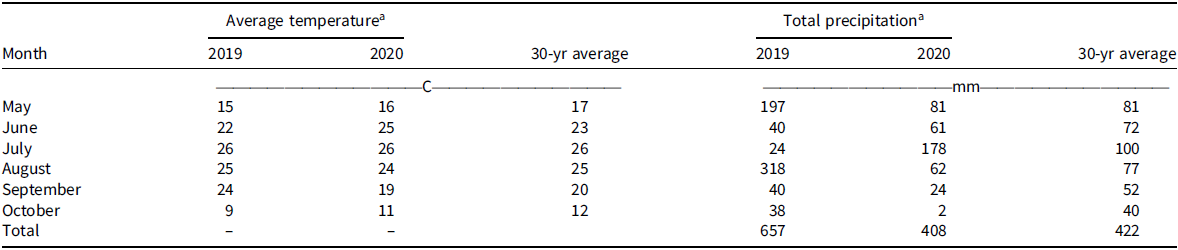Introduction
Palmer amaranth, a native to the desert regions of the southwestern United States and northern Mexico, is one of the most troublesome summer annual broadleaf weeds in agronomic crops in the United States (Van Wychen Reference Van Wychen2017; Vencill et al. Reference Vencill, Grey, Culpepper, Gaines and Westra2008). Palmer amaranth is a dioecious species that possesses unique characteristics, including an extended period of emergence, rapid growth rate, C4 photosynthetic pathway, and high seed production (Horak and Loughin Reference Horak and Loughin2000; Keeley et al. Reference Keeley, Carter and Thullen1987; Ward et al. Reference Ward, Webster and Steckel2013). Palmer amaranth exhibits a high genetic diversity within and among field populations and a high propensity to evolve herbicide resistance (Chahal et al. Reference Chahal, Aulakh, Jugulam and Jhala2015; Heap 2021; Ward et al. Reference Ward, Webster and Steckel2013). Palmer amaranth populations with resistance to multiple herbicide sites of action (SOAs), including inhibitors of acetolactate synthase, 5-enolpyruvyl shikimate-3-phosphate synthase, dinitroanilines, photosystem II, 4-hydroxyphenyl pyruvate dioxygenase, protoporphyrinogen oxidase, and synthetic auxins have been reported in the United States (Chahal et al. Reference Chahal, Varanasi, Jugulam and Jhala2017; Garetson et al. Reference Garetson, Singh, Singh, Dotray and Bagavathiannan2019; Heap 2021; Jhala et al. Reference Jhala, Sandell, Rana, Kruger and Knezevic2014; Kumar et al. Reference Kumar, Liu, Boyer and Stahlman2019, Reference Kumar, Liu and Stahlman2020). Reduced sensitivity to glyphosate, chlorsulfuron, atrazine, and mesotrione has been detected in several Palmer amaranth populations in southcentral Kansas (Kumar et al. Reference Kumar, Liu and Stahlman2020). Furthermore, five-way resistance to 2,4-D, glyphosate, chlorsulfuron, atrazine, and mesotrione has also been confirmed in a single Palmer amaranth population (Kumar et al. Reference Kumar, Liu, Boyer and Stahlman2019, Reference Kumar, Liu and Stahlman2020). Increased occurrence of multiple-herbicide-resistant Palmer amaranth poses a serious threat to no-tillage (NT) dryland cropping systems of the Central Great Plains (CGP).
Wheat (Triticum aestivum L.)–chemical fallow (2-yr) or wheat–summer crop–fallow (3-yr) are two predominant crop rotations in the NT semiarid CGP region (Lenssen et al. Reference Lenssen, Johnson and Carlson2007; Peterson and Westfall Reference Peterson and Westfall2004) where Palmer amaranth has become a serious problem (Kumar et al. Reference Kumar, Liu and Stahlman2020). Palmer amaranth cohorts begin to emerge during May and June in wheat with extended emergence through July and August, resulting in depletion of available soil water and replenishment of the soil seedbank after wheat harvest. If not controlled, high seed production potential of Palmer amaranth cohorts that escape in-season herbicide applications or those emerging late in the season can enhance the risk of herbicide resistance evolution (Bagavathiannan and Norsworthy Reference Bagavathiannan and Norsworthy2012). Timely weed control in postharvest wheat stubble is also critical to conserve soil water for successful production of subsequent crops in this region (Anderson and Nielsen Reference Anderson and Nielsen1996). Haag and Schlegel (Reference Haag and Schlegel2018) reported that delaying weed control from July to August after wheat harvest resulted in soil water depletion and ultimately reduced subsequent corn (Zea mays L.) grain yields, biomass production, water use, and water use efficiency in a long-term study in Kansas. Additionally, late-season weed control at or near flowering/seed set stage in postharvest wheat stubble has the advantage of depleting the soil seedbank, an important strategy for mitigating herbicide resistance in weed populations (Jha and Norsworthy Reference Jha and Norsworthy2012; Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos and Witt2012; Taylor and Oliver Reference Taylor and Oliver1997; Walker and Oliver Reference Walker and Oliver2008).
