Introduction
In comparison with other domestic species, the development of new tests to improve the spermiogramme in canines have been scarce (Peña et al., Reference Pena, Núñez Martinez and Morán2006). These studies have focused on the evaluation of sperm membranes, acrosomes or capacitation status; IVF tests have also been successfully used (Peña et al., Reference Peña, Johannisson, Wallgren and Rodriguez Martinez2004). Recent studies have examined the sperm DNA (Núñez Martinez et al., Reference Núñez Martinez, Moran and Peña2005) and the sperm subpopulation structure (Núñez Martinez et al., Reference Núñez Martinez, Moran and Peña2005a,b, Reference Núñez Martinez, Moran and Peña2006). The importance of the so-called, ‘apoptotic-like’ phenomena is receiving increasing interest both in human and veterinary andrology. Apoptotic-like phenomena are demonstrated to be involved in human infertility (Said et al., Reference Said, Paasch, Glander and Agarwal2004) and in the damage induced by cryopreservation procedures both in humans (Paasch et al., Reference Paasch, Sharma, Gupta, Grunewald, Mascha, Thomas, Glander and Agarwal2004) and animals (Peña et al., Reference Peña, Johanisson, Wallgren and Rodriguez Martinez2003, Martin et al., Reference Martin, Sabido, Durand and Levy2005)
Apoptosis is a complex phenomenon regulating cellular proliferation that can be divided into three phases: induction, execution and degradation. After induction of apoptosis, mitochondrial pores are opened, leading to a decreased mitochondrial membrane potential (ΔΨm). Opening of mitochondrial pores causes the release of pro-apoptotic factors in the cytoplasm where they are activated, leading to the degradation phase. During degradation, the sperm membrane permeability increases, and phosphatidylserine (which triggers a non-inflammatory recognition of the apoptotic cells by phagocytes) is externalized (Desagher & Martinou, Reference Desagher and Martinou2000). Mitochondria play a major role in the control of apoptosis. In addition, mitochondria seem to be the subcellular structures that are more sensitive to the stress of cryopreservation (Peña et al., Reference Peña, Johannisson, Wallgren and Rodriguez Martinez2004; Ortega Ferrusola et al., Reference Ortega-Ferrusola, Sotillo-Galán, Varela–Fernández, Gallardo-Bolaños, González-Fernández, Tapia and Peña2008). Improvement of cryo-preservation protocols requires an in-depth knowledge of the subcellular and molecular mechanisms involved in cryo-damage, and the development of new techniques able to detect these injuries. Flow cytometry is a highly accurate, and repeatable, technique suitable to detect these subcellular and molecular injuries allowing a rapid clinical application of this technique in assessing cryopreservation (Peña et al., Reference Pena, Núñez Martinez and Morán2006)
The aim of the present study was to investigate the presence of active caspases in frozen thawed canine sperm using a cell permeable fluorescent derivative of the inhibitor peptide VAD–FMK (FITC–VAD–FMK) and assess the correlation of the presence of active caspases with other apoptosis markers, ΔΨm and early changes on membrane permeability. Additionally, we investigated the possible correlation of these parameters with sperm motility (CASA).
Material and methods
Animals
Eight privately owned dogs (one German shepherd, three golden retrievers, and four Labrador retrievers) were used in this study. All dogs were of known fertility (all had sired at least one litter). The range of the dogs' weight was from 25 to 35 kg, with ages ranging from 2 to 6 years.
Semen collection and processing
Semen was manually collected (three ejaculates per dog) in a prewarmed graduated test tubes. After collection, sperm samples were kept at 37°C in a water bath for less than 10 min. An aliquot was removed for sperm concentration measurement and evaluation of motility using computer-assisted sperm analysis (CASA) and morphology analysis (phase contrast, microscopy of wet smears using a ×100 objective). Only samples with at least 70% progressive motility and 80% morphologically normal spermatozoa were included in the study.
