Introduction
As interest in implementing sustainable farming practices has increased during the past few decades, cover crops have also increased in popularity. Cover crops are typically grown during fallow periods after cash crop harvest; however, as living mulches growing alongside cash crops, they can provide additional benefits. Cropping system benefits of living mulches include improved soil health, resource-use efficiency and organic matter return, and reduced soil erosion (Hall et al. Reference Hall, Hartwig and Hoffman1984; Hartwig and Ammon Reference Hartwig and Ammon2002; Leary and DeFrank Reference Leary and DeFrank2000; Masiunas Reference Masiunas1998; Teasdale et al. 2007). Living mulches can also suppress weeds in both grain and vegetable crops more effectively than cover crop residue (Liebman and Dyck Reference Liebman and Dyck1993; Teasdale 1996, Reference Teasdale1998; Teasdale et al. 2007).
The benefits of living mulches, including as alternatives to interrow cultivation, occur in both annual and perennial living mulch systems (Bhaskar et al. Reference Bhaskar, Bellinder, DiTommaso and Walter2018; Grabber and Jokela Reference Grabber and Jokela2013; Hartwig and Ammon Reference Hartwig and Ammon2002; Liebman and Dyck Reference Liebman and Dyck1993; Teasdale et al. 2007). Research has often focused on perennial living mulches because they do not require annual seeding (Teasdale 1996), but annual living mulches merit further study because they may be more easily controlled with lower herbicide inputs (Hall et al. Reference Hall, Hartwig and Hoffman1984; Moomaw and Martin Reference Moomaw and Martin1976). In herbicide-managed living mulch systems, excessive herbicide injury reduces living mulch biomass and soil cover (Bhaskar et al. Reference Bhaskar, Bellinder, Reiners and DiTommaso2020; Linscott and Hagin Reference Linscott and Hagin1975; Robertson et al. Reference Robertson, Lundy, Prine and Currey1976). When living mulch injury is not severe, weed suppression by the living mulch can be acceptable, but greater injury usually provides weeds with the competitive advantage (Bhaskar et al. Reference Bhaskar, Bellinder, Reiners and DiTommaso2020; Echtenkamp and Moomaw Reference Echtenkamp and Moomaw1989; Hughes and Sweet Reference Hughes and Sweet1979). However, living mulches may not adequately suppress weeds on their own and may need to be accompanied by other management techniques such as the application of herbicides (Brainard et al. Reference Brainard, Bakker, Noyes and Myers2012; Kunz et al. Reference Kunz, Sturm, Peteinatos and Gerhards2016; Vrabel et al. Reference Vrabel, Minotti and Sweet1980). Thus, herbicides should augment the level of weed suppression provided by living mulches without markedly affecting their vigor. Herbicides must also limit living mulch growth and thereby diminish competition between the living mulch and the crop. Competition is a primary risk associated with living mulch systems (Brainard and Bellinder Reference Brainard and Bellinder2004; Masiunas Reference Masiunas1998; Teasdale 1996; Teasdale et al. 2007), especially under resource-limited conditions (Bhaskar et al. Reference Bhaskar, Bellinder, DiTommaso and Walter2018; Brainard et al. Reference Brainard, Bellinder and Miller2004; Paine et al. Reference Paine, Harrison and Newenhouse1995; Pfeiffer et al. Reference Pfeiffer, Silva and Colquhoun2016).
It is difficult to identify chemical control strategies that compromise neither living mulch function nor crop yield (Echtenkamp and Moomaw Reference Echtenkamp and Moomaw1989; Hughes and Sweet Reference Hughes and Sweet1979; Martin et al. Reference Martin, Greyson and Gordon1999). One approach to managing these competing goals involves combining different herbicides at low rates that can facilitate quick living mulch recovery. When rates are decreased, herbicide combinations can be used to control a greater number of weed species while maintaining, or even reducing, overall herbicide input. When herbicide combinations have been used to manage living mulch, they have traditionally been sprayed in single applications (Cardina and Hartwig 1980; Hartwig 1976; Linscott and Hagin Reference Linscott and Hagin1975). Spacing out herbicide applications temporally may provide greater consistency in soil cover (by limiting canopy loss resulting from living mulch injury) and longer-lasting weed control. Sequential applications also provide the opportunity to apply herbicides with different properties at different growth stages. Through appropriate combinations and application timing, potential differences between herbicide effects on weeds and living mulches could be used to give living mulches competitive advantages over weeds.
