Cogongrass, a rhizomatous perennial grass native to the Old World, is considered one of the ten worst weeds in the world (Holm et al. Reference Holm, Plucknett, Pancho and Herberger1991). It continues to invade more lands and is regarded as the worst invasive threat in the southern United States (Miller Reference Miller2007). Today, cogongrass management depends heavily on chemical treatment, but success is limited unless other practices are integrated into the management plan (Johnson et al. Reference Johnson, Gaffney and Shilling1997; MacDonald Reference MacDonald2004). Extensive research showed that glyphosate and imazapyr are the most successful herbicides for cogongrass control in the market (Barnett et al. Reference Barnett, Byrd and Mask2001; Dickens and Buchanan Reference Dickens and Buchanan1975; Dozier et al. Reference Dozier, Gaffney, McDonald, Johnson and Shilling1998; MacDonald Reference MacDonald2004; Udensi et al. Reference Udensi, Akobundu, Ayeni and Chikoye1999; Willard et al. Reference Willard, Gaffney and Shilling1997). Imazapyr activity is usually slower than that of glyphosate, but it provides better long-term control due to residual soil activity (Dozier et al. Reference Dozier, Gaffney, McDonald, Johnson and Shilling1998; Willard et al. Reference Willard, Gaffney and Shilling1997). Imazapyr is used to control annual and perennial weeds, trees, and vines in rights-of-way and other noncropland areas, as well as to release conifers in forestry plantations (Shaner Reference Shaner2014).
The mode of action of imazapyr is associated with the inhibition of acetolactate synthase (ALS; also called acetohydroxyacid synthase, AHAS), an enzyme in the pathway to the production of the branched chain amino acids leucine, isoleucine, and valine (Duke Reference Duke1990; Shaner Reference Shaner2014). The death of the plants is a consequence from events occurring as a result of the ALS inhibition, such as the accumulation of phytotoxic compounds (2-oxybutyrate and transamination of 2-amino butyrate), but the sequence of events that causes plant death is uncertain (Duke and Dayan Reference Duke and Dayan2011). Soil type and environmental conditions affect imazapyr persistence, with field half-life ranging from 25 to 142 d; soil adsorption is influenced by organic matter and clay content as well as soil pH (Shaner Reference Shaner2014).
The primary dissipation mechanisms for imidazolinones in aerobic conditions are microbial degradation and, to a lesser degree, photolysis (Loux and Reese Reference Loux and Reese1993). Cultural practices, including tillage after herbicide application, can alter persistence and distribution of an herbicide in the soil (Wixson and Shaw Reference Wixson and Shaw1992).
General recommendations for cogongrass management include sequential herbicide applications to achieve long-term control (Byrd Reference Byrd2007; MacDonald Reference MacDonald2004). However, integrating weed management practices is essential to increase efficacy and achieve long-term control (Johnson et al. Reference Johnson, Gaffney and Shilling1997). Integrating cover crops for cogongrass management is ideal to the process of establishing desirable plants, avoiding the use of herbicides in excess, and introducing vegetation that will resist cogongrass reinvasion (Shilling and Gaffney Reference Shilling and Gaffney1995). Cover crops reduce soil erosion, increase organic matter, and provide or conserve nitrogen for subsequent crops (Hartwig and Ammon Reference Hartwig and Ammon2002). According to Brook (Reference Brook1989), annual crops or perennials and legume cover crops could be used as an intermediate stage between land clearance and establishment of cash crops.
An important step is to establish plants as soon as residual herbicide levels in the soil become tolerable to the revegetation species (Dozier et al. Reference Dozier, Gaffney, McDonald, Johnson and Shilling1998). It is important to consider herbicide persistence to predict the timing to effectively establish vegetation and accomplish effective cogongrass suppression. Usually, the injury symptoms caused by imidazolinone herbicides are stunted plants, shortened internodes, and yield reductions (Ulbrich et al. Reference Ulbrich, Souza and Shaner2005). Therefore, the verification of how plant species respond to imazapyr residues in soil would benefit restoration of many cogongrass-infested ecosystems in the southeastern United States (MacDonald et al. Reference MacDonald, Johnson, Shilling, Miller and Brecke2002).