Cryopreservation procedure
Semen was processed using the Uppsala method (Peña & Linde Forsberg., Reference Peña and Linde-Forsberg2000) modified by Nuñez Martinez et al. (Reference Núñez-Martínez, Morán and Peña2006). In brief, collected semen was extended 1:1 in Tris–glucose Extender I (Tris 200 mM, glucose 70 mM, citric acid 63 mM, BSA 3%, penicillin 1000 UI/ml, dihydroestreptomicin 1 mg/ml) and centrifuged for 8 min at 700 g. The seminal plasma was then removed and the sperm pellet resuspended in Extender II at room temperature (Tris 200 mM, 70 mM glucose, 63 mM citric acid, glycerol 3% v/v, egg yolk 20% v/v, penicillin 1000 UI/ml, dihydroestreptomicin 1 mg/ml), and cooled to 5°C over a period of 1 h, resulting in a concentration of 300–400 × 106 sperm per ml. After equilibration, an equal volume of Extender III (Tris 200 mM, 70 mM glucose, 63 mM citric acid 7% v/v, glycerol 20% v/v, egg yolk 1% v/v, Equex STM paste [Nova Chemical Sales Inc. Sciutate] penicillin dihydroestreptomicin) was added at 5°C to a final sperm concentration of 150–200 × 106 spermatozoa per ml. The sperm samples were then loaded into 0.5 ml straws and frozen horizontally in racks by placement of samples 4 cm above the surface of liquid N2 in a closed Styrofoam box for 10 min and then plunging directly in liquid N2. After 4 weeks of storage, the straws were thawed in a 70°C water bath for 8 s.
Motility analysis
Motility was measured after cryopreservation using a CASA system (ISAS® Proiser, Valencia, Spain). Analysis was based on the examination of 25 consecutive, digitalized images obtained from a single field using a ×10 negative phase-contrast objective. Images were taken at a time lapse of 1 s and the image capture speed was, therefore, once every 40 ms. The number of objects incorrectly identified as spermatozoa were minimized on the monitor by the playback function. With respect to the setting parameters for the programme, sperm with a mean velocity (VAP) < 10 μm/s were considered immotile, while sperm with a velocity > 15μm/s were considered motile. Spermatozoa deviating < 10% from a straight line were designated as linear motile. Sperm motion kinematics measured by CASA included the following:
• curvilinear velocity (VCL) (μm/s): measures the sequential progression along the true trajectory;
• linear velocity (VSL) (μm/s): measures the straight trajectory of the spermatozoa per unit time;
• mean velocity (VAP) (μm/s): measures the mean trajectory of the spermatozoa per unit time;
• linearity coefficient (LIN) (%): VSL/VCL × 100;
• straightness coefficient (STR) (%): VSL/VAP × 100;
• wobble coefficient (WOB) (%): VAP/VCL × 100;
• mean lateral head displacement (ALH) (μm): measures the mean head displacement along the curvilinear trajectory;
• BCF (beat cross frequency) (Hz): number of times the sperm head crosses the mean path/s.
Staining for cytofluorometric assessment of activated caspases
The caspase FITC–VAD–FMK in situ marker (Molecular Probes) was used to detect active caspases. This cell permeable caspase inhibitor peptide conjugated to FITC covalently binds activated caspases, thus serving as an in situ marker for apoptosis. Spermatozoa (1 × 106) were diluted in 1 ml of PBS, 1 μl of FITC–VAD–FMK was added, and samples were incubated at room temperature in the dark for 20 min. The spermatozoa were then washed in PBS and resuspended to the initial cell concentration, 1 μl of ethidium homodimer (1.167 mM) (Molecular Probes) was added, and samples were assessed by flow cytometry and fluorescence microscopy within 10 min.
