Introduction
Canada is the world leader in flax production and exports (Flax Council of Canada 2016). Effective weed management is still challenging for flax growers, as weeds can reduce flax yield by as much as 20% (Friesen Reference Friesen1986; Stevenson and Wright Reference Stevenson and Wright1996). At today’s current market prices this would equate to a $136 CAD loss per hectare (Agriculture and Agri-food Canada 2018; Statistics Canada 2018). It is important, therefore, to maintain a weed-free environment during the early stages of crop growth to help maximize crop yield potential.
Of the eight most troublesome weeds in Canada (WSSA 2017), six of these species are also considered the most problematic weeds in flax production. These include wild oat (Avena fatua L.), wild buckwheat [Fallopia convolvulus (L.) Á. Löve], redroot pigweed (Amaranthus retroflexus L.), Canada thistle [Cirsium arvense (L.) Scop.], green foxtail [Setaria viridis (L.) P. Beauv.], and common lambsquarters (Chenopodium album L.) (Flax Council of Canada 2016; Leeson et al. Reference Leeson, Thomas, Hall, Brenzil, Andres, Brown and Van Acker2005; Zollinger Reference Zollinger2016). Despite the recurrent yield losses associated with these weedy species, there remains a lack of herbicides registered to control them in flax crops. Currently, only Group 1 (acetyl-CoA carboxylase inhibitors), Group 4 (synthetic auxins), and Group 6 (photosystem II [PSII] inhibitors) herbicides are registered for POST in-crop weed control.
Beckie et al. (Reference Beckie, Lozinski, Shirriff and Brenzil2013) estimated that 7.7 million hectares in Alberta, Saskatchewan, and Manitoba contain weeds resistant to Group 1, 2, or/and 8 herbicides. The presence of Group 1–resistant wild oat and green foxtail are especially challenging for flax growers. Registration of new herbicides for flax production would help in the management of resistant weeds and, with appropriate mixes, could help to delay the evolution of future cases of resistance.
Herbicides such as fluthiacet-methyl (Group 14, POST), flumioxazin (Group 14, PRE), pyroxasulfone (Group 15, PRE), and topramezone (Group 27, POST) all control multiple weed species and provide novel sites of action that are not widely used in crop production on the Canadian prairies. Fluthiacet-methyl and flumioxazin are traditionally used for control of broadleaf weeds in corn (Zea mays L.) and soybean [Glycine max (L.) Merr.] (Senseman Reference Senseman2007; Taylor-Lovell et al. Reference Taylor-Lovell, Wax and Bollero2002). Pyroxasulfone (PRE) and topramezone (POST) can control both grassy and broadleaf weed species in corn (Anonymous 2012; Gitsopoulos et al. Reference Gitsopoulos, Melidis and Evgenidis2010; Government of Saskatchewan 2016). Tolerance of crops to topramezone, pyroxasulfone, fluthiacet-methyl, and flumioxazin has been largely attributed to rapid metabolism of these herbicides (Grossmann and Ehrhardt Reference Grossmann and Ehrhardt2007; Price et al. Reference Price, Pline, Cranmer and Danehower2004; Senseman Reference Senseman2007; Shimizu et al. Reference Shimizu, Hashimoto, Nakayama, Nakao, Mizutani, Unai, Yamaguchi and Abe1995; Tanetani et al. Reference Tanetani, Ikeda, Kaku, Shimizu and Matsumoto2013). Furthermore, susceptibility to topramezone has been related to the sensitivity of the target enzyme, as is the case in corn (Grossmann and Ehrardt Reference Grossmann and Ehrhardt2007). The selectivity of fluthiacet-methyl and flumioxazin is based on differences in uptake and translocation of the herbicide between plant species (Price et al. Reference Price, Pline, Cranmer and Danehower2004; Shimizu et al. Reference Shimizu, Hashimoto, Nakayama, Nakao, Mizutani, Unai, Yamaguchi and Abe1995). Because tolerance to these herbicides is largely attributed to the plant’s ability to metabolize, or “deactivate” the herbicide, there is potential to use these herbicides in flax.
Flax demonstrates plasticity during growth and development in response to the surrounding environment. Specifically, Diederichsen and Richards (Reference Diederichsen, Richards, Muir and Westcott2003) noted that flax can recover from herbicide damage through the production of apical branches. Therefore, it is plausible that the underlying driver of this physiological response in flax is its metabolism. It is possible that fluthiacet-methyl, flumioxazin, pyroxasulfone, and topramezone could be safely used in flax. However, none of these products has been tested for use in flax production. The objective of this study was to evaluate the tolerance of flax to topramezone, pyroxasulfone, flumioxazin, and fluthiacet-methyl applied alone, as well as in mixture with currently registered herbicides.