Asymmetric herbicide responses could result from morphological and physiological differences between weeds and living mulches. In annual living mulches, some of these factors are dependent on seedling age and are therefore sensitive to planting time. Living mulch–cash crop competition can be high when living mulches are planted too early relative to the cash crop (Brainard and Bellinder Reference Brainard and Bellinder2004; Brainard et al. Reference Brainard, Bellinder and Miller2004; Vrabel et al. Reference Vrabel, Minotti and Sweet1980). Other benefits associated with living mulches, including weed suppression and soil cover, may be greater when living mulches are planted early (Brainard et al. Reference Brainard, Bellinder and Miller2004; Brainard and Bellinder Reference Brainard and Bellinder2004). In some cases, the optimal time for living mulch planting is close to the time of cash crop planting (Vrabel et al. Reference Vrabel, Minotti and Sweet1980).
In a recently published study (Bhaskar et al. Reference Bhaskar, Bellinder, Reiners and DiTommaso2020), we tested the effects of several herbicide treatments on weeds in a tomato crop with a sunn hemp living mulch. Each treatment consisted of two temporally separated POST applications, each of which included a single herbicide and rate from the following list: fomesafen (0.012 kg ai ha−1), halosulfuron (0.05 to 0.053 kg ai ha−1), metribuzin (0.08 to 0.15 kg ai ha−1), and rimsulfuron (0.007 to 0.017 kg ai ha−1). Our results indicated that treatments that began with metribuzin, a primarily PRE herbicide with its POST activity enhanced by a surfactant, were the most effective in suppressing weeds by killing newly emerged weed-seedlings and preventing future emergence through residual activity, with minimal adverse impact on crop yield. The use of residual herbicides may reduce herbicide inputs (Moomaw and Martin Reference Moomaw and Martin1976) and damage to crops and living mulch. Metribuzin was most effective when followed by a herbicide with greater POST activity.
The study presented here builds upon our earlier report by describing the effects of a greater variety of herbicides and rates on a simplified system containing only sunn hemp and weeds. Using two-step herbicide treatments (similar to that reported by Bhaskar et al. Reference Bhaskar, Bellinder, Reiners and DiTommaso2020), we tested combinations of fomesafen, halosulfuron, imazethapyr, metribuzin, rimsulfuron, and S-metolachlor. Based on preliminary greenhouse and field evaluations, we hypothesized that a primarily PRE herbicide such as metribuzin followed by a primarily POST herbicide would provide more effective weed control with lower living mulch injury than other sequences/combinations. We also hypothesized that some herbicide treatments would have disproportionate effects on weeds relative to the living mulch at the relatively low application rates evaluated here. The objective of the study was to identify such treatments, which would represent an important step toward improved control of competitive dynamics in living mulch systems.
Materials and Methods
Field trials were conducted in 2015 and 2016 at the Homer C. Thompson Vegetable Research Farm in Freeville, NY (42.5298°N, 76.3126°W). Soils at this location are Howard gravelly loam (Loamy-skeletal mixed mesic Glosoboric Hapludalf) with a pH range of 6.0 to 6.6. Different fields were used each year. Although cash crops were not planted in this experiment, the sunn hemp is referred to as living mulch because we evaluated management practices for that purpose.