Staining for evaluation of mitochondrial membrane potential (ΔΨm)
The lipophilic cationic compound, 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzymidazolyl carbocianyne iodine (JC-1), possesses the unique ability to differentially label mitochondria with low and high membrane potentials. In mitochondria with high membrane potential, JC-1 forms multimeric aggregates emitting in the high orange wavelength (590 nm) when excited at 488 nm. In mitochondria with low membrane potential, JC-1 forms monomers, emitting in the green wavelength (525 to 530 nm) when excited at 488 nm. A 3 mM stock solution of JC-1 (Molecular Probes) in DMSO was used for staining, and a 500 μl aliquot of each semen sample was stained with 0.5 μl of the JC-1 stock solution. The samples were incubated at 38°C in the dark for 40 min before flow cytometric and fluorescence microscopy analysis.
Staining for assessment of subtle membrane changes and viability
Early membrane changes and sperm viability were determined as described by Peña et al. (Reference Peña, Johannisson, Wallgren and Rodriguez Martinez2005) with modifications for adaptation to canine semen. In brief, the following stock solutions in DMSO were prepared: YO–PRO-1 (25 μM) and ethidium homodimer-1 (1.167 mM) (Molecular Probes). A total of 500 μl of a sperm suspension containing 5 × 106 spermatozoa/ml in PBS was stained with 3 μl of YO–PRO-1 and 1 μl of ethidium homodimer. After thorough mixing, the sperm suspension was incubated at 37°C in the dark for 16 min. This staining distinguishes four sperm subpopulations. The first subpopulation of unstained spermatozoa are considered live and in the absence of any membrane alteration. A second subpopulation is the YO–PRO-1-positive cells emitting green fluorescence. In early stages of apoptosis, the membrane permeability is known to be modified, selectively allowing the entry of several non-permeable DNA-binding molecules (Ormerod et al., Reference Ormerod, Sun, Snowden, Davies, Fearnhead and Cohen1993; Wronsky et al., Reference Wronski, Golob, Grygar and Winsdish2002). This subpopulation comprises the spermatozoa that may show early damage or a shift to another physiological state. Membranes become slightly permeable during the first steps of cryoinjury, enabling YO–PRO-1 but not ethidium homodimer to penetrate the plasma membrane (Izdiorek et al., Reference Idziorek, Estaquier, de Bels and Ameisen1995). Neither of the probes enters intact cells. Finally, two subpopulations of cryoinjury-induced necrotic spermatozoa were easily detected by staining: early necrotic spermatozoa stained with both YO–PRO-1 and ethidium homodimer (emitting both green and red fluorescence), and late necrotic spermatozoa stained only with ethidium homodimer (emitting red fluorescence). The staining patterns were visually checked under combined phase-contrast fluorescence microscopy (Nikon-E80).
Flow cytometry
Flow cytometric analyses were carried out with a Coulter EPICS XL (Coulter Corporation Inc.) flow cytometer equipped with standard optics. An argon-ion laser (Cyonics, Coherent, Santa Clara, CA, USA) performing 15 mW at 488 nm and the EXPO 2000 software was included. Subpopulations were divided by quadrants, and the frequency of each subpopulation was quantified. Non-sperm events (debris) were gated out based on the forward scatter and side scatter dot plot by drawing a region enclosing the cell population of interest. Events with scatter characteristics similar to sperm cells but without reasonable DNA content were also gated out. Forward and sideways light scatter were recorded for a total of 10 000 events per sample; samples were measured at flow rate of approximately 300 cells/s. Green fluorescence was detected in FL1, red fluorescence was detected in FL3, and orange fluorescence in FL2.
Statistical analysis
The data were first examined using the Kolmogorov–Smirnov test to determine their distribution. In view of the non-Gaussian distribution of most of the data gathered, multivariate analysis of variance was performed, and when significant differences were found, the non-parametric Mann–Whitney U-test was used to directly compare pairs of values. The Spearman non-parametric test was used to study the correlation between sperm analysis prefreezing and sperm quality post-thaw. All analyses were performed using SPSS ver. 15.0 for Windows (SPSS Inc.). Significance was set at p ≤ 0.05.
