Vegetable grafting is common practice in Asiatic and European countries for production of solanaceous and cucurbitaceous crops (Kubota et al. Reference Kubota, McClure, Kokalis-Burelle, Bausher and Rosskopf2008; Lee et al. Reference Lee, Kubota, Tsao, Bie, Hoyos Echevarria, Morra and Oda2010). However, grafting is a relatively new technique in the United States (Sakata et al. Reference Sakata, Ohara and Sugiyama2007). Tomato grafting has emerged in the United States as an alternative to methyl bromide to address soil borne diseases and other pests (Louws et al. Reference Louws, Rivard and Kubota2010). Surveys conducted by the University of Arizona in 2002 and 2006 showed that more than 40 million grafted tomato seedlings are used annually in North American greenhouses (Kubota et al. Reference Kubota, McClure, Kokalis-Burelle, Bausher and Rosskopf2008). Grafting is successfully used in tomato production to manage wilt caused by Fusarium oxysporum f. sp. lycopersici (Sacc.) W.C. Snyder and H.N. Hans or Verticillium dahliae Kleb., southern blight caused by Sclerotium rolfsii Sacc., bacterial wilt caused by Ralstonia solanacearum, and root-knot nematodes (Meloidogyne spp.) (Barrett et al. Reference Barrett, Zhao and McSorley2012; McAvoy et al. Reference McAvoy, Freeman, Rideout, Olson and Paret2012; Rivard and O’Connell et al. Reference Rivard, O’Connell, Peet and Louws2010; Rivard et al. Reference Rivard, O’Connell, Peet, Welker and Louws2012). Grafting with certain rootstocks has resulted in greater fruit yield and enhanced tolerance to abiotic stresses such as thermal, salt, and water stress, as well as organic pollutants (Colla et al. Reference Colla, Suãrez, Cardarelli and Rouphael2010; Proietti et al. Reference Proietti, Rouphael, Colla, Cardarelli, De Agazio, Zacchini, Rea, Moscatello and Battistelli2008; Schwarz et al. Reference Schwarz, Rouphael, Colla and Venema2010). Djidonou et al. (Reference Djidonou, Zhao, Simonne, Koch and Erickson2013) has reported improved nitrogen- and water-use efficiency using grafted plants in tomato production systems.
Metribuzin, a triazinone and photosystem II inhibitor herbicide (Senseman Reference Senseman2007), is registered PRE and POST at 280 to 550 g ha−1 for controlling annual broadleaf weeds (Anonymous 2009), but can cause injury to tomato. Injury is typically reported as stunting and marginal leaf chlorosis and necrosis under certain environmental conditions (Fortino and Splittstoesser Reference Fortino and Splittstoesser1974a, Reference Fortino and Splittstoesser1974b; Friesen and Hamill Reference Friesen and Hamill1978). Tomato plants are more sensitive to metribuzin injury when growing under stress conditions such as drought, flooding, low (<15 C) or high (>30 C) temperature, high relative humidity (80%), or low light intensity (6,500 lx) before metribuzin application (Anonymous 2009; Fortino and Splittstoesser Reference Fortino and Splittstoesser1974a, Reference Fortino and Splittstoesser1974b; Phatak and Stephenson Reference Phatak and Stephenson1973). McNaughton (Reference McNaughton2013) reported that, in greenhouse studies, injury to tomato exposed to glyphosate drift followed by metribuzin application was less under drought-stress treatments than it was under non-stress treatments.
Metribuzin injury to tomato also depends on tomato plant size and cultivar (Fortino and Splittstoesser Reference Fortino and Splittstoesser1974a; Gawronski Reference Gawronski1983). Small plants are more sensitive to metribuzin injury than are larger plants. Differential metribuzin tolerance of tomato cultivars had been attributed to metabolism, which has been reported to be at least two-fold greater in tolerant tomato seedling compared to susceptible seedlings (Stephenson et al. Reference Stephenson, McLeod and Phatak1976). Frear et al. (Reference Frear, Mansager, Swanson and Tanaka1983) reported that in tolerant tomato seedlings 80% of absorbed 14C-metribuzin was metabolized within 24 hr after application.