Field Preparation and Planting
To prepare for planting, fields were moldboard-plowed, disked, harrowed, and fertilized with nitrogen, phosphorus, and potassium, each applied at the standard rate of 100 kg ha−1 using 13-13-13 (N-P-K). Sunn hemp (Hancock Seed Co., Dade City, FL) was seeded at a row spacing of 23 cm using a grain drill (2010, Model KED-72, Eco-Drill, Kasco Mfg., Shelbyville, IN) on June 4, 2016. In 2015, sunn hemp was replanted on July 6, after the first planting in late May failed to establish following unusually heavy rains. Sunn hemp was seeded at rates of 65 and 90 kg ha−1 in 2015 and 2016, respectively, in 1.8-m-wide strips. The variation occurred because the drill lacked a setting appropriate for sunn hemp seeds. For the same reason, final seeding rates exceeded the intended rate of 55 kg ha−1 (which was already slightly higher than typically recommended rates because we considered it necessary to achieve rapid and complete establishment). Within the seeded strips, treatments were randomly assigned to 1.8-m by 3.1-m plots. The experiment was set up as a randomized complete block design with three replicates. No irrigation was provided in 2015 because it was a wet year. In 2016, irrigation (total for the season of approximately 60 mm) was provided several times due to severe drought conditions.
Herbicide Treatments
We tested the effects of fomesafen, halosulfuron, imazethapyr, metribuzin, rimsulfuron, and S-metolachlor (Table 1) on living mulch and weeds. All herbicide applications included a nonionic surfactant at 0.25% of spray volume to increase POST activity by facilitating flow onto and absorption into plant tissues (Hess and Foy Reference Hess and Foy2000). Treatments consisting of the herbicides listed above were assumed to be inadequate for grass weed control, so sethoxydim (0.27 kg ai ha−1; Table 1) was also applied across the entire field. These sethoxydim applications did not have any visible effects on sunn hemp. Weed data reported here do not differentiate between broad-leaved and grass weeds and may include grasses not killed by the sethoxydim application. No other methods of weed control, including hand-weeding, were used.
Table 1. Application rates and product details of herbicides used in 2015 (July to October) and 2016 (June to September) living mulch (sunn hemp) trials in Freeville, NY.
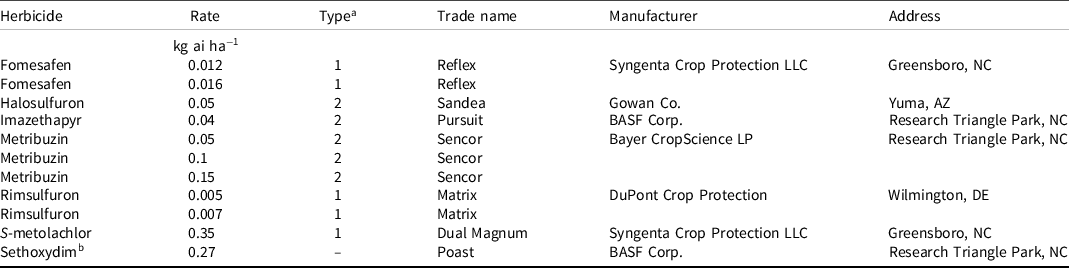
a At the rates used in this study, herbicides were classified as Type 1 or Type 2 based on the sensitivity of sunn hemp to these applications. Herbicide applications that caused severe injury to sunn hemp are Type 1; herbicide applications that did not cause severe injury are Type 2.
b Sethoxydim was applied to all plots because the herbicide treatments were considered to be inadequate for grass weed control. Therefore, sethoxydim is not considered as a treatment.
Herbicide treatments included two herbicides applied individually in two separate applications (Table 2). They are referred to as “(first herbicide) fb (second herbicide),” where fb means “followed by” in a sequential application. Based on greenhouse and preliminary field evaluations of living mulch sensitivity, the herbicides were classified into two types. At the rates used, Type 1 [fomesafen (at both rates), rimsulfuron (at both rates), and S-metolachlor (single rate)] herbicides were more injurious than Type 2 [halosulfuron, imazethapyr, and metribuzin (at all rates)] herbicides. To illustrate the effects of different combinations of the two herbicide types, results are also presented in terms of the following treatment groups: Type 1 fb Type 1, Type 1 fb Type 2, Type 2 fb Type 1, and Type 2 fb Type 2. We included two controls: an untreated sunn hemp check (living mulch without herbicides) and a weedy check (neither living mulch nor herbicides). Both these check plots received the sethoxydim applications applied to treatment plots.