Hurst (Reference Hurst1987) reported that legumes, and especially Lespedeza species, show tolerance to imazapyr. Shaner (Reference Shaner1989) reported that legume species were able to metabolize imazapyr to an inactive compound. Therefore, legume tolerance to imazapyr generated an interest for uses in conservation reserve programs and rights-of-way to control vegetation and promote the establishment of plants for wildlife food (Shaw et al. Reference Shaw, Watkins and Cole2001).
The objective of this study was to evaluate tolerance of selected legume species to soil-applied imazapyr at selected rates and various planting times after application. It was hypothesized that the residual impact of imazapyr would be detrimental to these species, and that delayed planting would improve plant development.
Materials and Methods
Experiments were conducted from 2013 through 2015 in a greenhouse located at the Mississippi State University R. R. Foil Plant Science Research Center in Starkville, Mississippi (33.47°N, 88.78°W). Longview silt loam (fine-silty, siliceous, thermic Aqueptic Fragiudalfs, Alfisols) topsoil was mixed with sand in a 2:1 proportion of sand:topsoil, which resulted in a loamy sand soil mixture with 86% sand, 11.5% silt, 2.5% clay, 1.0% organic matter, 8.1 CEC, and pH 6.6 (Mississippi State University Soil Testing Lab, Mississippi State, MS 39762). Round plastic containers of size 11 by 9 cm (diameter×height) (Thinwall Rounds, Dillen Products, Middlefield, OH 44062) were filled with 800 cm3 of this soil mix and surface-irrigated to soil water holding capacity. Imazapyr herbicide treatments of Arsenal® 2L (BASF Company, Research Triangle Park, NC 27709) at 0, 70, 140, and 280 g ae ha−1 were made with a compressed air spray chamber equipped with a single 8002EVS TeeJet® (Spraying Systems Company, Wheaton, IL 60189) flat-fan nozzle at an application rate of 234 L ha−1 and a pressure of 140 kPa. Soybean ‘AG4934’ Roundup Ready and Sulfonylurea tolerant (RR/STS) (Asgrow®, Monsanto Company, St. Louis, MO 63167), Korean lespedeza (Oktibbeha County Co-op., Starkville, MS 39759), crimson clover (Oktibbeha County Co-op.) and white clover ‘Durana’ (Rackmaster® Wildlife Seed; Pennington Seed Co., Madison, GA 30650) were planted at 0 (same day as the application), 1, 3, and 6 months after treatment (MAT). All pots were kept in the greenhouse until the plants were harvested. Approximately 100 ml of water was added on the surface of all pots, regardless of planting date, every 2 to 3 days, based on the response of untreated seedlings. Table 1 provides more information about planting dates following application.
Table 1 Exact dates on which the legumes were planted in each of the two experiments (A and B).
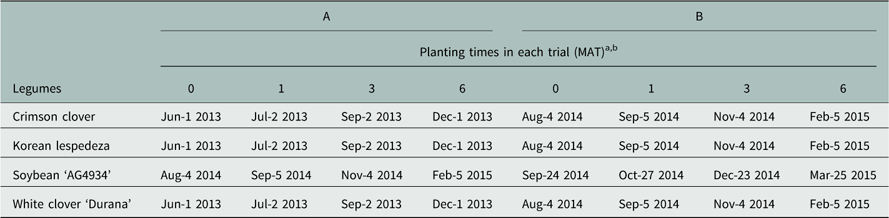
a Abbreviations: MAT, months after treatment; Feb, February; Jun, June; Aug, August; Jul, July; Sep, September; Nov, November; Dec, December.
b The dates which correspond to planting time 0 MAT also correspond to the dates which imazapyr was applied.
All treatment combinations had 15 seeds planted at a 1-cm depth and maintained at 25/30 C night/day temperature and natural light conditions. At 6 weeks after each planting, the number of emerged seedlings and average height were measured. Shoot biomass samples were collected by cutting all plants in each pot at soil level. Biomass samples were dried in a force-draft oven at 65 C for 4 days and then weighted. Biomass data were converted to a per plant basis prior to analysis. Furthermore, emergence, height, and shoot biomass reductions were calculated as percentage of the untreated at each planting date.