The effect of metribuzin on nongrafted tomato under various environmental conditions has been well studied (Fortino and Splittstoesser Reference Fortino and Splittstoesser1974a, Reference Fortino and Splittstoesser1974b; Phatak and Stephenson Reference Phatak and Stephenson1973). Given that grafted tomato is gaining popularity in the United States, it is important to understand the effect of metribuzin on grafted tomato. Grafted tomato plants cost US$0.43 to US$0.74 more per plant than do nongrafted plants because of the extra investment in potting media, seeding trays, rootstock seeds, grafting supplies, and manual labor required to perform grafting (Rivard and Olha et al. Reference Rivard, Olha, O’Connell, Peet and Louws2010), hence metribuzin injury could become an even greater economic risk when using grafted plants. Therefore, the objective of this experiment was to determine the response of grafted and nongrafted tomato under drought-stress and non-drought-stress conditions to metribuzin applied POST in the greenhouse.
Materials and Methods
Experiments were conducted at the Marye Anne Fox Science Teaching Laboratory Greenhouse at North Carolina State University (35.79°N, 78.67°W) in Raleigh, North Carolina in March 2014 and were repeated in May 2014. Transplant type included nongrafted ‘Amelia’ (Harris Moran, PO Box 4938, Modesto, CA) tomato, and Amelia scion grafted on ‘Beaufort’ or ‘Maxifort’ (DeRuiter Seeds, 800 North Lindbergh Blvd, St. Louis, MO) tomato rootstocks (hereafter Amelia, A-Beaufort, and A-Maxifort, respectively). Maxifort and Beaufort are commercially available interspecific hybrid tomato rootstocks. These rootstocks confer resistance against tomato mosaic virus, fusarium wilt, corky root, verticillium wilt, and root knot nematodes (Rivard and Louws Reference Rivard and Louws2006). Grafted plants were produced at North Carolina State University’s phytotron using the tube grafting technique (Rivard and Louws Reference Rivard and Louws2006). Plants were transplanted into 20 cm wide by 15 cm deep polyethylene pots (ITML Horticultural Products, Brantford, ON, Canada) using a 1:1 (v/v) mix of sand (Screened Topsoil, Rex H. Frazier Page Rd. Garden Center, NC) and commercial potting mix (Fafard 4P potting mix, Conrad Fafard Inc., Agawam, MA). The resulting soil mix had organic matter 4.2%, CEC 4.9 meq 100g−1, and pH 6. Plants were watered on a twice daily basis, which was designed to bring soil to saturation except when drought stress was applied.
At 15 to 18 d after transplanting, drought stress was induced when plants were 27 to 32 cm tall (first experiment) and 38 to 42 cm tall (second experiment). Drought-stress treatments were no drought stress (no-stress), 3 d of drought stress before metribuzin application with no drought stress after application (3 d DSB), and 3 d of drought stress before metribuzin application with 3 d of drought stress after application (3 d DSBA). Plants subjected to drought-stress conditions did not receive water for at least 2 d. After visible wilting occurred, drought stress was maintained by providing limited water (160 to 180 mL per pot per day) to the soil surface. A similar method was used by Zhou et al. (Reference Zhou, Tao, Messersmith and Nalewaja2007) and McNaughton (Reference McNaughton2013) to produce drought-stressed plants. Drought-stress symptoms of tomato included yellowing, stunting, and decreased leaf size and number (Figure 1). Metribuzin (TriCor DF, United Phosphorus Inc., King of Prussia, PA) at 0 or 550 g ha−1 was applied POST (over the top of the tomato plant) in a spray chamber with a CO2-pressurized sprayer fitted with an 8002EVS nozzle (Teejet Technologies, Springfield, IL) calibrated to deliver 187 L ha−1 at 275 kPa pressure. After metribuzin application, 3 d DSB plants received similar amounts of water as did no-stress plants. However, 3 d DSBA plants received limited water (160 to 180 mL per pot per day) for the next three consecutive days after metribuzin application and received similar amount of water as did no-stress plants thereafter. After POST metribuzin application, pots were watered on the soil surface and care was taken to avoid washing the metribuzin from the leaves. After transplanting, all plants were fertilized with 100 mL per pot of a 4 g L−1 fertilizer solution (Miracle-Gro® Fertilizer, Scotts Company LLC, Marysville, OH) as required to ensure optimum plant growth. The greenhouse was maintained at 25±5 C under natural sunlight. The experiment was conducted in a randomized complete block design with a three-way factorial (3×3×2) arrangement of transplant type (Amelia, A-Beaufort, and A-Maxifort), drought-stress treatment (no-stress, 3 d DSB, and 3 d DSBA), and metribuzin rate (0 and 550 g ha−1), with five replications of each treatment.