Previous research documented that glufosinate, 2,4-D, or dicamba applied at the inflorescence initiation stage reduced seed production of glyphosate-resistant (GR) Palmer amaranth by 95%, and seed viability by 39% to 51% (Jha and Norsworthy Reference Jha and Norsworthy2012). Apart from Palmer amaranth, Ganie et al. (Reference Ganie, Kaur, Jha, Kumar and Jhala2018) reported ≥96% inflorescence injury and seed reduction of GR giant ragweed (Ambrosia trifida L.) with single or sequential, late-season applications of 2,4-D or dicamba. Up to 99% seed reduction has been documented in common lambsquarters (Chenopodium album L.), redroot pigweed (Amaranthus retroflexus), velvetleaf (Abutilon theophrasti L.), and sicklepod (Senna obtusifolia L.) with late-season applications of glyphosate, 2,4-D, dicamba, or glufosinate (Biniak and Aldrich Reference Biniak and Aldrich1986; Fawcett and Slife Reference Fawcett and Slife1978; Taylor and Oliver Reference Taylor and Oliver1997).
Although previous research tested the effectiveness of late-season herbicides on GR Palmer amaranth, only limited postemergence herbicide options (glyphosate, glufosinate, 2,4-D, dicamba, and pyrithiobac) were investigated (Jha and Norsworthy Reference Jha and Norsworthy2012). In addition, the study was conducted in a water-enriched environment in Arkansas. Information on the efficacy of late-season postemergence herbicides (containing two or three herbicide SOAs) for Palmer amaranth control in a water-limited environment (NT dryland CGP region) is sparse. Furthermore, the evolution of Palmer amaranth resistant to glyphosate, chlorsulfuron, mesotrione, atrazine, and 2,4-D in Kansas, and recent reports of dicamba-resistant Palmer amaranth in Tennessee and glufosinate-resistant Palmer amaranth in Arkansas (Barber et al. Reference Barber, Norsworthy and Butts2021; Kumar et al. Reference Kumar, Liu, Boyer and Stahlman2019, Reference Kumar, Liu and Stahlman2020) necessitate the need for late-season alternative postemergence herbicide programs for effective control. The objective of this research was to evaluate the efficacy of late-season herbicides applied alone or in a mixture on Palmer amaranth control, shoot biomass, and seed production in postharvest wheat stubble.
Materials and Methods
Field studies were conducted at the Kansas State University Agricultural Research Center (KSU-ARC) in Hays, KS, during the 2019 and 2020 growing seasons, to determine the effectiveness of late-season herbicides for Palmer amaranth control in postharvest wheat stubble. The soil type at the study site was a Roxbury silt loam (fine-silty, mixed, superactive, mesic Cumulic Haplustolls) with 10% sand, 56% silt, and 34% clay with a pH of 7.6 and 2.1% organic matter. The study site was under a NT dryland wheat–sorghum [Sorghum bicolor (L.) Moench ssp. bicolor]–fallow rotation prior to study initiation. The study site had a natural Palmer amaranth population that was known to be susceptible to herbicides tested in this study. Winter wheat variety ‘Joe’ (67 kg ha−1) was drilled using an NT drill at a row spacing of 15 cm on October 10, 2018, and October 14, 2019. The study was established under NT dryland wheat production and fertilized with nitrogen-phosphorus-potash per KSU recommendations for winter wheat production (Lollato and Edwards Reference Lollato and Edwards2015). Wheat plots were harvested using a commercial combine (JD 6620; John Deere, Deere & Company, Moline, IL). Detailed information on tested herbicides, their application rates, SOAs, and manufacturer is summarized in Table 1. A nontreated control was included for treatment comparison. Herbicides were applied using a handheld CO2-pressurized backpack boom sprayer equipped with flat-fan nozzles (Turbo Teejet AIXR 110015-VP; Spraying Systems Co., Wheaton, IL), calibrated to deliver 140 L ha−1 of spray solution at 276 kPa. Treatments were applied about 3 wk after wheat harvest on August 2, 2019, and August 14, 2020, when Palmer amaranth plants had attained inflorescence initiation stage (average height of 60 to 90 cm). Treatments were arranged in a randomized complete block design with four replications. Individual test plots were 9 m long by 3 m wide. Palmer amaranth control was assessed visually at 2, 4, and 8 wk after treatment (WAT) on a scale of 0% to 100% (where 0% = no control and 100% = complete control/plant death). Palmer amaranth control was based on symptoms such as chlorosis, stunting, and/or necrosis. At 8 WAT, Palmer amaranth plants were harvested at the soil level using a 1-m2 quadrat from the center of each plot. The harvested samples from each plot were oven-dried at 65 C for 3 d to determine aboveground shoot biomass. The oven-dried plant material was manually threshed, and seeds were separated from the inflorescence using different sized sieves, and an air-propelled column blower. Total seed weight and number of seeds per square meter were determined using an average 1,000-seed weight calculated from three subsamples per plot.