The experimental design was a four-by-four factorial arrangement of treatments by plant species in a randomized complete block design with four replications of each treatment combination. The experiment was replicated in time. Factor A was imazapyr rate and factor B was planting time. Data were analyzed with PROC GLIMMIX (Statistical Analysis Systems®, version 9.4; SAS Institute Inc., Cary, NC 27513) to determine differences between treatments and interactions between factors. Data were pooled across experimental replications because experimental replication was considered a random variable. Appropriate means were separated using least square means (LSMEANS) comparison with the PDIFF option, at α=0.05 significance level (SAS Institute Inc 2013).
Results and Discussion
Statistical analysis revealed a significant herbicide rate by planting time interaction for number of emerged seedlings, average height, and biomass per plant for each species. Therefore, a description of the results was made in separate tables.
Crimson Clover
Emergence of Seedlings
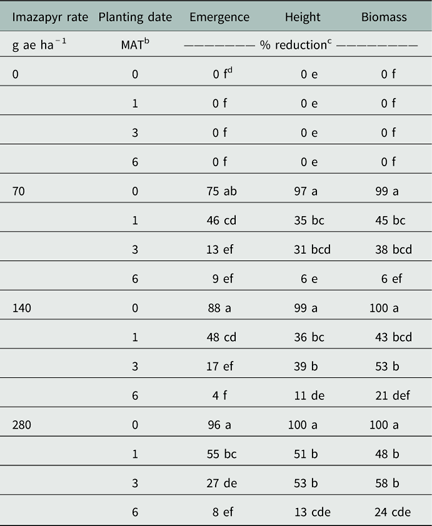
Treatment combinations of 70 and 140 g ae ha−1 of imazapyr planted 3 or 6 MAT, as well as treatments of 280 g ae ha−1 of imazapyr planted 6 MAT, did not reduce crimson clover emergence in comparison to untreated seedlings. Treatments of imazapyr rate at 70, 140, and 280 g ae ha−1 and planted 0 MAT reduced crimson clover emergence by 75%, 88%, and 96%, respectively. Seedlings planted 1 MAT with imazapyr level of 70, 140, and 280 g ae ha−1 reduced seedling emergence by 46%, 48%, and 55%, respectively. Treatments planted 3 MAT with imazapyr at 280 g ae ha−1 resulted in 27% reduction of emergence of crimson clover (Table 2).
Table 2 Crimson clover emergence, height, and biomass reductions as affected by imazapyr rate and planting date.Footnote a
a Data pooled over experiments, with differences detected using LSMEANS procedure and PDIFF option.
b Abbreviation: MAT, months after treatment.
c Means calculated as percent reduction compared to the untreated at each planting date.
d Means followed by same letter, within the same column, are not different from each other at 0.05 significance level.
Height Reduction
No height reduction was observed when crimson clover was planted 6 MAT for any imazapyr rate tested. Treatment combinations of imazapyr at 70, 140, and 280 g ha−1 and planted 0 MAT resulted in 97%, 99%, and 100% height reduction of seedlings, respectively. Treatments planted 1 and 3 MAT with imazapyr rate of 70 g ae ha−1 reduced seedling height by 35% and 31%, respectively. Similarly, when planting was delayed 1 and 3 MAT with imazapyr at 140 g ae ha−1, seedling height was reduced by 36% and 39%, respectively. Seedlings planted 1 and 3 MAT with imazapyr at 280 g ha−1 were 51% and 53% shorter than the nontreated, respectively (Table 2).
Biomass Reduction
Treatment combinations of imazapyr rates at 70 and 140 g ha−1 planted 6 MAT resulted in no change of biomass in crimson clover seedlings. Seedlings planted 0 MAT with imazapyr rates at 70, 140, and 280 g ae ha−1 resulted in 99%, 100%, and 100% biomass reduction, respectively. Seedling biomass was reduced by 45% and 38%, respectively, when treatments were planted 1 and 3 MAT with imazapyr at 70 g ae ha−1. Seedlings planted 1 and 3 MAT with imazapyr at 140 g ae ha−1 resulted in 43% and 53% reduction of biomass, respectively. Treatments planted 1 and 3 MAT with imazapyr rate at 280 g ae ha−1 reduced biomass of seedlings by 48% and 58%, respectively. Biomass was reduced by 24% when seedlings were planted 6 MAT with the higher rate of imazapyr (Table 2).