Figure 1 Tomato plant at time of metribuzin application: (A) Amelia (B) A-Beaufort (C) A-Maxifort. In each panel, the plant on left experienced no drought stress and the plant on the right has been subjected to 3 d of drought stress.
Photosynthetic rate (μmol CO2 m−2 s−1) and stomatal conductance (mol H2O m−2 s−1) of tomato were measured with a LI-COR 6400 Portable Photosynthesis System (LI-COR Biosciences, PO Box 4425, Lincoln, NE) before starting drought-stress treatments, after 3 d of drought stress but before metribuzin application, 3 d after metribuzin application (last day of drought stress of 3 d DSBA plants), and 7 d after metribuzin application (3 d after rewatering of 3 d DSBA plants). Measurements were recorded to document the physiological stress associated with drought and metribuzin treatments in grafted and nongrafted tomato. The light source was set to 1,500 μmol m−2 s−1 and the reference CO2 was set to 400 μmol. Measurements were taken between 10:00 AM and 3:00 PM Eastern Standard Time on the third fully expanded leaf from the top of each plant. Measurements were taken from a 2-cm2 leaf area over a 30 sec period and from three replicate plants per treatment with six measurements per plant at a time.
Tomato injury (marginal leaf chlorosis and necrosis) was estimated visually at 7 and 14 d after metribuzin treatment (DMT), and plant height was measured at 7 and 21 DMT. The scale for tomato injury was 0% to 100%, where 0% indicates no injury and 100% indicates crop death (Frans et al. Reference Frans, Talbert, Marx and Crowley1986). At 21 DMT, plants were harvested by cutting the stem at the soil surface and dried at 55 C for 4 d using a forced air oven and then weighed to determine aboveground shoot dry weight.
All data were subjected to ANOVA using the PROC MIXED procedure of SAS 9.2 (SAS Institute, Cary, NC) to test for treatment effects and interactions. Means were separated using Fisher’s Protected LSD test at P=0.05 when appropriate. Data were checked for homogeneity of variance and normality using residual plots. Transplant type, drought stress, metribuzin treatment, and their interactions were considered fixed effects in the model. Experiment run, replication within run, and treatment by run interaction were considered random effects when data were combined for both experimental runs. However, only replication was considered a random effect when data for each experiment run were analyzed separately.
Results and Discussion
In both experiment runs, the drought-stress symptoms partially disappeared when stressed plants were provided with adequate water, and the new growth that developed later did not show any stress symptoms. No injury to tomato from metribuzin was observed in the second run. Larger plants at the time of metribuzin application in the second run could be the reason for the absence of observed injury. A possible reason for the larger plant size during second run is the availability of more natural light for plant growth in May compared to March. Fortino and Splittstoesser (Reference Fortino and Splittstoesser1974a) reported that small tomato plants are more sensitive to metribuzin injury than are larger plants. However, metribuzin labels indicate that, irrespective of tomato size, crop injury may result from broadcast or directed spray applications if tomatoes are growing under stress conditions such as drought (Anonymous 2009). In the first experiment run, no difference in injury was observed between grafted and nongrafted tomato plants, however, injury was lower in 3 d DSBA plants (5% and 2% at 7 and 14 DMT, respectively) as compared to no-stress plants (18% and 11% at 7 and 14 DMT, respectively) (Table 1). The 3 d DSB plants showed a similar level of injury as did no-stress plants.