Materials and methods
Experimental location and design
Field experiments were conducted in 2015 and 2016 at three locations across western Canada. The Kernen Research Farm (52.2°N, 106.5°W) and the Goodale Research Farm (52.1°N, 106.5°W) are located near Saskatoon, SK. One site was located in Manitoba at the Ian N. Morrison Crop Research Farm in Carman, MB (49.5°N, 98.0°W). Most of the sites were located on Black Chernozemic soils. Carman (MB) is located on a Gleyed Black, and Kernen (SK) on a Black Chernozem. The soil at Goodale (SK) was a Dark Brown Chernozemic loam. The pH and soil organic matter content at each location can be found in Table 1. The trial was seeded with the flax variety ‘CDC Glas’ (Booker et al. Reference Booker, Rowland and Rashid2014) at a target rate of 800 seeds m−2. CDC Glas is a popular flax variety in western Canada (Flax Council of Canada 2016). Plots at Kernen were 2-m wide by 6 m-long, while plots at Carman were 2-m wide by 8-m long. Border plots were seeded on both sides of the trial at all locations.
Table 1. Soil classification, soil descriptions, and rate of nitrogen, phosphorous, and sulfur applied at Kernen Crop Research Farm (Saskatoon, SK), Goodale Research Farm (Saskatoon, SK), and Ian N. Morrison Research Farm (Carman, MB).

a Abbreviation: OM, organic matter.
b In 2015 and 2016, 14 kg ha−1 of 11–52–0 was applied with the seed. Nitrogen (56 kg N ha−1) was applied as a liquid formulation (28–0–0) before seeding in 2015 and 2016.
c In 2015, 56 kg ha−1 of 16–20–0–14 and 139 kg ha−1 of 40–0–0 were applied. In 2016, 84 kg ha−1 of 16–20–0–14 and 129 kg ha−1 of 46–0–0 were applied.
d In 2016, 14 kg ha−1 of 11–52–0 was applied with the seed. Nitrogen (67 kg N ha−1) and sulfur (13 kg S ha−1) were applied as a liquid formulation before seeding.
The trial was established on fallow and was maintained weed free for the duration of the growing season. Before seeding, glyphosate (675 g ae ha−1) was applied to control emerged weeds. A tractor-mounted sprayer equipped with Turbo TeeJet® 110015 nozzles (TeeJet Technologies, P.O. Box 7900 Wheaton, IL 60187-7901 USA) calibrated to deliver 100 L ha−1 at 275 kPa was used to apply both the preseed burnoff and the treatments at the Saskatoon locations. For overspray of the entire trial at Carman, a sprayer equipped with XR TeeJet® 8002 tips was calibrated to deliver 111.25 L ha−1 at 262 kPa. At Carman, a hand boom sprayer equipped with TeeJet® 80015 XR sprayer tips calibrated to deliver 100 L ha−1 at 275 kPa was used to apply all treatments.
Fertilizer requirements at all sites were determined via preseeding soil tests with a yield goal of 2,200 kg ha−1. Details on soil type and descriptions and fertilizer rates applied at each site-year are presented in Table 1. The trial was seeded with a planter equipped with disk openers on 19-cm row spacing at Carman, while a box drill with hoe openers on 23-cm row spacing was used at the Saskatoon locations. A summary of field operations is presented in Table 2.
Table 2. Field operation for flax herbicide trials at the Kernen Crop Research Farm (Saskatoon, SK), Goodale Research Farm (Saskatoon, SK), and Ian N. Morrison Research Farm (Carman, MB) in 2015 and 2016.

Experimental procedure
The trial was designed as a randomized complete block with four replications, with a total of 18 treatments (Table 3). Treatments consisted of seven herbicides not currently registered for flax production applied at 1X and 2X rates. Three registered herbicides were included as industry standards for comparison, and all treatments were compared to the nontreated control. Treatments were applied either PRE or POST based on label recommendations for other crops. PRE treatments were applied 5 to 7 d before seeding. All POST treatments were applied when the flax was 5- to 10-cm tall.
Table 3. Herbicide common name, herbicide group, herbicide concentration, herbicide rate, application timing, surfactant/adjuvant used, and adjuvant rate for the flax tolerance trials at Kernen Crop Research Farm (Saskatoon, SK), Goodale Research Farm (Saskatoon, SK), and Ian N. Morrison Research Farm (Carman, MB) in 2015 and 2016.

a NIS, nonionic surfactant. Merge® = 50% solvent/50% surfactant blend. BASF Canada Inc.
b Industry standards.
Flax was monitored throughout the growing season for any symptoms of herbicide injury, including reductions in stand variability, chlorosis, and stunting. Crop damage (phytotoxicity) was assessed using the CWSS–SCM rating scale for crop tolerance with comparisons to the nontreated plots (Canadian Weed Science Society 2018). This rating scale is a percentage scale ranging from 0% to 100%. In cases where crop tolerance is being evaluated, the scale is largely focused on the range of 0% to 30%. Phytotoxicity was rated at three separate time intervals: 7 to 14, 21 to 28, and 56+ d after treatment (DAT).