In general, data indicated a trend that if imazapyr application rates were 70 g ha−1 or higher, emergence and development of crimson clover increased when planting was delayed at least 1 MAT. In addition, seedlings planted 6 MAT with imazapyr rates between 70 and 280 g ha−1 had lower growth reductions in comparison to treatments planted earlier. However, differences were observed between planting dates when no imazapyr was applied. This indicated that differences in environmental conditions, such as photoperiod or temperature in the greenhouse, at different planting times likely affected crimson clover seedling emergence and development.
Differences were also observed between planting dates with respect to emergence and height reductions, but no differences were detected in biomass measurements. Although fewer plants emerged, those that did emerge produced more biomass per individual plant, which masked differences among treatments. This indicated that even though emergence failure occurred, seedlings that emerged had metabolized imazapyr and maintained similar biomass production. Therefore, planting of crimson clover should be delayed at least 1 month after applications of imazapyr from 70 to 280 g ae ha−1 to reduce emergence, height, and biomass reductions.
Korean lespedeza
Emergence of Seedlings
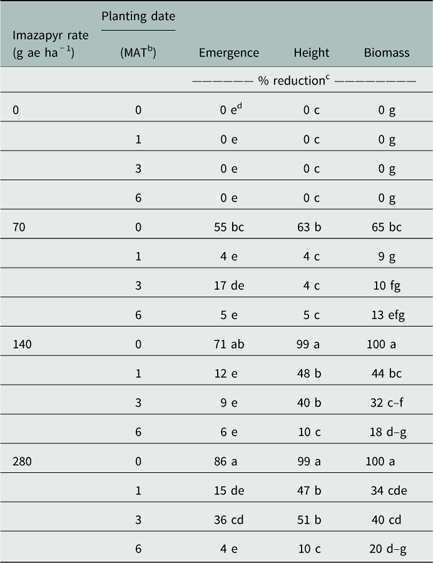
Treatment combinations seeded 1, 3, or 6 MAT with all levels of imazapyr resulted in no change in emergence of Korean lespedeza, except for seedlings planted 3 MAT with imazapyr at 280 g ha−1 (36% reduction in comparison to untreated). Seedlings planted 0 MAT with imazapyr at 70, 140, and 280 g ha−1 showed 55%, 71%, and 86% emergence reduction, respectively (Table 3).
Table 3 Korean lespedeza emergence, height, and biomass reductions as affected by imazapyr rate and planting date.Footnote a
a Data pooled over experiments, with differences detected using LSMEANS procedure and PDIFF option.
b Abbreviation: MAT, months after treatment.
c Means calculated as percent reduction compared to the untreated at each planting date.
d Means followed by same letter, within the same column, are not different from each other at 0.05 significance level.
Height Reduction
Treatment combinations planted 1, 3, or 6 MAT with imazapyr at 70 g ha−1 did not reduce height of seedlings. Similarly, seedlings planted 6 MAT with imazapyr at 140 or 280 g ha−1 showed no height reduction in comparison to untreated. Seedlings planted 0 MAT with imazapyr at 70, 140, and 280 g ha−1 reduced plant height by 63%, 99%, and 99%, respectively. Seedlings planted 1 and 3 MAT with imazapyr at 140 g ha−1 resulted in 48% and 40% height reduction, respectively. Furthermore, plants seeded 1 and 3 MAT with imazapyr at 280 g ha−1 showed 47% and 51% height reductions, respectively (Table 3).
Biomass Reduction
Treatment combinations planted 1, 3, or 6 MAT with imazapyr at 70 g ae ha−1, and seedlings planted 6 MAT with imazapyr at 140 or 280 g ae ha−1, resulted in no change in biomass production in comparison to untreated. Treatments planted 0 MAT with imazapyr at 70, 140, and 280 g ha−1, reduced biomass by 65%, 100%, and 100%, respectively. Plants seeded 1 and 3 MAT with imazapyr at 140 and 280 g ha−1 reduced biomass from 32% to 44% (Table 3).
Results indicated that if imazapyr was applied at 70 g ha−1 or higher, Korean lespedeza emergence and development increased if planting was delayed at least 1 MAT or more. However, differences were observed between planting times when no imazapyr was applied. This indicated that environmental conditions, such as temperature or photoperiod at different planting times, most likely affected seedling emergence and development. Therefore, if the establishment of Korean lespedeza is to follow imazapyr applications of 70 to 280 g ha−1, planting should be delayed at least 1 MAT to reduce stand loss and growth reduction.