Table 1 Effect of transplant type and drought-stress treatments on tomato injury from metribuzin applied postemergence.Footnote a
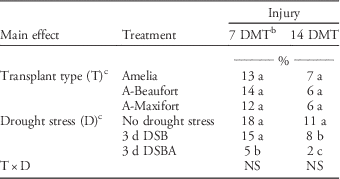
a Data from first run of greenhouse study.
b Means within columns for main effects (transplant type or drought stress) followed by same letters not significantly different according to Fisher’s protected LSD test at P≤0.05.
c Abbreviations: 3 d DSB, 3 d of drought stress before metribuzin application with no drought stress after application; 3 d DSBA, 3 d of drought stress before metribuzin application with 3 d of drought stress after application; A-Beaufort, Amelia scion grafted onto Beaufort rootstock; A-Maxifort, Amelia scion grafted onto Maxifort rootstock; DMT, d after metribuzin treatment; NS, not significant.
Treatment (transplant type, drought stress, and metribuzin rate) by experiment run interactions for plant height and biomass were significant; therefore, data for these parameters were analyzed by experiment run. Differences between the two experiments may have been due to differences in injury level or difference in plant size at the time of metribuzin application. The interactions between main effects of transplant type, drought stress, and metribuzin treatment were not significant for plant height and biomass, except for plant height at 7 DMT in the first experiment run (Table 2). At 7 DMT, transplant type and metribuzin treatment had no effect on plant height; however, both drought-stress treatments (3 d DSB and 3 d DSBA) reduced plant height relative to the no-stress treatment. At 21 DMT, the effect of transplant type and drought stress on plant height was inconsistent between runs. Transplant type and drought-stress treatment did not affect plant height in the first run, but did have significant effects in the second run (Table 2). In the second run, the grafted plants A-Beaufort and A-Maxifort (67 and 68 cm, respectively) were shorter than were nongrafted plants (73 cm), and 3 d DSBA plants (65 cm) had reduced height compared to plants that received no drought stress (73 cm). The metribuzin treatment reduced plant height at 21 DMT in both experiment runs. The effect of transplant type was only significant in the first run, where the dry weight of grafted A-Beaufort (23 g) was greater than that of nongrafted Amelia (21 g), and that of grafted A-Maxifort (19 g) was less than that of nongrafted Amelia. Drought stress affected plant dry weight only in the second run, where the 3 d DSBA treatment reduced plant dry weight by 20% relative to the no-stress treatment.
Table 2 Effect of transplant type, drought stress, and metribuzin on tomato plant height and dry weight.

a Means within columns for main effects (transplant type, drought stress, and metribuzin) followed by same letters are not significantly different according to Fisher’s Protected LSD test at P≤0.05.
b *denotes significance at P=0.05.
c Abbreviations: 3 d DSB, 3 d of drought stress before metribuzin application with no drought stress after application; 3 d DSBA, 3 d of drought stress before metribuzin application with 3 d of drought stress after application; A-Beaufort, Amelia scion grafted onto Beaufort rootstock; A-Maxifort, Amelia scion grafted onto Maxifort rootstock; DMT, d after metribuzin treatment; NS, not significant; Exp., experiment run.
Photosynthesis and stomatal conductance of grafted and nongrafted tomato before drought stress ranged from 21.7 to 23.6 µmol CO2 m−2 s−1 and 0.49 to 0.58 mol H2O m−2 s−1, respectively (data not shown). Photosynthesis and stomatal conductance measured before metribuzin application were similar for all transplant types (Table 3). However, photosynthesis and stomatal conductance levels prior to metribuzin application were reduced in plants subjected to 3 d of drought stress relative to those of non-stressed plants (Table 3). This reduction confirms that tomato in the drought-stress treatments was stressed before metribuzin application.