Table 2. Herbicide treatments used on living mulch (sunn hemp) in 2015 (July to October) and 2016 (June to September) in Freeville, NY. Reference plots are also listed.a, b, c

a All herbicide applications included a non-ionic surfactant at 0.25% spray volume.
b Abbreviation: fb, followed by.
c Sunn hemp was planted at 23-cm row spacing.
d At the rates used in this study, herbicides were classified as Type 1 or Type 2 based on the sensitivity of sunn hemp to these applications. Herbicide applications that caused severe injury to sunn hemp are Type 1; herbicide applications that did not cause severe injury are Type 2.
e First applications were made approximately 25 d after living mulch emergence (at approximately 75% soil cover); second applications were made approximately 15 d later.
Herbicides were applied using a CO2-pressurized backpack sprayer fitted with a four-nozzle [8003 EVS flat fan (TeeJet Technologies®, Spraying Systems Co., P.O. Box 7900, Wheaton, IL)] boom of 1.8-m swath, operating at 200 to 240 kPa pressure and delivering a spray volume of approximately 320 L ha−1. The first set of herbicides was applied when sunn hemp seedlings were 20 cm tall at approximately 25 d after emergence (late June in 2016; late July in 2015), and the second set was made approximately 15 d later.
Data Collection
Percent cover of living mulch, percent cover of weeds, and living mulch height were measured four times at 2- to 3-wk intervals between mid-July and early October in 2015 and mid-July and late August in 2016. In 2016, aboveground biomass and density were measured at the end of August, aimed to coincide with the typical start of vegetable harvests in the region. Due to late planting in 2015, aboveground biomass and density data were collected in early October that year. Biomass and density of living mulch were determined from two randomly selected 50-cm-long segments of living mulch row, at least 60 cm away from plot edges. Biomass and density of weeds were determined from two randomly selected 0.25-m2 (50 cm by 50 cm) areas at least 60 cm away from plot edges. Living mulch and weeds were cut at ground level and oven-dried for 2 wk at 75 C to estimate dry biomass.
Data Analyses
Living mulch (cover, height, density, and biomass) and weed (cover, density, and biomass) parameters were subjected to ANOVA and regression analyses at the 5% level of significance. ANOVA was used to test whether any treatment means differed and linear mixed-effects models followed by a post hoc test, Tukey’s honestly significant difference test, were used to determine treatment differences. We also used regression to test whether variation in living mulch parameters were associated with variation in weed parameters. Data were not combined across years due to very different climatic conditions in 2015 and 2016. In regression models, herbicide treatments were included as fixed effects and block was included as a random effect. No data transformations were needed to meet model assumptions. Statistical analyses were performed using JMP Pro 12.0.1 (2013 SAS Institute Inc., Cary, NC) software.
Results and Discussion
Weather and Interannual Variation
At the trial site, the 30-yr average for rainfall from May to August was 404 mm (Northeast Regional Climate Center 2020). In 2015, 451 mm rainfall were recorded during this period, so soil moisture was not deemed limiting. In 2016, rainfall was only 239 mm, which included a prolonged drought (120 mm rainfall from May to July, compared with 395 mm in 2015). In both years, temperatures were low enough by late September to curb the growth of the warm-season sunn hemp. Flowering in sunn hemp was negligible and no seed set was observed.
