Introduction
The Willamette Valley is located in western Oregon. More than 400,000 ha are dedicated to diverse agricultural production, including over 170 economically viable crops and livestock, with tree nuts and fruits and grass grown for seed (USDA 2020). At 99% of US hazelnut production, this enterprise is almost exclusively limited to the Willamette Valley. With the introduction of eastern filbert blight–resistant cultivars, hazelnut hectarage has expanded over the last decade, and in 2018 over 31,800 ha of hazelnut were cultivated in the state (USDA 2020). Hazelnuts have replaced previously cultivated crops like grass grown for seed. Hazelnuts are mechanically harvested by picking nuts from the orchard floor. A firm soil enhances the process without debris or plant material. Weed interference is most noticeable during harvest, but weeds compete with trees reducing yield even in older orchards (Kaya-Altop et al. Reference Kaya-Altop, Haghnama, Sarıaslan, Phillippo, Mennan and Zandstra2016). Herbicides are the primary weed management method in hazelnut orchards. A mixture of PRE and POST herbicides is typically used in fall or winter and followed by POST applications in the spring and summer (Olsen and Peachey Reference Olsen and Peachey2013). Weed species that are not controlled in the fall or winter continue to grow, becoming less susceptible to herbicides in later growth stages (Kudsk Reference Kudsk2002).
Italian ryegrass is an important weed species in multiple crops, including hazelnuts. Management of Italian ryegrass has become more complex because of resistant and multiple-resistant populations (Avila-Garcia and Mallory-Smith Reference Avila-Garcia and Mallory-Smith2011; Perez-Jones et al. Reference Perez-Jones, Park, Colquhoun, Mallory-Smith and Shaner2005). Based on a recent survey in the area, 88% of the 75 tested Italian ryegrass populations were herbicide-resistant; among the resistant populations, three-quarters displayed resistance to more than one herbicide (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021). Documented cases of herbicide-resistant Italian ryegrass include resistance to the inhibitors of acetyl-CoA carboxylase (ACCase), acetolactate synthase (ALS), 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), glutamine synthetase, long-chain fatty acids, and photosystem I diverter (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021; Heap Reference Heap2021).
The widespread presence of multiple resistances in the region may result from resistance genes spread through gene flow. Italian ryegrass is an annual, obligately outcrossing wind-pollinated species (Fearon et al. Reference Fearon, Hayward and Lawrence1983), and gene flow and population admixture are to be expected. Gene flow drove resistance spread in California orchards and vineyards, and suppression of pollen and seed production was deemed essential to contain resistance spread (Karn and Jasieniuk Reference Karn and Jasieniuk2017). Although gene flow is often observed in outcrossing species, habitat fragmentation influenced by variable landscapes and diverse agricultural management practices may cause the structuring of a weed population. The structuring of a population indicates its adaptation to a new environment; such adaptation may have profound implications for weed management. The genetic diversity in a small population may provide new functional variants to a weed population that is better fit to thrive in a specific agroecosystem (Dekker Reference Dekker1997). Population structuring has been reported in Italian ryegrass. The genetic differentiation was not associated with geographic isolation but possibly with site-specific crop management actions (Karn and Jasieniuk Reference Karn and Jasieniuk2017). Evidence for potential Italian ryegrass population structuring was noted on the clustered distribution of multiple herbicide resistances (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021). Because herbicide-resistant Italian ryegrass in the Willamette Valley is ubiquitous yet locally unique, site-specific recommendations are needed to optimize weed control. This study was initiated in response to several Italian ryegrass escapes in hazelnut orchards after herbicide application. The objective of this study was to identify effective POST treatments to control Italian ryegrass in hazelnut orchards.