Table 3 Effect of transplant type and drought stress on photosynthesis and stomatal conductance of tomato plants 3 d after drought stress, but before metribuzin application.Footnote a
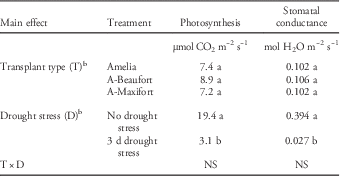
a Data pooled over two experiment runs. Means within columns for main effects (transplant type and drought stress) followed by same letters are not significantly different according to Fisher’s Protected LSD test at P≤0.05.
b Abbreviations: A-Beaufort, Amelia scion grafted onto Beaufort rootstock; A-Maxifort, Amelia scion grafted onto Maxifort rootstock; NS, not significant.
The main effect of transplant type and its interaction with either drought stress or metribuzin rate were not significant for either photosynthesis or stomatal conductance at 3 and 7 DMT. This result indicated that grafting had no apparent effect on photosynthesis and stomatal conductance of tomato when metribuzin was applied to tomato under drought stress in the greenhouse. However, an interaction between drought stress and metribuzin was observed for photosynthesis at 3 and 7 DMT (Table 4). At 3 DMT, nontreated plants subjected to no drought stress and 3 d DSB plants had higher rates of photosynthesis (16.8 and 16.5 μmol CO2 m−2 s−1, respectively) than did 3 d DSBA plants (5.3 μmol CO2 m−2 s−1). However, metribuzin-treated plants subjected to no drought stress and 3 d DSBA plants had lower levels of photosynthesis (11.6 and 5.2 μmol CO2 m−2 s−1, respectively) than did 3 d DSB plants (16.7 μmol CO2 m−2 s−1). The main effect of metribuzin and the interaction between drought stress and metribuzin did not affect stomatal conductance at 3 DMT (Table 5). However, drought stress had a significant effect: tomato in both no-stress and 3 d DSB treatments had higher stomatal conductance than did 3 d DSBA plants (Table 5). At 7 DMT, 3 d DSBA plants recovered from drought stress and had similar or higher photosynthesis rates than did 3 d DSB or no-stress plants (Table 5). No effect of drought stress or metribuzin treatments was observed on stomatal conductance of tomato plants 7 DMT (3 d after rewatering) (Table 5).
Table 4 Effect of drought stress and metribuzin on photosynthesis of tomato plant measured at 3 and 7 d after metribuzin applied postemergence.Footnote a

a Data pooled over experiment runs and transplant types. Means within columns followed by same letters are not significantly different according to Fisher’s Protected LSD test at P≤0.05.
b Abbreviations: 3 d DSB, 3 d of drought stress before metribuzin application with no drought stress after application; 3 d DSBA, 3 d of drought stress before metribuzin application with 3 d of drought stress after application; DMT, d after metribuzin treatment.
c denotes significance at P=0.05.
Table 5 Effect of drought stress on stomatal conductance of tomato plants 3 and 7 d after metribuzin applied postemergence.Footnote a

a Data pooled over experiment run, metribuzin rate, and transplant type. Means within columns followed by same letters are not significantly different according to Fisher’s Protected LSD test at P≤0.05.
b Abbreviations: 3 d DSB, 3 d of drought stress before metribuzin application with no drought stress after application; 3 d DSBA, 3 d of drought stress before metribuzin application with 3 d of drought stress after application; DMT, d after metribuzin treatment.