Major weeds in the experimental plots were common lambsquarters, common purslane, hairy galinsoga, Powell amaranth, and shepherd’s purse [Capsella bursa-pastoris (L.) Medik.]. In 2015, replanting of sunn hemp during early July, compared with June planting in 2016, likely reduced overall weed pressure in the field by eliminating the impact of a potentially greater weed pressure at the beginning of the season. Maximum weed emergence may have occurred during the typical vegetable planting time in June. In a previous study at the same farm, rye (Secale cereale L.) interseeded in broccoli (Brassica oleracea L. var. italica) a few weeks later than typical planting time had no pronounced effect on weeds because the later weed emergence was weak (Brainard and Bellinder Reference Brainard and Bellinder2004). Above-average rainfall in 2015 also likely moderated the effects of the living mulch and herbicides on weeds. The abundance of soil moisture could have eliminated competition for water between the living mulch and weeds and hastened recovery from herbicide injury.
In 2016, severe drought conditions enhanced the competitive effects of living mulch on weeds, potentially increasing differences between treatments. Soil moisture is an important influence on competitive dynamics in living mulch systems (Bhaskar et al. Reference Bhaskar, Bellinder, DiTommaso and Walter2018; Echtenkamp and Moomaw Reference Echtenkamp and Moomaw1989). The difference in seeding rate represents a less likely explanation for interannual variation, given that living mulch stand density was similar between years (Tables 3 and 4). However, eliminating this possibility would require experiments on seeding rates, which would also help improve management recommendations.
Table 3. Living mulch (sunn hemp; cover, height, stand density, and biomass) and weed (cover, density and biomass) measurements in 2015 (July to October) in Freeville, NY.a, b, c

a Values within a column followed by a same letter are not significantly different according to Tukey’s test (α = 0.05).
b Abbreviations: LM, living mulch; harv., harvest during late August, typical vegetable harvest time in the region; fb, followed by.
c Sunn hemp was planted at 23-cm row spacing.
d Average values are the means of four time points 2 to 3 wk apart, including the late August (harv.) time-point.
e Visual estimations (percentages) are in absolute terms, so living mulch and weed cover may not add to 100%.
f Oven-dried (2 wk at 75 C) dry matter.
g All herbicide applications included a nonionic surfactant at 0.25% spray volume.
h First applications were made approximately 25 d after living mulch emergence (at approximately 75% soil cover); second applications were made approximately 15 d later.
i Fomesafen, rimsulfuron, and S-metolachlor are referred to in the text as Type 1 herbicides; halosulfuron, imazethapyr, and metribuzin are Type 2 herbicides. At the rates used in this study, herbicides were classified as Type 1 or Type 2 based on the sensitivity of sunn hemp to these applications. Herbicide applications that caused severe injury to sunn hemp are Type 1; herbicide applications that did not cause severe injury are Type 2.
j Herbicide rates in kg ai ha−1 are given within parentheses.
Table 4. Living mulch (sunn hemp; cover, height, stand density, and biomass) and weed (cover, density and biomass) measurements in 2016 (June to September) in Freeville, NY.a, b, c

a Values within a column followed by a same letter are not significantly different according to Tukey’s test (α = 0.05).
b Abbreviations: LM, living mulch; harv., harvest during late August, typical vegetable harvest time in the region; fb, followed by.
c Sunn hemp was planted at 23-cm row spacing.
d Average values are the means of four time points 2 to 3 wk apart, including the late August (harv.) time-point.
e Visual estimations (percentages) are in absolute terms, so living mulch and weed cover may not add to 100%.
f Oven-dried (2 wk at 75 C) dry matter.
g All herbicide applications included a nonionic surfactant at 0.25% spray volume.
h First applications were made approximately 25 d after living mulch emergence (at approximately 75% soil cover); second applications were made approximately 15 d later.
i Fomesafen, rimsulfuron, and S-metolachlor are referred to in the text as Type 1 herbicides; halosulfuron, imazethapyr, and metribuzin are Type 2 herbicides. At the rates used in this study, herbicides were classified as Type 1 or Type 2 based on the sensitivity of sunn hemp to these applications. Herbicide applications that caused severe injury to sunn hemp are Type 1; herbicide applications that did not cause severe injury are Type 2.
j Herbicide rates in kg ai ha−1 are given within parentheses.