Materials and Methods
Site Description
Experiments were conducted at six hazelnut orchards located in the northern part of the Willamette Valley (Figure 1) during the early spring of 2017 and 2018. The sites were not more than 64 km apart. Fields were selected based on a history of survival of Italian ryegrass with various POST herbicides. In 2017, two adjacent locations in Canby, OR (45.26° N 122.69° W) were chosen. The hazelnut grower had reported Italian ryegrass escapes after treatment with glufosinate. Density of Italian ryegrass ranged from 35 to 50 plants m–2; plants were 22 to 35 cm in height at initiation of the experiment. In 2018, four additional sites were selected. The first site was an orchard located in Amity, OR (45.11° N 123.20° W) selected because of poor Italian ryegrass control with glyphosate, glufosinate, and sethoxydim. Italian ryegrass plants were 20 to 35 cm in height with 10 to 17 plants m–2. The second site was in Dayton, OR (45.22° N 123.07° W), in an orchard with a history of reduced Italian ryegrass control efficacy with either glyphosate or paraquat. Italian ryegrass plants were between 15 to 25 cm in height with 6 to 14 plants m–2. The third site, in Mount Angel, OR (45.06° N 122.80° W), was a 2-yr-old hazelnut orchard located in a field that had formerly been planted to Italian ryegrass grown for seed. Plants were 8 to 15 cm in height with a density of 15 to 22 plants m–2. The fourth site was in Salem, OR (44.94° N 123.03° W) in a newly planted hazelnut orchard known to be infested with glyphosate-resistant Italian ryegrass. Plants were 8 to 15 cm in height with a density of 35 to 50 plants m–2.

Figure 1. Map of the northern Willamette Valley, indicating research site locations. Triangles represent research sites in 2017 and circles are research sites in 2018. The sites are located within 40 mi (about 64 km) of each other as shown in the red rectangle in the map insert of the state of Oregon, USA.
The experiments were conducted as a randomized complete block design with four replicates. Each experimental plot measured 4 by 9 m. The POST herbicides used in the study are listed in Table 1. The 2017 studies include two experimental sites, and the tested herbicides were glyphosate, paraquat, glufosinate, rimsulfuron, sethoxydim, and selected mixtures for a total of nine treatments, including a nontreated plot (Table 2). Treatments containing flazasulfuron were included in the 2018 study for a total of 13 treatments, including a nontreated plot (Table 3).
Table 1. Herbicides used in 2017 and 2018 field studies in hazelnut orchards of Oregon.

a Abbreviations: AMS, ammonium sulfate (BroncMax; Wilbur Ellis, Aurora, CO) added at 1% v/v; COC, crop oil concentrate (Mor-act Crop Oil; Wilbur Ellis) added at 1% v/v; NIS, nonionic surfactant (Rainier; Wilbur Ellis) added at 0.25% v/v.
Table 2. Italian ryegrass control, coverage reduction, and biomass reduction 28 d after a single treatment with POST herbicides in hazelnut orchards in Canby, OR, in 2017.
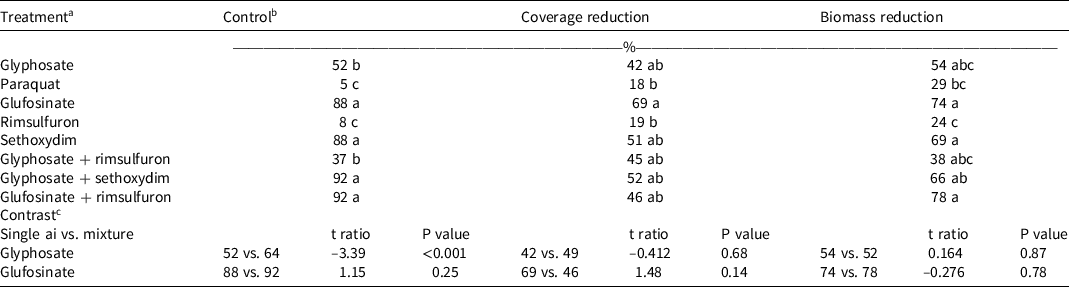
a Treatment means of two field trials (n = 8) conducted in 2017. Glyphosate (1740 g ae ha–1), paraquat (840 g ai ha–1), glufosinate (1,680 g ai ha–1), rimsulfuron (70 g ai ha–1), sethoxydim (315 g ai ha–1). Ammonium sulfate (BroncMax; Wilbur Ellis, Aurora, CO) was added to all treatments at 1% v/v. Nonionic surfactant (Rainier, Wilbur Ellis) was added at 0.25 % vol/vol to all treatments containing glyphosate, flazasulfuron, rimsulfuron, and paraquat. Crop oil concentrate (Mor-act Crop Oil; Wilbur Ellis) was added at 1% v/v to treatments containing sethoxydim.
b
Means within a column followed by the same letter are not different based on Šidák’s significance test (P
![]() $$ \le $$
0.05). Weed control, coverage, and biomass are presented as percent reduction relative to nontreated control.
$$ \le $$
0.05). Weed control, coverage, and biomass are presented as percent reduction relative to nontreated control.
c Contrast tests comparing treatments with single active ingredient to mixtures.