Results
Presence of active caspases in frozen–thawed sperm
Typical cytograms of FT canine spermatozoa labelled with FITC–VAD–FMK/Eth are shown in Fig. 1. Four cell patterns were detected by cytometric analysis: live cells without caspase activity, live cells with caspase activity, and two groups of dead cells, dead with caspase activity and dead cells without caspase activity. Staining of spermatozoa for cellular caspase activity revealed two patterns of caspase localization; caspase activity (green fluorescence) was observed in the mid piece, tail and over the acrosomal region, or observed only in the midpiece and tail (Fig. 2). On average, a high proportion of FT canine sperm showed caspase activity (Table 1), ranging from with 30.2 to 70.7% of live sperm and 7.3 to 24.0% of dead sperm as caspase positive.

Figure 1 Flow cytometry analysis of activation of caspase family members in frozen–thawed dog sperm. Representative cytograms in two dogs. Left panel indicates a freezer that worked, while right panel shows results from samples stored in a defective freezer. Events in the lower left region represent intact sperm, and events in the lower right region represent spermatozoa showing caspase activity. Events in the upper right and left regions are ethidium positive, thus dead spermatozoa.

Figure 2 Localization of FITC–VAD–FMK fluorescence in FT dog spermatozoa. Active caspases are present in (a) the midpiece, tail and acrosome or (b) in the midpiece and tail.
Table 1 Mean percentages of caspase-positive and -negative live and dead spermatozoa stained with FITC–VAD–FMK and ethidium homodimer.

ZVAD–/Eth– (non-stained cells corresponding to viable spermatozoa); ZVAD+/Eth– spermatozoa (early apoptotic, confirmed caspase activity); ZVAD+/Eth+ (late apoptotic caspase activity in dead sperm); ZVAD–/Eth+ spermatozoa (necrotic cells).
a,bWithin a column values with different superscripts differ significantly (p < 0.05).
Mitochondrial membrane potential
The mean per cent of sperm cells showing low ΔΨm ranged from 8 to 39.8%, and significant differences among the dogs were not identified. The proportion of mitochondria showing high and low ΔΨm varied significantly (Fig. 3).

Figure 3 Patterns of JC-1 florescence in FT dog spermatozoa. (a) Spermatozoa with high ΔΨm; and (b) spermatozoa with low ΔΨm. (c) Spermatozoa showing an intermediate situation (mitochondria showing low and high ΔΨm within the same spermatozoan).
Presence of apoptotic and dead spermatozoa (increase in sperm membrane permeability)
No significant differences were observed among dogs either in the percent of live spermatozoa or in the presence of spermatozoa showing early changes in sperm membrane permeability (Table 2). However, significant differences were observed among dogs in the per cent of ethidium positive spermatozoa.
Table 2 Mean percentages of live and dead spermatozoa with and without early membrane changes in frozen–thawed semen. Flow cytometric evaluation of early membrane changes and changes in membrane integrity.

YO–PRO-1–/Eth– (non-stained cells corresponding to viable spermatozoa); YO–PRO+/Eth– (early apoptotic, spermatozoa with increased membrane permeability); YO–PRO-1+/Eth+ (late apoptotic); and YO–PRO–/Eth+ spermatozoa (necrotic cells).
a,bWithin a column values with different superscript differ significantly (p < 0.05).
Motility and kinematic parameters
Significant differences were observed in the per cent of linear motile spermatozoa, VCL VAP, BCF and VSL among dogs (Tables 3 and 4).
Table 3 Mean percentages of sperm motility and velocity after thawing.

LM, linear motile sperm; NLM, non-linear motile sperm; VCL, circular velocity; VSL, straight line velocity; VAP, average velocity.
a,bWithin a column values with different superscript differ significantly (p < 0.05).
Table 4 Mean kinematic parameters after thawing.