Effects on Living Mulch
In 2015, living mulch biomass was relatively consistent across herbicide treatments (3,800 to 5,800 kg ha−1) and did not differ between the herbicide treatments and the untreated check (7,000 kg ha−1), except for the metribuzin fb fomesafen treatment (3,800 kg ha−1; P = 0.027; Table 3; Figure 1A). Similarly, living mulch density in 2015 did not differ across herbicide treatments (P = 0.9) or herbicide-type combinations (P = 0.86). In 2016, most herbicide treatments did not decrease living mulch biomass and cover or increase living mulch mortality (i.e., reduce stand density; Table 4). Taken together, these findings demonstrate that appropriate herbicide applications can avoid living mulch mortality and maintain biomass and cover. It is desirable that herbicides do not cause living mulch mortality because the density of living mulch stands is crucial to their function; gaps in a stand can allow weed outbreaks (Echtenkamp and Moomaw Reference Echtenkamp and Moomaw1989; Hughes and Sweet Reference Hughes and Sweet1979).

Figure 1. Living mulch (sunn hemp) cover (top), weed cover (center), and living mulch height (bottom; ± SE) at different times in 2015 (July to October; A) and 2016 (June to September; B) in Freeville, NY. Sunn hemp was planted at 23-cm row spacing. Abbreviations: T, type; fb, followed by (in a sequential application); UC, untreated living mulch check (no hand-weeding, no herbicide treatments); WC, weedy check (no living mulch, no hand-weeding, no herbicide treatments); M fb H, metribuzin followed by halosulfuron (0.05 and 0.05 kg ai ha−1, respectively); M fb R I, metribuzin followed by rimsulfuron rate I (0.05 and 0.005 kg ai ha−1, respectively); M fb F, metribuzin followed by fomesafen (0.1 and 0.012 kg ai ha−1, respectively); M fb R II, metribuzin followed by rimsulfuron rate II (0.1 and 0.007 kg ai ha−1, respectively); I fb R, imazethapyr followed by rimsulfuron (0.04 and 0.007 kg ai ha−1, respectively); I fb F, imazethapyr followed by fomesafen (0.04 and 0.012 kg ai ha−1, respectively); s-M fb R, S-metolachlor followed by rimsulfuron (0.35 and 0.007 kg ai ha−1, respectively); s-M fb F, S-metolachlor followed by fomesafen (0.35 and 0.016 kg ai ha−1, respectively); R fb M, rimsulfuron followed by metribuzin (0.007 and 0.15 kg ai ha−1, respectively); F fb M, fomesafen followed by metribuzin (0.012 and 0.15 kg ai ha−1, respectively). First herbicide applications were made approximately 25 d after living mulch emergence (at approximately 75% soil cover); second applications were made approximately 15 d later. Herbicide applications were classified as Type 1 or Type 2: at the rates used in this study, herbicide applications that caused severe injury to sunn hemp are Type 1; those that did not cause severe injury are Type 2. Fomesafen, rimsulfuron, and S-metolachlor applications are Type 1; halosulfuron, imazethapyr, and metribuzin applications are Type 2.