Table 3. Italian ryegrass control 28 d after treatment in hazelnut orchards of the Willamette Valley, OR, in 2018.
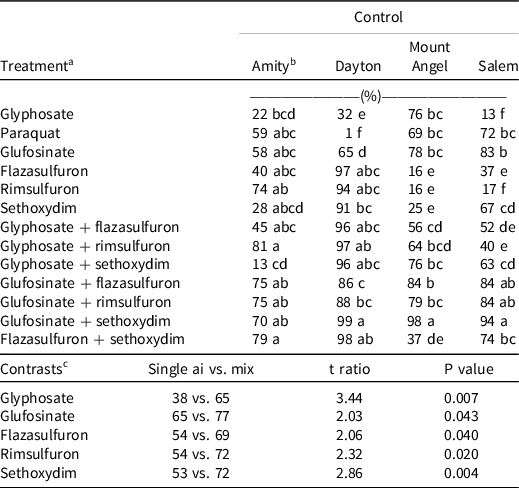
a Treatment means of one field trial (n = 4) conducted in 2018. Glyphosate (1,740 g ae ha–1), paraquat (840 g ai ha–1), glufosinate (1,680 g ai ha–1), rimsulfuron (70 g ai ha–1), sethoxydim (315 g ai ha–1). Ammonium sulfate (BroncMax; Wilbur Ellis, Aurora, CO) was added to all treatments at 1% v/v. Nonionic surfactant (Rainier; Wilbur Ellis) was added at 0.25 % v/v to all treatments containing glyphosate, flazasulfuron, rimsulfuron, and paraquat. Crop oil concentrate (Mor-act Crop Oil; Wilbur Ellis) was added at 1% v/v to treatments containing sethoxydim.
b
Means within a column followed by the same letter are not different based on Šidák’s significance test (P
![]() $$ \le \;$$
0.05). Coverage reduction and biomass reduction are presented as percent reduction relative to nontreated control.
$$ \le \;$$
0.05). Coverage reduction and biomass reduction are presented as percent reduction relative to nontreated control.
c Contrast tests comparing treatments with single active ingredient to mixtures. Means were averaged across all experimental sites.
Treatments were applied with a CO2-pressurized backpack sprayer connected to a four-nozzle boom equipped with AI11002 nozzles (TeeJet®; Spraying Systems Co., Wheaton, IL 60187), calibrated to deliver 187 L ha–1 at 275 kPa at 4.8 km h–1. The 2017 treatments were applied on May 2 and May 4, respectively, at the first and second sites. The 2018 sites were treated on April 18 at the Amity site, on March 30 at the Dayton site, and on March 7, 2018 at the Salem and Mount Angel sites. The phenological stage of the hazelnut trees ranged from bud swell (BBCH 02) for application made in early March, bud break (BBCH 07) in late March, and first true leaf (BBCH 11) (Meier et al. Reference Meier, Bleiholder, Brumme, Bruns, Mehring, Proll and Wiegand2009).
Assessments and Statistical Analysis
Visual estimates of weed control were performed 28 d after treatment using a scale of 0% (no control) to 100% (plant death). Groundcover by Italian ryegrass was measured using digital image analysis, as described by Ali et al. (Reference Ali, Streibig, Duus and Andreasen2013). Field images were collected with a point-and-shoot camera (COOLPIX AW110; Nikon Inc.) using automatic focal adjustments. A single picture per plot was recorded at 1.2 m above the ground under daylight conditions. Images were analyzed using ImageJ software (Ferreira and Rasband Reference Ferreira and Rasband2012). The green color of the field images was segmented using the HSB values on a scale of 0 to 255 as Hue 46 to 120, saturation 0 to 255, and brightness 20 to 255. A subset of pictures was ground-truthed by visual validation. File output was in binary format, and the procedure analyze particle in ImageJ was used to estimate the relative (%) area of the image covered by green color. Coverage reduction was calculated relative to the nontreated control plots. The difference between values recorded on the treated and nontreated plots was divided by the measured value of nontreated plots. Italian ryegrass biomass was collected by harvesting the aboveground biomass from a 0.25-m–2 quadrat per plot in the center of the plot. Biomass was dried for 96 h at 52 C and the weights recorded. Biomass reduction was calculated using the procedure described for coverage reduction.