In addition to phytotoxicity ratings, flax population, height, yield, and thousand-seed weight (TSW) were measured. Flax population was determined by counting the total number of seedlings in two 1-m paired rows in the front and back of each plot 2 to 3 wk after crop emergence. Flax height was assessed on five random plants in each plot once the crop had reached the mid- to late boll stage. The trial was harvested when the crop was at harvest maturity. Flax seed yield was collected using a plot combine at all locations. Samples were cleaned and weighed to determine flax yield and TSW. At both Saskatoon locations, TSW was determined by counting and weighing 1,000 individual seeds, while at Carman, 250 seeds were counted, weighed, and multiplied by 4 to determine TSW.
Statistical analysis
Linear mixed models were constructed using the MIXED model procedure of SAS v. 9.4 (SAS/STAT). Residuals were initially tested for normality with the UNIVARIATE procedure, while homogeneity of error variance was confirmed using the Shapiro-Wilk test in SAS (SAS/STAT). Crop population, crop height, yield, and TSW were all analyzed using PROC MIXED with a normal distribution, because the residual data were normally distributed. Herbicide treatments were included as fixed effects in the model, while site, replication (nested within site), and their interactions with fixed effects were treated as random effects. Random effects and their interactions with herbicide treatments (fixed effect) were assessed with a COVTEST to determine whether site-years could be combined for analysis (SAS/STAT). A Dunnett’s test was used to compare the means of all treatments to the nontreated control for crop population, crop height, yield, and TSW. The calculated minimum significant difference (MSD) was used to determine whether the mean of the treatment significantly differed from the nontreated control (α = 0.05).
Data that were collected for phytotoxicity ratings are a proportion and are often skewed (Bowley Reference Bowley2015). Such data need to be analyzed using a different distribution in the PROC GLIMMIX procedure. A beta distribution with a log-link function provided the best fit for analyses. To conduct the beta analysis, all data were converted from percentages into decimal fractions. Because the beta distribution is between 0 and 1, any values equal to 0 were changed to 0.0001, and all values of 1 were changed to 0.9999 to account for the restrictions of the model. Data were back-transformed for presentation of results.
Results and discussion
Covariance analysis showed that site data could not be combined across all site-years due to significant interaction between site-year and herbicide treatment (unpublished data). In addition, crop phytotoxicity also varied between sites in all years (unpublished data). Therefore, data were analyzed within site but across years for all response variables.
Crop phytotoxicity
Treatments applied PRE caused the greatest crop damage at Carman in both 2015 and 2016. Pyroxasulfone, pyroxasulfone + sulfentrazone, and flumioxazin caused unacceptable (10% to 30%), and in some cases severe (>30%), crop damage when evaluated at 7 to 14 and 21 to 28 d after treatment (Table 4). Crop damage was less severe and less frequent at Kernen in 2015 and 2016 and at Goodale in 2016. Because these were soil-applied herbicides, symptoms of injury included plant stand reduction, delayed emergence, and stunting. Flumioxazin consistently caused the greatest amount of crop injury at Carman and was always severe. On average, treatments containing flumioxazin caused 90% crop injury at 7 to 14 d after treatment at both low and high rates and in both site-years. Crop damage subsided over time for all treatments (<10%), except for flumioxazin, which continued to cause injury ranging from 66% and 93% at the 1X and 2X rates at Carman in 2016. In 2015 at Carman, all PRE treatments continued to cause severe (>30%) crop injury at 21 to 28 DAT. Crop recovery continued past 56 DAT, and initial damage subsided substantially for the majority of treatments in both site-years. However, the crop continued to exhibit symptoms of severe crop injury ranging between 26% and 82% as a result of flumioxazin (unpublished data).
Table 4. Mean flax visual phytotoxicity ratings and SEs (0%–100% scale) at 7 to 14 and 21 to 28 d after treatment (DAT) at Kernen Crop Research Farm (Saskatoon, SK), Goodale Research Farm (Saskatoon, SK), and Ian N. Morrison Research Farm (Carman, MB) in 2015 and 2016. a

a Values derived from beta-analysis of visible ratings at individual site-years. Any rating below 10% is considered acceptable crop damage, between 10% and 30% is considered unacceptable injury. Ratings above 30% are considered severe injury and are bolded.
b Abbreviations: Flum, flumioxazin; FluMCP, fluthiacet-methyl + MCPA; Fluth, fluthiacet-methyl; Pyrox, pyroxasulfone; PyrSft, pyroxasulfone + sulfentrazone; SFT, sulfentrazone; Top, topramezone; TopBx, topramezone +bromoxynil.