Soybean
Emergence of Seedlings
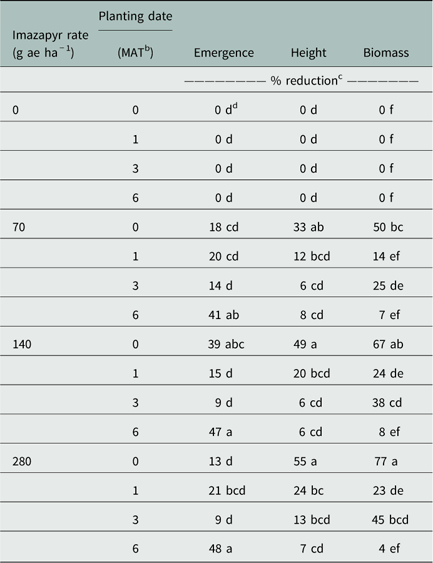
Treatment combinations planted 0, 1, or 3 MAT with imazapyr at 70 or 280 g ha−1, as well as treatments planted 1 or 3 MAT with imazapyr at 140 g ha−1, resulted in no change of soybean emergence. These results indicated a level of soybean tolerance to the rates tested. Seedlings planted 0 MAT with imazapyr at 140 g ha−1 showed 39% emergence reduction in comparison to the nontreated. Seedlings planted 6 MAT with imazapyr at 70, 140, and 280 g ha−1 resulted in 41%, 47%, and 48% emergence reduction, respectively (Table 4). These results may be related to the fact that at 6 MAT, light and temperature conditions in the greenhouse were not ideal for soybean development (December to February).
Table 4 Soybean emergence, height, and biomass reductions as affected by imazapyr rate and planting date.Footnote a
a Data pooled over experiments, with differences detected using LSMEANS procedure and PDIFF option.
b Abbreviation: MAT, months after treatment.
c Means calculated as percent reduction compared to the untreated at each planting date.
d Means followed by same letter, within the same column, are not different from each other at 0.05 significance level.
Height Reduction
Treatment combinations planted 1, 3, or 6 MAT with imazapyr at 70 or 140 g ha−1, as well as treatments planted 3 or 6 MAT with imazapyr at 280 g ha−1, resulted in no difference in seedling height. Soybean planted 1 MAT with imazapyr 280 g ha−1 showed 24% height reduction in comparison to the nontreated. Seedlings planted 0 MAT with imazapyr at 70, 140, and 280 g ae ha−1 resulted in 33%, 49%, and 55% emergence reduction, respectively (Table 4).
Biomass Reduction
Treatment combinations planted 6 MAT with imazapyr at 70, 140, or 280 g ae ha−1, as well as treatments planted 1 MAT with imazapyr at 70 g ae ha−1, resulted in no change in seedling biomass. Soybean seeded 0 MAT with imazapyr 70, 140, and 280 g ae ha−1 resulted in 50%, 67%, and 77% biomass reduction, respectively. Seedlings planted 1 or 3 MAT with imazapyr at 140 and 280 g ha−1, as well as soybean seeded 3 MAT with imazapyr at 70 g ha−1, reduced biomass from 23% to 45% (Table 4).
In general, when imazapyr applications were made at rates of 70 g ha−1 or higher, soybean development was improved when planting was delayed at least 1 MAT. However, differences were observed between planting times when no imazapyr was applied. This indicated that environmental conditions, such as greenhouse temperature and photoperiod, at different planting dates probably influenced seedling development. Therefore, planting of soybeans should be delayed at least 1 month after application of imazapyr at rates of 70 to 280 g ha−1 to minimize height and biomass reductions. Other imidazolinone herbicides, such as imazamox and imazethapyr, are labeled for postemergence broadleaf and grass weed control in soybean (Shaner Reference Shaner2014). However, these herbicides might still injure the crop. According to Nelson et al. (Reference Nelson, Renner and Penner1998), soybean injury from imazamox at 35 g ai ha−1 and imazethapyr at 70 g ai ha−1 was 16% and 14%, respectively.