LIN, linearity coefficient (VSL/VCL × 100); STR, straightness coefficient (VSL/VAP × 100); WOB, wobble coefficient (VAP/VCL × 100); ALH, mean lateral head displacement; BCF (beat cross frequency).
a,bWithin a column values with different superscript differ significantly (p < 0.05)
Correlations among caspase activity, ΔΨm, membrane integrity and motility
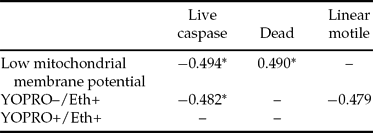
Significant negative correlations were observed among sperm showing low ΔΨm and live spermatozoa showing caspase activity. Ethidium positivity was negatively correlated with live spermatozoa showing caspase activity. Significant positive correlations were observed among the per cent of sperm showing low ΔΨm and dead spermatozoa. The percentage of YO–PRO-1+/ Eth+ sperm was negatively correlated with the percentage of linear motile sperm (Table 5).
Table 5 Correlation among caspase activity and other sperm characteristics in frozen–thawed sperm.
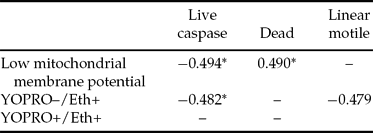
*p < 0.05.
Discussion
Cryopreservation induces major damage to sperm. During this process, crystallization of intracellular and extracellular water leads to mechanical damage, and chemical damage due to osmotic stress also occurs (Mazur, Reference Mazur1984) Recently, biochemical damage, such as lipid peroxidation, premature ageing and phase membrane transitions, have been recognized as important factors that contribute to the sublethal damage of the spermatozoa that survive freezing and thawing (Watson., Reference Watson2000). The mitochondrion has been recently recognized as the subcellular structure of the spermatozoa most sensitive to this biotechnology (Peña et al., Reference Peña, Johannisson, Wallgren and Rodriguez Martinez2003, Ortega Ferrusola et al., Reference Ortega-Ferrusola, Sotillo-Galán, Varela–Fernández, Gallardo-Bolaños, González-Fernández, Tapia and Peña2008). Mitochondria have a major role in apoptosis, and recently the role of apoptosis in the cellular injury induced by the cryopreservation procedure has been characterized (Martin et al., 2003; Grunewald et al., Reference Grunewald, Paasch, Said, Sharma, Glandler and Agarwal2005; Ortega Ferrusola et al., Reference Ortega-Ferrusola, Sotillo-Galán, Varela–Fernández, Gallardo-Bolaños, González-Fernández, Tapia and Peña2008). Even under the best conditions, only 50% of the spermatozoa remain motile after thawing and, of these, only 5% are considered completely competent spermatozoa for fertilization (Holt & Van Lock, Reference Holt and Van Look2004). This observation implies that the identification of subtle changes in the spermatozoa is of utmost importance to the identification of competent spermatozoa and the improvement of current cryopreservation protocols.
We identified, for the first time, active caspases in frozen–thawed canine sperm. Active caspases have been previously identified in frozen–thawed bovine sperm (Martín et al., Reference Martin, Sabido, Durand and Levy2004), human sperm, mainly in infertile patients (Marchetti et al., Reference Marchetti, Gallego, Defossez, Formstecher and Marchetti2004; Hu et al., Reference Hu, Xu, Qiao, Wu, Sa, Fu, Yu, Zhang, Zhang, Gu, Chen and Xie2006), rodents (Cisternas & Moreno, Reference Cisternas and Moreno2006), rams (Martí et al., Reference Marti, Perez Pe, Muiño Blanco and Cebrian Perez2006) and horses (Ortega Ferrusola et al., Reference Ortega-Ferrusola, Sotillo-Galán, Varela–Fernández, Gallardo-Bolaños, González-Fernández, Tapia and Peña2008).
The mitochondrial apoptotic pathway seems to be involved in caspase activation in spermatozoa. Caspase activation has been induced with betulinic acid (Grunewald et al., Reference Grunewald, Paasch, Said, Sharma, Glandler and Agarwal2005) and bovine spermatozoa have been demonstrated to contain the apoptotic machinery, especially the one related to mitochondrial pathway.