Statistical analysis was performed in RStudio 1.4.1103 (RStudio Team 2021) using a generalized linear mixed model (GLMM) with the package glmmTMB version 1.0.2.11 (Brooks et al. Reference Brooks, Kristensen, van Benthem, Magnusson, Berg, Nielsen, Skaug, Machler and Bolker2017), using a beta distribution with a logit error distribution (Stroup Reference Stroup2015) with significance value of P ≤ 0.05. The herbicide treatment and sites were treated as fixed factors, as the intent was to identify site-specific responses. The 2017 and 2018 data were analyzed separately. No interaction between experimental site and treatment was observed in 2017, and the data were combined for analysis. A significant effect of experimental site was observed in the 2018 studies. Therefore, data were analyzed independently for each 2018 site. Means were compared using a Šidák test, with a 95% confidence interval using the emmeans package version 1.5.4 and the cld function (Lenth Reference Lenth2019; Šidák Reference Šidák1967). Preplanned contrast tests were developed to compare the effect of mixing active ingredient to the active ingredients alone, and statistical significance was corrected with Bonferroni adjustment. Contrast comparisons were made as a combined analysis across the experimental locations in 2018 by treating the experimental locations as a random factor. This approach allows for the comparison of mixtures to single active ingredients as a resistance management strategy in a broader scope, as proposed previously (Moore and Dixon Reference Moore and Dixon2015).
Results and Discussion
Control of Italian ryegrass in 2017 in Canby, OR ranged from 5% to 92% among treatments (Table 2), suggesting that this was an herbicide-resistant population. Glyphosate applied alone resulted in 52% control, whereas negligible control was observed with paraquat (5%) and rimsulfuron (8%). Glufosinate and sethoxydim controlled 88% of the Italian ryegrass. Not all mixtures provided adequate control of Italian ryegrass. Glyphosate with rimsulfuron resulted in 37% control, whereas glyphosate with sethoxydim provided 92% control of Italian ryegrass. Treatments containing either glufosinate or sethoxydim resulted in a greater reduction of ground coverage (51% to 69%) and plant biomass (69% to 74%) relative to the nontreated control (Table 2). Mixtures with glyphosate improved control of Italian ryegrass (P < 0.05) but did not affect reduction of biomass coverage based on contrast. Glufosinate was similar when used alone or in a mixture for all measured parameters. Resistances to glyphosate and rimsulfuron, ALS-inhibiting herbicides, are among the most frequently detected resistances in the Willamette Valley (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021). This population was probably resistant to paraquat as well.
In the 2018 field studies, results were dependent on the herbicide treatment and the experimental site. No treatment containing a single active ingredient satisfactorily controlled Italian ryegrass (>80%) across all sites (Table 3). Control of Italian ryegrass with glyphosate was lowest in Salem (13%); glyphosate-resistant Italian ryegrass was expected to be present based on grower-reported treatment failures. Poor control with glyphosate was observed in most locations except in Mount Angel (76% control, Table 3). Likewise, paraquat provided 1% control in Dayton and 59% to 72% control in other locations. Glufosinate provided the most consistent control among the single-active-ingredient treatments (58% to 83%). The ALS-inhibiting herbicides flazasulfuron and rimsulfuron and the ACCase-inhibiting herbicide sethoxydim controlled only the Dayton Italian ryegrass population (97%, 94%, and 91% control, respectively). Italian ryegrass from other locations were only suppressed (40% to 74%) or minimally affected (<37% control) with these herbicides (Table 3).
Efficacy with mixtures also depended on the experimental site, indicating the presence of multiple-resistant populations. When considering mixtures of glyphosate, glyphosate plus flazasulfuron improved control in Italian ryegrass at Dayton and Salem (P ≤ 0.05) but not in other locations (Table 3). Notably, glyphosate plus sethoxydim failed to control the Amity populations (13%). The highest levels of control were observed with mixtures containing glufosinate (70% to 99%). Combining across all experimental sites, mixtures improved Italian ryegrass control compared to single active ingredients. Mixtures improved control with glyphosate by 34%, glufosinate by 14%, flazasulfuron by 30%, rimsulfuron by 26%, and sethoxydim by 23% (Table 3).