At Kernen, pyroxasulfone and flumioxazin caused less injury than at Carman. Treatments containing pyroxasulfone did not cause unacceptable injury at 7 to 14 DAT in 2015 and 2016. However, unacceptable damage in 2016 was caused by both the 1X and 2X rates of flumioxazin (12% and 17%, respectively) at 7 to 14 DAT. At the later evaluation timing at Kernen in 2016, 21 to 28 DAT, pyroxasulfone, pyroxasulfone + sulfentrazone, and flumioxazin continued to cause unacceptable or severe crop injury, ranging between 13% and 51%, with fumioxazin causing the greatest crop injury. PRE treatments did not cause any unacceptable crop damage at Goodale.
While several of the PRE herbicide treatments caused unacceptable and severe injury, fluthiacet-methyl (POST) and topramezone (POST) showed acceptable crop safety in both years at all locations with only a few exceptions. At Kernen in 2016, fluthiacet-methyl caused 15% and 22% damage at the 1X and 2X rates, respectively, at 7 to 14 DAT. Damage from the mixture of topramezone + bromoxynil was not consistent among site-years and resulted in severe injury at Kernen and Goodale in 2016. At Goodale, POST treatments containing fluthiacet-methyl + MCPA and topramezone + bromoxynil at both high and low rates caused unacceptable crop damage at 7 to 14 DAT, with injury ranging between 47% and 81%. Crop injury from POST treatments was transient for most treatments and began to subside by 21 to 28 DAT in both site-years. Treatments containing fluthiacet-methyl + MCPA continued to cause 13% to 28% crop injury at Kernen in both site-years and 29% to 60% injury at Goodale. All POST herbicide treatments were given acceptable crop injury ratings by 56 DAT (unpublished data).
Differences in environmental conditions and soil characteristics may explain why PRE herbicides such as pyroxasulfone, pyroxasulfone + sulfentrazone, and flumioxazin caused unacceptable crop injury, particularly when spring moisture conditions were conducive for high herbicidal activity (Table 5). Under dry conditions, efficacy of PRE herbicides declines (Jursík et al. Reference Jursík, Soukup, Holec, Andr and Hamouzová2015). While PRE herbicides require moisture for activation and plant uptake (Stewart et al. Reference Stewart, Nurse, Hamill and Sikkema2010), under very high moisture conditions there is an increased risk of crop injury from soil-applied herbicides (Jursík et al. Reference Jursík, Soukup, Holec, Andr and Hamouzová2015). At Kernen, precipitation in May was 94% lower and 14% higher than the long-term average in 2015 and 2016, respectively. The lack of spring precipitation at Kernen in 2015 explains why there was minimal injury caused by PRE treatments. In 2016 at Kernen and Goodale, precipitation was near normal and, as such, there was greater activation of pyroxasulfone, sulfentrazone, and flumioxazin. In contrast, spring precipitation events at Carman were 42% and 55% higher than the long-term average in 2015 and 2016, respectively. Consequently, PRE herbicides caused the greatest amount of crop injury at this location as a function of spring moisture conditions.
Table 5. Mean monthly temperature and precipitation data at the Kernen Crop Research Farm (Saskatoon, SK), Goodale Research Farm (Saskatoon, SK), and Ian N. Morrison Research Farm (Carman, MB) in 2015 and 2016.

a Long-term average (1981–2010) temperature and precipitation recorded at nearby weather stations: http://climate.weather.gc.ca/climate_normals/index_e.html#1981.
Flumioxazin efficacy is highly influenced by soil organic matter, soil texture, and soil moisture (Sebastian et al. Reference Sebastian, Nissen, Westra, Shaner and Butters2017). The herbicide binds tightly to soil colloids and will not readily disassociate without adequate soil moisture (Alister et al. Reference Alister, Rojas, Gómez and Kogan2008; Sebastian et al. Reference Sebastian, Nissen, Westra, Shaner and Butters2017). Hence, in years of drought or low soil moisture, flumioxazin will remain inert in the soil, which results in little crop damage and reduced weed control. Furthermore, flumioxazin has been found to be readily adsorbed in soils with a high clay and organic matter content (Alister et al. Reference Alister, Rojas, Gómez and Kogan2008). The soil at Carman had an organic matter content of 6% and consisted of 31% clay. These soil characteristics combined with high-rainfall events in the months of May and June may have created conditions conducive to herbicide persistence in the soil (Table 5). This could explain why flumioxazin, and potentially other PRE herbicides, caused such severe damage at Carman compared with other locations, where injury tended to be minimal.