Under physiological situations, pro-apoptotic factors remain inactivated within the mitochondria. Caspases are unrelated to sperm survival or capacitation and the acrosome reaction (Wundrich et al., Reference Wundrich, Paasch, Leicht and Glander2006). Cytochrome c and the apoptosis inducing factor (AIF) have been detected in bovine spermatozoa (Martin et al., Reference Martin, Cagnon, Sabido, Sion, Grizard, Durand and Levy2007), and in addition, pro-caspase 9 and activated caspase 9, involved in the mitochondrial pathway, were detected in bovine (Martin et al., Reference Martin, Cagnon, Sabido, Sion, Grizard, Durand and Levy2007) and human sperm (Wundrich et al., Reference Wundrich, Paasch, Leicht and Glander2006) Cryopreservation induces an increase in mitochondrial permeability leading to the release of proapoptotic factors. Also, apoptosis plays an important role in spermatogenesis (Konrad et al., Reference Konrad, Keilani, Laible, Nottelmann and Hofmann2006; Zheng et al., Reference Zheng, Turner and Lysiak2006) reflecting the important role of this mechanism in the male reproductive sphere.
Among the interesting findings of our study were the identification of various degrees of membrane injury and the detection of active caspases both in live and dead sperm. Two patterns of caspase activity in dead spermatozoa were observed in our analyses: (1) caspase activity in the midpiece tail; and (2) in the midpiece tail and acrosomal region. In live (ethidium-negative) spermatozoa, caspase activity was detected only in the midpiece tail region. In previous reports, caspase activity was detected only in the midpiece and no differentiation was made between live and dead sperm showing caspase activity (Martín et al., Reference Martin, Sabido, Durand and Levy2005).
The presence of caspase activity in the acrosomal region is not easy to interpret. One possibility is that the staining is simply an artefact, while the presence of caspase activity in the midpiece supports the theory of the mitochondrial pathway of apoptosis (Cyali et al., Reference Cyali, Sakkas, Vigue, Demir and Huszar2004) Also the presence of live (ethidium-negative) caspase-positive spermatozoa supports the theory of an apoptotic-like mechanism involved in sublethal damage after freeze-thawing.
From a practical point of view the differentiation between caspase activity in dead and live spermatozoa may be a simple method to disclose subtle differences in sperm quality. This staining allowed us to find statistically significant differences in the sperm characteristics among dogs. In fact, the sample that showed overall better results in all the sperm parameters studied after thawing was the one with a lower percentage of active caspases, both in dead and live spermatozoa. Also, the human study that found significant correlations between the per cent of sperm with active caspases and the outcome of IVF (Marchetti et al., Reference Marchetti, Gallego, Defossez, Formstecher and Marchetti2004) strengthen this assay.
JC-1 staining showed high variations among and within dogs. This fact was related to numerous intermediate situations (different percentages of low and high ΔΨm within the same spermatozoa) that made it difficult to properly gate the different subpopulations in the flow cytometric dot plots. Experiments are in progress in our laboratory to improve the identification of these intermediate situations. Analysis of correlations among different parameters of sperm quality provided important information. The percentage of live sperm that showed caspase activity was negatively correlated with sperm that showed low ΔΨm. This fact seems contradictory, however it can be interpreted when taking into account that low ΔΨm was positively correlated with the percentage of dead sperm. Bearing that in mind, we distinguished two categories of caspase positive spermatozoa. Live, caspase-positive spermatozoa may represent those with a very early stage of cell injury; the negative correlation of YO–PRO–/Eth+ (necrotic sperm) with live sperm that showed caspase activity supports this theory.
On the other hand, it is noteworthy that apoptotic sperm were negatively correlated with the per cent of linear motile spermatozoa. This subpopulation may represent spermatozoa with subtle damage, leading in this case to a reduction or compromised linear pattern of movement.
In short, we successfully used flow cytometric analysis to detected active caspases in frozen–thawed canine sperm. This may represent a novel and simple test to assess the quality of a semen sample, as this technique allows the differentiation among semen samples of similar quality with conventional analysis.
Acknowledgements
This study was supported by the Ministry of Education and Science of Spain, Grants AGL 2007–60598 (GAN), BFU-2007–62423 (BFI) and Fundación ONCE del Perro Guía, Boadilla del Monte Madrid, Spain.