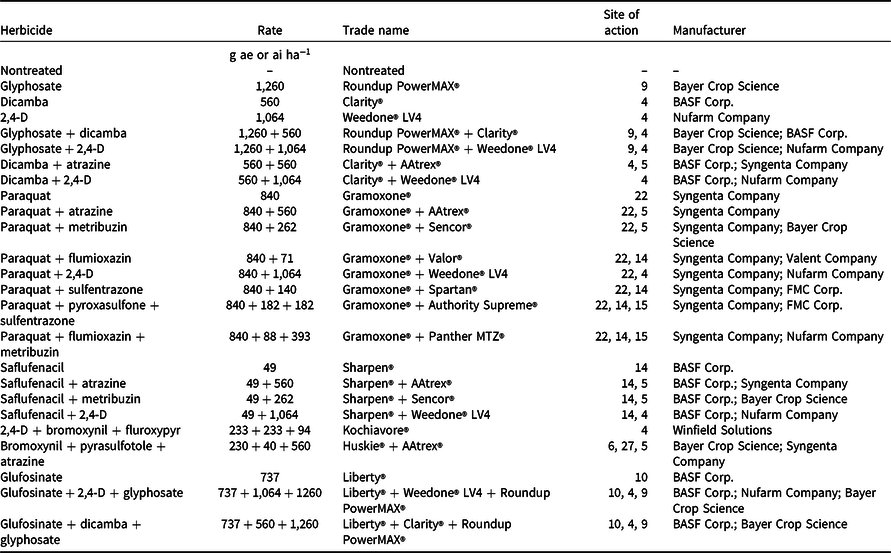
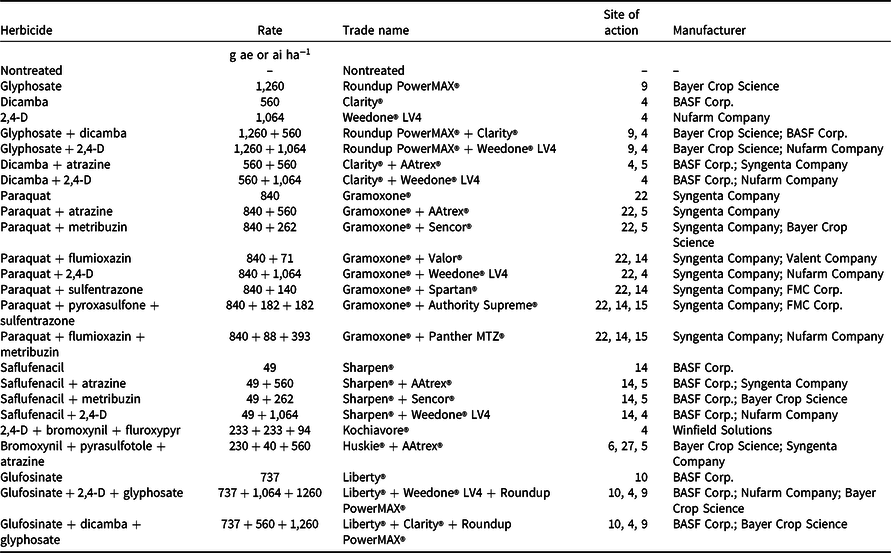
Table 1. List of herbicide treatments, application rates, trade names, and manufacturers used in this study.
Statistical Analyses
Data were checked for ANOVA assumptions using the UNIVARIATE procedure in SAS® version 9.3 software (SAS Institute Inc., Cary, NC). Data of Palmer amaranth control and seed production were square root–transformed, and data for shoot biomass were arcsine-transformed in order to improve the normality of residuals and homogeneity of variance. Nontransformed means were presented in tables based on the interpretation from the transformed data. Data were subjected to ANOVA using the MIXED procedure in SAS. Year, herbicide treatment, and their interaction were fixed effects in the ANOVA model, whereas replication was a random effect. Due to a nonsignificant year by herbicide interaction (P = 0.132), data were combined across experimental years. Zero percent control of Palmer amaranth from nontreated control plots were excluded from the analyses. Where the ANOVA indicated significant differences, means were separated using Fisher’s protected LSD test (α = 0.05).