Flax population, height, and yield
Flax population and height were not affected by any treatment at Kernen in 2015 or 2016, regardless of observed phytotoxicity (Figure 1; Table 4). In contrast, flax population was reduced by several of the PRE herbicides at Carman. Pyroxasulfone (2X rate) and pyroxasulfone + sulfentrazone (1X rate) reduced flax populations in 2015 by 246 and 186 plants m−2, respectively. Furthermore pyroxasulfone + sulfentrazone applied at the 2X rate and flumioxazin applied at the 1X and 2X rates reduced flax population on average by 233 plants m−2 in both site-years. In 2016, fluthiacet-methyl + MCPA applied at the 2X rate and bromoxynil + MCPA both reduced flax populations by an average of 54 plants m−2 at Carman. The reduction in plant population observed for these PRE treatments at Carman can be attributed to the severe phytotoxic damage observed for these treatments at Carman (Table 4). Topramezone + bromoxynil (2X rate) significantly reduced flax population by 194 plants m−2 at the Goodale site. However this reduction in flax population did not influence crop height (unpublished data).
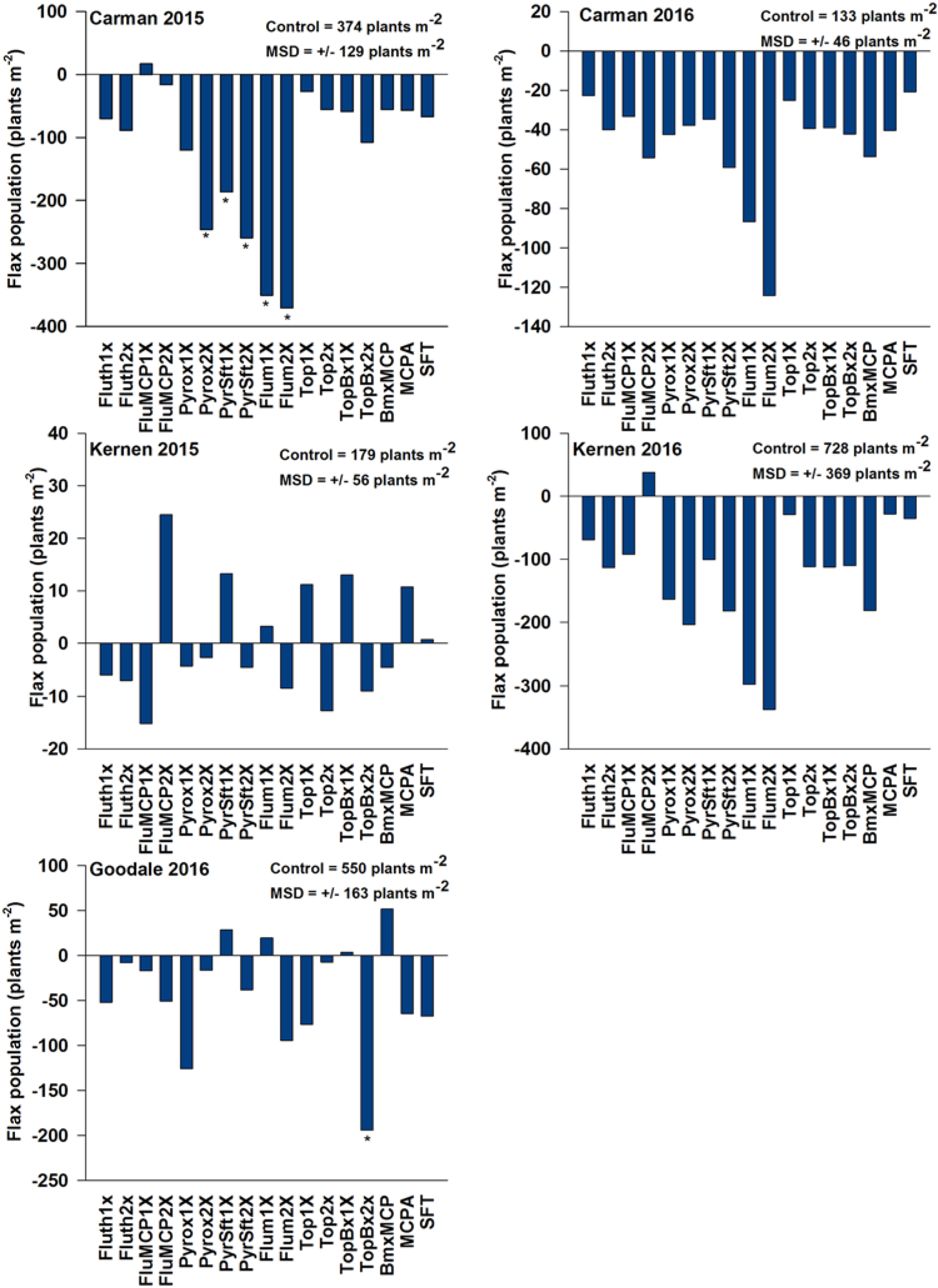
Figure 1. Effect of herbicide treatment on flax population (plants m−2) relative to the nontreated control. The Dunnett’s test was used to separate treatment means. An asterisk (*) below bars indicates a minimum significant difference (MSD) from the nontreated control. Abbreviations: BmxMCP, bromoxynil + MCPA; Flum, flumioxazin; FluMCP, fluthiacet-methyl + MCPA; Fluth, fluthiacet-methyl; Pyrox, pyroxasulfone; PyrSft, pyroxasulfone + sulfentrazone; SFT, sulfentrazone; Top, topramezone; TopBx, topramezone + bromoxynil. Kernen Crop Research Farm (Saskatoon, SK), Goodale Research Farm (Saskatoon, SK), and Ian N. Morrison Research Farm (Carman, MB).