No difference was observed between grafted and nongrafted tomato tolerance to metribuzin applied POST. Chaudhari et al. (Reference Chaudhari, Jennings, Monks, Jordan, Gunter and Louws2015) reported similar results: grafted and nongrafted tomato responded similarly to metribuzin applied POST and PRE under both field and greenhouse conditions. This observation indicates that the changed physiological conditions in the plant due to grafting did not affect plant processes when non-stressed tomato was treated with metribuzin POST. However, visible injury from metribuzin was more pronounced in the no-stress and 3 d DSB plants than it was in the 3 d DSBA plants. Previous research has also shown lower levels of herbicide injury in plants under drought-stress conditions, including velvetleaf (Abutilon theophrasti Medik.) treated with glyphosate (Zhou et al. Reference Zhou, Tao, Messersmith and Nalewaja2007) and green foxtail [Setaria viridis (L.) Beauv.] treated with fenoxaprop, fluazifop-P, haloxyfop, and sethoxydim (Boydston Reference Boydston1990). McNaughton (Reference McNaughton2013) reported a lower level of injury from glyphosate (90 g ae ha−1) drift followed by metribuzin (250 g ha−1) application on tomato grown under low soil moisture compared to that of tomato grown in non-limiting soil moisture conditions. This result may be due to decreased herbicide absorption and translocation in drought-stressed plants. Previous studies have shown that drought stress decreases absorption and translocation of picloram in Russian knapweed [Acroptilon repens (L.) DC.], haloxyfop in johnsongrass (Sorghum halepense (L.) Pers.] and large crabgrass [Digitaria sanguinalis (L.) Scop.], and glyphosate in common milkweed (Asclepias syriaca L.) (Morrison et al. Reference Morrison, Lownds and Sterling1995; Peregoy et al. Reference Peregoy, Kitchen, Jordan and Griffin1990; Waldecker and Wyse Reference Waldecker and Wyse1985). Therefore, the lower levels of injury in the 3 d DSBA plants in our experiment could be attributed to decreased metribuzin absorption and translocation in stressed plants. However, 3 d DSB plants showed the same level of injury as did no-stress plants. Both 3 d DSB and no-stress plants were watered similarly after metribuzin application. Photosynthesis and stomatal conductance of 3 d DSB and no-stress plants at 3 d after metribuzin treatment were similar, which shows that 3 d DSB plants recovered from drought stress. It is likely that 3 d DSB plants were not in stress after metribuzin application; therefore, foliar absorption and translocation of metribuzin could be similar in 3 d DSB and no-stress plants.
No differences in photosynthesis or stomatal conductance were observed between grafted and nongrafted tomato under either drought-stress or non-drought-stress conditions. Photosynthesis rate and stomatal conductance of plants were negatively impacted by drought stress; however, plants recovered after rewatering. Researchers have reported that drought stress reduces photosynthesis, stomatal conductance, respiration, and transpiration in tomato (Brix Reference Brix1962; Nguyen et al. Reference Nguyen, Fuentes and Marschner2012; Rao et al. Reference Rao, Bhatt and Sadashiwa2000).
These results indicate that grafted and nongrafted tomato under drought stress exhibit similar tolerance to metribuzin. Visible metribuzin injury was more pronounced in no-stress and 3 d DSB plants than it was in 3 d DSBA plants. Metribuzin application negatively affected plant height and dry weight in the 3 d DSBA plants. Photosynthesis rate and stomatal conductance of plants were negatively impacted by drought stress; however, this effect disappeared after rewatering plants. Our results suggest that the risk of metribuzin injury after drought stress in grafted tomato appears to be similar to that of nongrafted tomato. Previous studies have shown that tomato is more sensitive to metribuzin injury when it is grown under environmental stress conditions before metribuzin application. However, few studies have evaluated the effect of metribuzin application to stressed tomato plants on tomato yield. Therefore, field or greenhouse studies are needed to determine the impact of metribuzin, both alone and in mixtures with other pesticides, on the yield of grafted tomato plants subjected to stresses such as low light, low or high temperature, nutrient deficiency, or low or high humidity.
Acknowledgments
The authors thank Nicholas Basinger, Shawn Beam, and Samuel McGowen for their assistance with conducting this research. The authors thank Dr. Kent Burkey, who provided the LI-6400 Portable Photosynthesis System and valuable suggestions for the experiment, and Walt Pursley for assisting with the instrumentation. The authors thank the USDA-NIFA-2009-2376 and USDA-SCRI-2011-51181-30963 for providing funding. The authors thank Dr. Cavell Brownie for her assistance with data analysis.