Throughout the 2016 season, in mid-July (18%; P < 0.0001), late July (13%; P < 0.0001), and early August (15%; P < 0.0001), Type 1 (fomesafen, rimsulfuron, or S-metolachlor) fb Type 2 (halosulfuron, imazethapyr, or metribuzin) herbicides had lower living mulch cover than all other herbicide-type combinations and the untreated check (Figure 1B). By late August, living mulch in Type 1 fb Type 2 had regrown to provide only 52% cover, which was still lower (P = 0.0004) than in the other herbicide-type combinations (93% to 98%). In early August, weed cover in Type 1 fb Type 2 and the weedy check (74% and 92%, respectively) were similar (P = 0.59). These results are consistent with other reports of increased weed pressure following severe herbicide damage to living mulch (Bhaskar et al. Reference Bhaskar, Bellinder, Reiners and DiTommaso2020; Echtenkamp and Moomaw Reference Echtenkamp and Moomaw1989; Hartwig Reference Hartwig1977; Hughes and Sweet Reference Hughes and Sweet1979). When applied first, Type 1 herbicides caused severe injury to the young living mulch with prolonged recovery times. For example, living mulch biomass in fomesafen fb metribuzin (among the herbicides, fomesafen caused the most severe living mulch injury) was only 1700 kg ha−1 (Table 4), which was lower than living mulch biomass in treatments with a first application of a Type 2 herbicide (P = 0.0002). In some cases, weeds that emerged following Type 1 herbicide applications could have avoided serious injury because these herbicides may have weaker residual soil activity. The resulting increase in weed pressure could have further inhibited living mulch growth.
Living mulch height in 2016 exhibited the same pattern (Table 4; Figure 1B). Type 1 fb Type 1, Type 2 fb Type 1, and Type 2 fb Type 2 were similar to each other and to the untreated check throughout the season, while living mulch in Type 1 fb Type 2 was shorter (P = 0.0005 in mid-July; P < 0.0001 in late July; P < 0.0001 in early August; P < 0.0001 in late August). Reductions in living mulch height are expected from herbicide applications and are positive outcomes because living mulch height has considerable influence on crop yield (Greenland Reference Greenland2000; Hinds et al. Reference Hinds, Wang and Hooks2016; Zandstra and Warncke Reference Zandstra and Warncke1993). Tall living mulches can easily become too competitive with crops by shading both the side and top portions of the crop canopy. In an earlier study (Echtenkamp and Moomaw Reference Echtenkamp and Moomaw1989), crop yields were reduced by 15% in the presence of a shorter chewings fescue [Festuca rubra L. ssp. fallax (Thuill.) Nyman] but by 46% in the presence of a taller rye plus oats (Avena sativa L.) plus vetch (Vicia villosa Roth) living mulch, even though both living mulches produced similar biomass. In our study, however, the reduction in height was associated with reductions in living mulch biomass and cover, which are antithetical to some of the objectives of living mulch systems.
Effects on Weeds
In 2015, weed biomass in herbicide-type combinations (60 to 440 kg ha−1) was not different from weed biomass in the untreated check (180 kg ha−1) but was lower than in the weedy check (2180 kg ha−1; P < 0.0001; Table 3). However, weed density was similar in all treatments (11 to 28 plants m−2), including the weedy check (27 plants m−2; P = 0.5). In 2016, weed cover decreased (P < 0.0001) with increasing living mulch biomass, but there was no relationship between weed density and living mulch biomass (P = 0.6). In late August 2016, weed biomass was negatively associated with both living mulch biomass (P = 0.002) and living mulch cover (P = 0.007). These findings suggest that the living mulch-herbicide treatments stunted weed growth through competitive and chemical stresses rather than killing them.
Negative relationships between weed biomass or cover and living mulch biomass or cover are consistent with reports of greater weed suppression by more vigorous living mulch stands and are considered to be evidence of living mulch efficacy (Bhaskar et al. Reference Bhaskar, Bellinder, DiTommaso and Walter2018; Mohammadi Reference Mohammadi2012; Teasdale et al. 2007). In chemical management of living mulches, a major constraint is that herbicide rates designed to maximize crop yield often cause severe damage to living mulches (Hartwig and Hoffman Reference Hartwig and Hoffman1975) and thereby reduce their efficacy. Unless crop-mulch competition is stronger than crop-weed competition (Chase and Mbuya Reference Chase and Mbuya2008), this problem may be tractable. Herbicide treatments with greater effects on weeds than on the living mulch could help make satisfactory crop yields possible without excessively compromising living mulch function. Some of our results would be consistent with such asymmetrical effects: several herbicide treatments consistently resulted in high living mulch biomass and cover in addition to low weed biomass and cover. However, these experiments did not provide statistical evidence to either support or reject our hypothesis that some herbicide treatments would injure weeds more than they injured the living mulch. More quantitative testing for asymmetry will involve collecting more data from untreated plots to determine whether the presence of herbicides affects the slope of the relationship between weed biomass and living mulch biomass.