At Carman, crop height and yield were impacted by several treatments. Topramezone + bromoxynil did not affect flax population or yield (Figure 2) at Carman; however, this combination reduced flax height by 7 cm in 2015 (unpublished data). Fluthiacet-methyl + MCPA also reduced crop height by an average of 4 cm. Likewise, flumioxazin treatments reduced crop height by 17 cm in 2015 and 15 cm in 2016. Flumioxazin was the only treatment to result in less yield then the control at Carman, and this only occurred in 2015 (Figure 2). In contrast, applications of flumioxazin and pyroxasulfone actually increased crop height by more than 5 cm at Kernen. Regardless of initial crop injury or developmental impairment, no treatment significantly affected crop yield at Kernen (2015, 2016) or Goodale (2016) (Figure 2). Similarly, despite treatments having varied effects on plant population and crop height, no treatment significantly impacted yield at Carman in 2016, as yields were generally quite low regardless of treatment (Figure 2). TSW was also not impacted by treatments in all site-years (unpublished data).
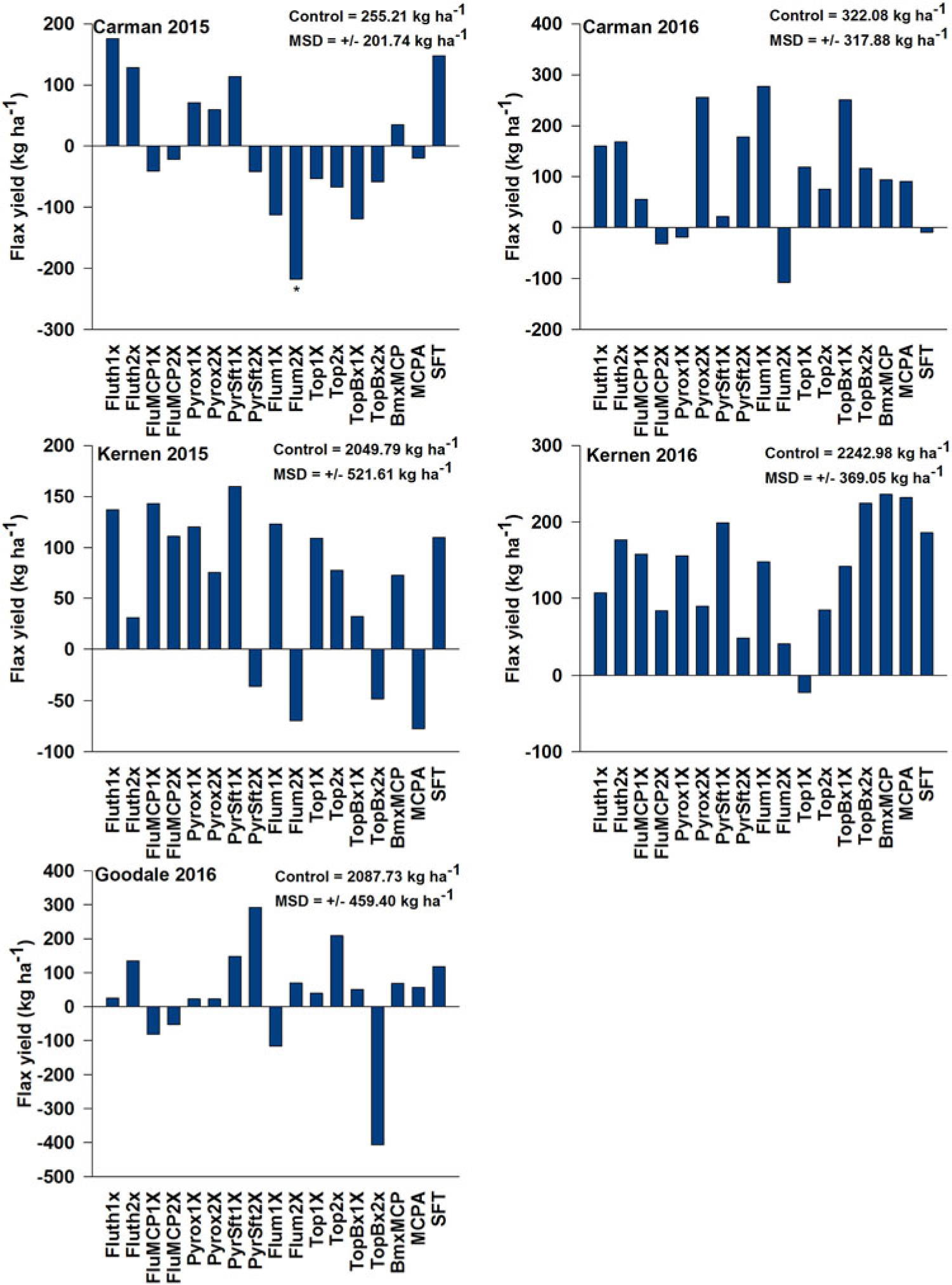
Figure 2. Effect of herbicide treatment on flax yield (kg ha−1) relative to the untreated check. Values were derived from yield of the entire plot, and a Dunnett’s test was used to separate treatment means. An asterisk (*) below the bar indicates a significant deviation from the nontreated control by more than the indicated minimum significant difference (MSD). Abbreviations: BmxMCP, bromoxynil + MCPA; Flum, flumioxazin; FluMCP, fluthiacet-methyl + MCPA; Fluth, fluthiacet-methyl; Pyrox, pyroxasulfone; PyrSft, pyroxasulfone + sulfentrazone; SFT, sulfentrazone; Top, topramezone; TopBx, topramezone + bromoxynil. Kernen Crop Research Farm (Saskatoon, SK), Goodale Research Farm (Saskatoon, SK), and Ian N. Morrison Research Farm (Carman, MB).
