and Alizarin red staining indicated that ADSC cells could be induced in vitro to differentiate into adipose cells, chondrocytes and osteoblasts (Fig. 1 B–D). Our results showed that rats whose endometrial injury had been injected with ADSCs demonstrated great improvement in endometrium regeneration and a higher expression of CK7/19, a marker protein for endometrial cells, than found in the PBS treated group (Fig. 3). The role of VEGF is important in wound angiogenesis. It was exciting to find a significant increase in VEGF in the ADSC treatment group compared with the PBS treatment group (Fig. 3). This finding indicated that ADSCs injected into the uterine cavities of these rats could differentiated into endometrial cells and improve endometrial injury.
Integrin and LIF play important roles in embryo implantation among regulators of endometrial function and are markers for endometrial receptivity (Achache and Revel, Reference Achache and Revel2006). Data from the present study showed that the expression levels of ITGAV, ITGB3 and LIF were significantly higher in rats after ADSCs transplantation, and data from qPCR showed that mRNA expression levels for ITGAV, ITGB3 and LIF were higher in rats that received ADSCs than rats in the PBS group (Fig. 4 D). Our data also showed higher expression of integrin beta 3 (ITGB3), cytokeratin, vimentin and LIF in the ADSC transplanted rats. Therefore ADSC transplantation may promote endometrial regeneration and improve endometrial receptivity.
Oestrogen increases the number of cells during endometrial injury and promotes regeneration of the uterine endometrium (Hyodo et al., Reference Hyodo, Matsubara, Kameda and Matsubara2011). Oestrogen has been widely used in the prevention of IUA following operative hysteroscopy. A previously study reported that oestrogen treatment reduced the incidence rate of IUA from 6.9 to 0% (Roy et al., Reference Roy, Negi, Subbaiah, Kumar, Sharma and Singh2014). In this study, the level of ERα was significantly increased in serum from rats with IUA compared with that from the control group, suggesting an effect of oestrogen on the repair of intrauterine adhesions. Furthermore, the level of protein expression of ERα/β and PR in the ADSC treatment group was higher than that in PBS group (Fig. 4 B). Furthermore, to investigate whether ADSCs can improve the fertility in vivo, the injured female rats were bred with male rats at day 30 after transplantation for 1 month and then isolated from the males. Our data showed that all the female rats were pregnant in normal group. However, there was a significant difference in number of pregnancies between the ADSCs group and the PBS group (Table 1).
Although our data demonstrated that ADSCs could help repair the injured endometrium and improve fertility in a rat endometrial injury model, therapeutic efficiency needs to be improved. Previous studies have shown that MSCs can release angiogenic and trophic factors crucially importance to proper wound regeneration (Kwon et al., Reference Kwon, Gao, Liu, Dulchavsky, Danyluk, Bansal, Chopp, McIntosh, Arbab, Dulchavsky and Gautam2008; Navone et al., Reference Navone, Pascucci, Dossena, Ferri, Invernici, Acerbi, Cristini, Bedini, Tosetti, Ceserani, Bonomi, Pessina, Freddi, Alessandrino, Ceccarelli, Campanella, Marfia, Alessandri and Parati2014). We speculated that the underlying mechanisms of ADSC repair of the injured endometrium might be associated with the inflammatory microenvironment; this remains to be explored in the future. Despite the advantages of stem cell and other exciting results, the safety of stem cell clinical application should be fully considered, including for ADSCs and other types of stem cells. The use of MSCs in cancer therapy still remains controversial (Bacakova et al., Reference Bacakova, Zarubova, Travnickova, Musilkova, Pajorova, Slepicka, Kasalkova, Svorcik, Kolska, Motarjemi and Molitor2018). Several studies have suggested that ADSCs promote the proliferation of cancer cells, possibly due to a wide spectrum of immune suppressors, growth factors or other cytokines secreted by ADSCs. Therefore, these disappointing results urged us to study ADSCs in greater detail (Mohr and Zwacka, Reference Mohr and Zwacka2018; Si et al., Reference Si, Wang, Sun, Kang, Xua, Wang and Hui2019). Taken together, our results confirmed that ADSCs, at least in part, could restore the injured endometrium and improve the fertility of endometrial injury rats. This study provides an experimental basis for clinical application of ADSCs.
Financial support
This work was supported by the Fundamental Research Funds for the Central Universities (no. 22120180388).
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S096719941900042X