Coverage reduction ranged from 6% to 99% (Table 4). As observed with the control, no single-active-ingredient treatment reduced coverage consistently across experimental sites. Coverage reduction by glyphosate and paraquat had the greatest range (12% to 88% and 6% to 83%, respectively; Table 4). Glufosinate alone was the most consistent, reducing Italian ryegrass ground coverage by 66% to 83%; in mixtures reduced ground coverage by 71% to 99 % (Table 4). Mixtures including glyphosate reduced coverage by 44 more than either glyphosate alone (P < 0.0001). A similar response was observed in Italian ryegrass biomass reduction (Table 5). Glufosinate alone or in mixture resulted in the most consistent reduction of biomass (73% and 98%). Regrowth of Italian ryegrass was observed with glufosinate (personal observation). Glufosinate with sethoxydim resulted in the greatest control, coverage, and biomass reduction, probably influenced by the crop oil adjuvant included in that treatment. Crop oil adjuvant has been shown to improved glufosinate efficacy in other weed species (Costa et al. Reference Costa, Kfouri, Fávaro, Jordao, da Silva, Rodrigues and Ferguson2019).
Table 4. Italian ryegrass ground coverage reduction 28 d after treatment in hazelnut orchards of the Willamette Valley, OR, in 2018.

a Treatment means of one field trial (n = 4) conducted in 2018. Glyphosate (1,740 g ae ha–1), paraquat (840 g ai ha–1), glufosinate (1,680 g ai ha–1), rimsulfuron (70 g ai ha–1), sethoxydim (315 g ai ha–1). Ammonium sulfate (BroncMax; Wilbur Ellis, Aurora, CO) was added to all treatments at 1% v/v. Nonionic surfactant (Rainier; Wilbur Ellis) was added at 0.25% v/v to all treatments containing glyphosate, flazasulfuron, rimsulfuron, and paraquat. Crop oil concentrate (Mor-act Crop Oil; Wilbur Ellis) was added at 1% v/v to treatments containing sethoxydim.
b
Means within a column followed by the same letter are not different based on Šidák’s significance test (P
![]() $$ \le \;$$
0.05). Coverage reduction and biomass reduction are presented as percent reduction relative to nontreated control.
$$ \le \;$$
0.05). Coverage reduction and biomass reduction are presented as percent reduction relative to nontreated control.
c Contrast tests comparing treatments with single active ingredient to mixtures. Means were averaged across all experimental sites.
Table 5. Italian ryegrass biomass reduction 28 d after treatment in hazelnut orchards of the Willamette Valley, OR, in 2018.

a Treatment means of one field trial (n = 4) conducted in 2018. Glyphosate (1,740 g ae ha–1), paraquat (840 g ai ha–1), glufosinate (1,680 g ai ha–1), rimsulfuron (70 g ai ha–1), sethoxydim (315 g ai ha–1). Ammonium sulfate (BroncMax; Wilbur Ellis, Aurora, CO) was added to all treatments at 1% v/v. Nonionic surfactant (Rainier; Wilbur Ellis) was added at 0.25 % v/v to all treatments containing glyphosate, flazasulfuron, rimsulfuron, and paraquat. Crop oil concentrate (Mor-act Crop Oil; Wilbur Ellis) was added at 1% v/v to treatments containing sethoxydim.
b
Means within a column followed by the same letter are not different based on Sidak’s significance test (P
![]() $$ \le $$
0.05). Coverage reduction and biomass reduction are presented as percent reduction relative to nontreated control.
$$ \le $$
0.05). Coverage reduction and biomass reduction are presented as percent reduction relative to nontreated control.
c Contrast tests comparing treatments with single active ingredient to mixtures. Means were averaged across all experimental sites.