Our results show that flax is tolerant to several potential new herbicides. Specifically, fluthiacet-methyl, pyroxasulfone, and topramezone showed the greatest potential to be registered in flax, as they did not negatively affect flax through phytotoxicity or by reducing density, height, or yield in the majority of site-years. Moreover the potential to apply these herbicides both alone and in a mix can greatly benefit in-crop weed management. For instance, the efficacy of fluthiacet-methyl can be enhanced through the addition of other herbicides into a mix. Studies have shown that fluthiacet-methyl can be mixed with 2,4-D, bromoxynil, or mesotrione (Group 27) to improve efficacy and weed control of problematic species such as glyphosate-resistant kochia [Bassia scoparia (L.) A.J. Scott] and common waterhemp [Amaranthus tuberculatus (Moq.) J.D. Sauer] (Ganie et al. Reference Ganie, Straman and Jhala2015; Reddy et al. Reference Reddy, Stahlman, Geier, Bean and Dozier2014). As such, because flax has acceptable tolerance to fluthiacet-methyl, there is potential for this herbicide to be applied in a mix to broaden the spectrum of weeds controlled in the field.
While some herbicide mixtures act synergistically to improve weed control, there is also the potential for antagonistic interactions to occur between herbicides (Liu et al. Reference Liu, Hsiao, Quick, Wolf and Hume1995; O’Sullivan et al. Reference O’sullivan, Friesen and Vanden Born1977; Qureshi and Vanden Born Reference Qureshi and Vanden Born1979). In winter wheat (Triticum aestivum L.), mixes of dicamba + MCPA + mecoprop caused notable crop injury, which in turn reduced crop height and crop yield (Sikkema et al. Reference Sikkema, Brown, Shropshire and Soltani2007). Similarly, Reddy et al. (Reference Reddy, Stahlman, Geier, Bean and Dozier2014) observed that while the addition of 2,4-D to fluthiacet-methyl increased weed control efficacy, it also damaged sorghum [Sorghum bicolor (L.) Moench] and negatively impacted crop yield. We observed similar results, as MCPA mixed with fluthiacet-methyl resulted in significant crop injury. In some instances, this injury further impeded normal crop growth and development. Moreover, this synergism can reduce the phytotoxic effects these herbicides have on weeds. As such, even though MCPA is registered for use in flax, combining MCPA with fluthiacet-methyl cannot be recommended due to the severe foliar injury observed in this study.
Treatments containing topramezone often produced phytotoxicity ratings lower than the industry standards in this study. These results demonstrate that topramezone is as safe as products currently registered for use in flax. However, when topramezone was combined with bromoxynil, some crop injury was observed. Nevertheless, this injury was not consistent across site-years, and the crop recovered with no significant reduction in yield or TSW. Additive interactions between 4-hydroxphenylpyruvate dioxygenase (HPPD; Group 27) and PSII (Group 6) inhibitors have been reported previously between another HPPD inhibitor (pyrasulfotole) and several PSII-inhibiting herbicides (Freigang et al. Reference Freigang, Laber, Lange and Schulz2008). Freigang et al. (Reference Freigang, Laber, Lange and Schulz2008) further suggested that when using an HPPD inhibitor and a PSII inhibitor, the PSII herbicide can have increased efficacy at much lower concentrations. This may partially explain the synergistic effects that we observed, as we applied the full rate of topramezone and bromoxynil together.