White Clover
Emergence of Seedlings
Treatment combinations planted 1, 3, or 6 MAT with imazapyr at 70 or 140 g ha−1, as well as treatments planted 1 or 6 MAT with imazapyr at 280 g ha−1, resulted in no difference in seedling emergence. White clover planted 0 MAT with imazapyr at 70, 140, and 280 g ha−1 reduced emergence by 84%, 97%, and 96%, respectively. Seedlings planted 3 MAT with imazapyr at 280 g ha−1 had 32% emergence reduction in comparison to the nontreated (Table 5).
Table 5 White clover emergence, height, and biomass reductions as affected by imazapyr rate and planting date.Footnote a

a Data pooled over experiments, with differences detected using LSMEANS procedure and PDIFF option.
b Abbreviation: MAT, months after treatment.
c Means calculated as percent reduction compared to the untreated at each planting date.
d Means followed by same letter, within the same column, are not different from each other at 0.05 significance level.
Height Reduction
No change in height of plants was observed when comparing treatment combinations planted 6 MAT with imazapyr at 70 or 140 g ha−1. White clover seeded 0 MAT with imazapyr at 70, 140, and 280 g ha−1, resulted in 95%, 100%, and 99% height reduction, respectively. Seedlings planted 1 and 3 MAT with imazapyr at 70 and 140 g ha−1, as well as treatments seeded 1, 3, and 6 MAT with imazapyr at 280 g ha−1, reduced plant height from 27% to 48% in comparison to the nontreated (Table 5).
Biomass Reduction
Treatment combinations planted 6 MAT with imazapyr at 70 or 140 g ha−1 resulted in no difference in biomass production. White clover planted 0 MAT with imazapyr at 70, 140, or 280 g ae ha−1 resulted in 100% biomass reduction in all treatments. Treatment combinations planted 1 and 3 MAT with imazapyr at 70 or 140 g ha−1, as well as treatments planted 1, 3, and 6 MAT with imazapyr at 280 g ha−1, reduced plant biomass from 32% to 45% (Table 5).
In general, when imazapyr levels were 70 g ha−1 or higher, white clover emergence and development improved when seeds were planted 1 MAT or later. However, differences were observed between planting times when no imazapyr was applied. This indicated that environmental conditions, such as photoperiod or temperature, at different planting times likely affected seedling emergence and development. Therefore, planting of white clover should be delayed at least 1 MAT with imazapyr from 70 to 280 g ha−1 to reduce emergence, height, and biomass reductions.
According to Franklin (Reference Franklin2009), light and temperature are two of the most important environmental stimuli that regulate plant development. Because Lespedeza and soybean are warm season legumes and the clover species are cool season, the ideal period to establish these plants would have been in spring and fall, respectively (Ball et al. Reference Ball, Hoveland and Lacefield2007). We hypothesized that timely planting for each respective crop would have minimized the observed differences among planting dates when the imazapyr rate was 0 g ha−1.
Our results were comparable to those of Bovey and Senseman (Reference Bovey and Senseman1998), who found that several forage grasses and herbs were affected by imazapyr carryover, resulting in significant biomass reductions. Shaw et al. (Reference Shaw, Watkins and Cole2001) reported that legume species have shown tolerance to imazapyr and imazapic, but also warned about the fact that planting timing is important. Ulbrich et al. (Reference Ulbrich, Souza and Shaner2005) successfully utilized a bioassay with cucumber (Cucumis sativus L.) to evaluate safe planting interval after imazapic plus imazapyr applications in two locations in southern Brazil. They found much faster soil dissipation time of imidazolinones under subtropical conditions compared to temperate regions. In addition, imidazolinone-tolerant crops could be part of the management system when utilizing imazapyr treatments to cogongrass, without the need to postpone planting time, because the carryover effect would not injure these crops (Burns Reference Burns2006).
Therefore, the hypothesis that imazapyr rate and planting dates significantly influenced seedling development of crimson clover, Korean lespedeza, soybean, and white clover was accepted. In conclusion, when utilizing imazapyr from 70 to 280 g ha−1 for weed management, recommendations about delayed planting at least 1 MAT should be made to reduce emergence failure and height and biomass reductions of legumes used for revegetation.
Acknowledgments
The authors wish to thank the Mississippi Department of Transportation and the Mississippi Agriculture and Forestry Experiment Station for partial support of this research. We would like to thank Debbie Boykin with the US Department of Agriculture Agricultural Research Service for help with statistical analysis.