Management Implications
The results demonstrate that sunn hemp may be an effective tool for weed control when used as a living mulch. The untreated check suppressed weeds in both 2015 and 2016, even more effectively than some herbicide treatments (Tables 3 and 4). For instance, in late August 2016, weed cover in the untreated check was 31%, whereas weed cover in the Type 1 fb Type 2 herbicide combination was 55% (Figure 1B). These results suggest that unless designed appropriately, herbicide applications may be redundant or even detrimental to weed control. However, improved weed control is not productive if crops suffer from excessive competition with the living mulch. Combining living mulches with properly designed herbicide regimes may provide the same effective weed control as an untreated living mulch (or more effective weed control under higher weed pressure) while also reducing competition between the living mulch and the cash crop.
The capacity of the living mulch to suppress weeds in the absence of herbicides, along with the effects of herbicides on living mulch and weeds in Type 1 fb Type 2, provide insight into the excellent weed control in Type 2 fb Type 1. In 2016, weed biomass in Type 2 fb Type 1 (600 kg ha−1) was lower than weed biomass in Type 1 fb Type 1 (P = 0.0009) and Type 1 fb Type 2 (P = 0.012; Table 4). Type 2 fb Type 1 (19%) and Type 2 fb Type 2 (8%) also had the lowest weed cover in late August (Table 4; Figure 1B). At the first herbicide application, living mulch seedlings were larger than weeds because the living mulch had emerged earlier and reached a height of approximately 20 cm by the time of weed emergence. A Type 2 herbicide applied along with a surfactant at the time of weed emergence therefore had more severe effects on weeds than on the living mulch. This injury to young weeds and the residual activity of the primarily PRE herbicides (such as metribuzin), classified as Type 2 may have jointly contributed to a period in which the living mulch could grow under low competition from weeds. This growth period may have helped the living mulch withstand the subsequent Type 1 herbicide application. At the second application, many weeds would still have been smaller than the living mulch due to residual activity from the Type 2 herbicide. Consequently, a second application of a Type 1 herbicide may again have injured weeds more than the living mulch. Thus, our findings corroborate previous work (Bhaskar et al. Reference Bhaskar, Bellinder, Reiners and DiTommaso2020) and support our hypothesis that treatments consisting of a Type 2 herbicide such as metribuzin followed by a more injurious herbicide would be more effective than other treatments.
Our results are consistent with the interpretation that Type 2 herbicides provide mild POST control of living mulch and weeds in addition to stronger soil residual activity against weeds. Some herbicide treatment combinations appeared to preferentially target weeds, successfully averting losses in living mulch cover, density, and biomass. Herbicides also have the potential to reduce living mulch height, although no treatment in this study reduced height without associated losses in living mulch biomass, cover, or weed suppression. Overall, the Type 2 fb Type 1 herbicide treatments appear to have the greatest potential to maintain healthy living mulch stands. In a planted field, variations on this treatment program could give producers finely tuned control over the interactions among crops, living mulches, and weeds. Findings from this study demonstrate that herbicide applications at reduced rates are a viable strategy for the management of living mulches and may promote sustainability by increasing the feasibility of living mulch systems.
Acknowledgments
We thank Peter Putriment, Rebecca Wilk, Steve McKay, Rick Randolph, and Ethan Tilebein for their assistance with the experiment. This project was supported in part by a grant from the Northeastern Sustainable Agriculture Research and Education (NE-SARE, award no. GNE15-095). No conflicts of interest have been declared.