Pyroxasulfone was one of the PRE herbicides that did not cause severe crop injury in all site-years. Despite any initial injury or reduction in flax density at most sites, pyroxasulfone did not significantly reduce crop height, yield, or TSW. While pyroxasulfone can control a broad range of weed species, efficacy can be largely influenced by site-year and environmental conditions (Tidemann et al. Reference Tidemann, Hall, Johnson, Beckie, Sapsford and Raatz2014). For example, pyroxasulfone had the greatest control of Italian ryegrass (Lolium perenne L. spp. multiflorum (Lam.) Husnot.) during wet growing conditions, whereas control was more variable during drier years (Hulting et al. Reference Hulting, Dauer, Hinds-Cook, Curtis, Koepke-Hill and Mallory-Smith2012). In addition, fields with higher soil organic matter require a higher dose of pyroxasulfone to achieve effective weed control, which can contribute to the variability in weed control between site-years (King and Garcia Reference King and Garcia2008; Nurse et al. Reference Nurse, Sikkema and Robinson2011). Unlike the addition of MCPA to fluthiacet-methyl or bromoxynil to topramezone, the addition of sulfentrazone to pyroxasulfone did not increase crop injury in our study. Similar effects have been observed by Niekamp et al. (Reference Niekamp, Johnson and Smeda1999) in soybean, where mixes of sulfentrazone + chlorimuron and sulfentrazone + imazaquin provided greater control of several broadleaved weed species than applications of sulfentrazone alone. Olson et al. (Reference Olson, Zollinger, Thompson, Peterson, Jenks, Moechnig and Stahlman2011) have shown that combining pyroxasulfone and sulfentrazone improved control of foxtail barley (Hordeum jubatum L.), prostrate pigweed (Amaranthus blitoides S. Watson), and wild buckwheat in sunflower (Helianthus annuus L.). While these products did provide effective weed control, crop injury was initially observed but subsided to acceptable levels by 7 wk after planting. Similar to our study, mixing pyroxasulfone with sulfentrazone did cause initial unacceptable crop injury, but the crop recovered over time without suffering a yield penalty. Therefore, because mixtures with sulfentrazone can improve the control of problematic broadleaf weeds, this combination could be a viable option for flax producers in the future.
Fluthiacet-methyl, pyroxasulfone, and topramezone offer more diversity for chemical weed control, because they represent three different modes of action that are not widely used in western Canada. Moreover, herbicides such as fluthiacet-methyl present the opportunity to control weedy species that have developed resistance to glyphosate (Sarangi et al. Reference Sarangi, Sandell, Knezevic, Aulakh, Lindquist, Irmak and Jhala2015). Mixes of fluthiacet-methyl + mesotrione have shown promise in controlling seedlings of glyphosate-resistant waterhemp and kochia (Ganie et al. Reference Ganie, Straman and Jhala2015). Pyroxasulfone has been shown to selectively control herbicide-resistant rigid ryegrass (Lolium rigidum Gaudin) populations in various crop types in Australia (Bajwa et al. Reference Bajwa, Walsh and Chauhan2017). Improved weed control in soybean has also been observed through mixing pyroxasulfone + sulfentrazone, as the combination of these two herbicides had a synergistic effect on weed control in soybean across multiple soil types (Belfry et al. Reference Belfry, McNaughton and Sikkema2015). These studies show that combining novel modes of action can improve control of herbicide-resistant weed species in multiple crops. Furthermore, the tolerance that flax exhibited to pyroxasulfone, topramezone, and fluthiacet-methyl in this study, combined with the capabilities of these groups to control herbicide-resistant weed populations, provides potential to improve weed management in flax.
In summary, fluthiacet-methyl, pyroxasulfone, and topramezone all have potential to be used in flax production for either PRE or POST weed control. Flumioxazin is not a viable option for use in flax because of the severe crop damage it can cause during growing seasons with high-moisture events. Pyroxasulfone can be safely mixed with sulfentrazone, which could add further diversity to the herbicides currently used for PRE weed management in flax. Utilizing a combination of protoporphyrinogen oxidase inhibitors, very-long-chain fatty-acid inhibitors, and HPPD inhibitors will help to reduce the selection pressure for the development of herbicide resistance to any one chemistry in high-risk weed species such as wild oat and green foxtail. Unfortunately, mixing fluthiacet-methyl and topramezone with MCPA or bromoxynil substantially increased crop phytotoxicity to unacceptable levels. These POST mixtures cannot be recommended until greater crop safety can be achieved.
Author ORCIDs
Moria E. Kurtenbach https://orcid.org/0000-0001-8867-6678
Acknowledgments
We would like to sincerely thank William May from the Agriculture Canada Research Station in Indian Head, SK, for his assistance during this research. As well, we would like to acknowledge the help and expertise of all technical staff involved with this project. The primary sources of funding for this research were provided by the Agriculture Development Fund, the Saskatchewan Flax Commission, Western Grains Research Foundation, BASF, and FMC. While BASF and FMC contributed to this work, these companies had no influence over any part of the project. No conflicts of interest have been declared.