Effective control of Italian ryegrass to avoid pollen and seed production is essential to contain further spread of herbicide resistance through gene flow (Karn and Jasieniuk Reference Karn and Jasieniuk2017). In hazelnuts, effective control is also needed for preharvest operations, as soil free of debris improves nut harvest. The results of this research indicate that none of the currently available POST herbicides can control Italian ryegrass in Oregon (Tables 2 and 3). Poor control was observed with inhibitors of ACCase, ALS, EPSPs, and photosystem I electron diverter. Italian ryegrass response to herbicides is intrinsically variable, perhaps because of its high genetic diversity. Variability in control with POST herbicides was reported even in commercially Italian ryegrass varieties grown as cover crops (Cornelius and Bradley Reference Cornelius and Bradley2017; Whalen et al. Reference Whalen, Bish, Young, Conley, Reynolds, Norsworthy and Bradley2019). In previous studies, glyphosate treatments provided 71% to 90% control of Italian ryegrass (Whalen et al. Reference Whalen, Bish, Young, Conley, Reynolds, Norsworthy and Bradley2019), and early-April applications treatments performed better because of smaller plant size (Cornelius and Bradley Reference Cornelius and Bradley2017). In the present study, herbicide failure in specific sites was observed even when smaller plants (8 to 15 cm) were treated.
The use of multiple modes of action in mixtures or in a sequence is a strategy often recommended for resistance management (Beckie and Reboud Reference Beckie and Reboud2009) and was confirmed in the present study. However, this approach was only effective because certain herbicides, notably glufosinate, maintain their efficacy. Glufosinate was effective against Italian ryegrass populations because it was applied at 1,680 g ai ha–1, a higher rate than what is used in other cropping systems (560 to 1,333 g ai ha–1) (Aulakh and Jhala Reference Aulakh and Jhala2015; Cornelius and Bradley Reference Cornelius and Bradley2017; Whalen et al. Reference Whalen, Bish, Young, Conley, Reynolds, Norsworthy and Bradley2019). The high rate of glufosinate used in this study (1,680 g ai ha–1) was probably able to control most glufosinate-resistant populations present, but survival and regrowth were noted. The glufosinate resistance level reported in the Italian ryegrass in Oregon was 2.4-fold based on growth reduction, with 72% to 89% of mortality observed after treatment with glufosinate at 2 kg ai ha–1 (Avila-Garcia and Mallory-Smith Reference Avila-Garcia and Mallory-Smith2011). Escaping plants are able to produce pollen and seeds, worsening the resistance problem.
In addition to POST herbicides, PRE herbicides are used to control weeds in hazelnuts, increasing the diversity of multiple modes of action. PRE herbicides are not used during the dry season, and they have limited efficacy in established plants. Because Italian ryegrass has a high degree of plasticity in germination and seed dormancy, it can germinate across a broad period of the year, allowing it to adjust to different environmental conditions or management practices. Consequently, Italian ryegrass germination and flowering can occur during an extensive period of time (Gundel et al. Reference Gundel, Martínez-Ghersa and Ghersa2008), allowing plants to escape PRE herbicides. Control of Italian ryegrass escapes with POST can be restricted if late-emerging plants have resistance traits. High seed dormancy in Italian ryegrass was correlated with resistance to POST herbicides in Italian ryegrass populations from Argentina and the United States (Gundel et al. Reference Gundel, Martínez-Ghersa and Ghersa2008; Maity et al. Reference Maity, Singh, Jessup and Bagavathiannan2021).
Effective nonchemical weed control tools are needed to mitigate herbicide resistance. The inconsistent response observed in Italian ryegrass to POST herbicides highlights this need. Nevertheless, currently available nonchemical weed control tools are neither practical nor effective in hazelnuts and other tree nut crops. Tillage is not suitable because of the negative impact on harvest. Mowing requires multiple and frequent operations (Ollerenshaw and Hodgson Reference Ollerenshaw and Hodgson1977) to only suppress weed growth and seed production (Donald Reference Donald2006), making it cost-prohibitive. A fundamental change is necessary to provide adequate nonchemical weed control in hazelnut and other tree crops. For the time being, Italian ryegrass can be controlled by glufosinate and its mixtures. Other treatments may be effective in a site-specific manner. The complexity of this site-specific recommendation emphasizes the importance of field efficacy to inform the selection of effective weed management programs in hazelnuts.
Acknowledgments
This work was supported by the Oregon Hazelnut Commission. No conflicts of interest have been declared. Thanks to Pratum Coop, Wilbur Ellis, Nutrien Ag, and others for the assistance provided during the execution of the project. The author would like to acknowledge the contribution of Andre Caixeta Consonni in data collection.