Results and Discussion
Monthly mean air temperature at the experimental site ranged from 9 C to 26 C in 2019 and 2020 growing seasons and 12 C to 26 C for 30-yr average (Table 2). Total precipitation received during the 2019 and 2020 growing seasons was 657 mm and 408 mm, respectively (Table 2). In comparison, the 30-yr averaged precipitation amount received at the experimental site was 422 mm (Table 2). A relatively high precipitation amount received in August 2019 (318 mm) and July 2020 (178 mm) coupled with optimum air temperature for Palmer amaranth seed germination resulted in a high infestation of Palmer amaranth in postharvest wheat stubble.
Table 2. Mean monthly air temperature and total precipitation during the 2019 and 2020 growing seasons and 30-yr average at Kansas State University Agricultural Research Center in Hays, KS.
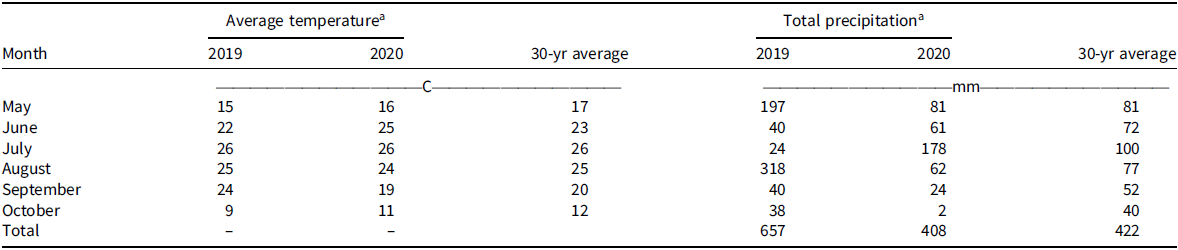
a Air temperature and precipitation data were obtained from the weather station located at Kansas State University Agricultural Research Center near Hays, KS (https://mesonet.k-state.edu/).
Palmer Amaranth Control
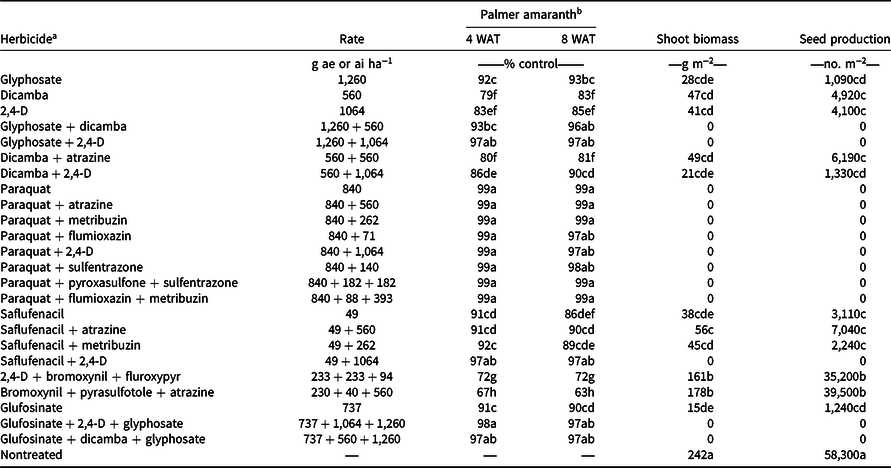
Palmer amaranth densities ranged from 28 to 44 plants m−2 in wheat stubble at the time of herbicide applications in both years (data not shown). Among tested herbicide programs, paraquat applied alone or in mixture with atrazine, metribuzin, flumioxazin, 2,4-D, sulfentrazone, pyroxasulfone + sulfentrazone, or flumioxazin + metribuzin provided 97% to 99% control of Palmer amaranth 8 WAT (Table 3). These results are consistent with those reported by Hay et al. (Reference Hay, Shoup and Peterson2019) that paraquat mixed with flumioxazin or flumioxazin + metribuzin can provide 90% to 93% control of Palmer amaranth 8 WAT in Kansas. Paraquat is a nonselective contact broad-spectrum herbicide that can control grass and broadleaf weeds (Anonymous 2021). Kumar and Jha (Reference Kumar and Jha2015) reported 98% control of kochia with 99% reduction in seed production with paraquat applied late-season when kochia was 45 cm to 50 cm tall. Furthermore, Palmer amaranth control with glyphosate + dicamba, glyphosate + 2,4-D, saflufenacil + 2,4-D, glufosinate + dicamba + glyphosate, and glufosinate + 2,4-D + glyphosate was excellent (96% to 97%) 8 WAT in the current study (Table 3). Control ranged from 89% to 93% 8 WAT with glyphosate, glufosinate, dicamba + 2,4-D, saflufenacil + atrazine, and saflufenacil + metribuzin treatments and from 81% to 86% control with dicamba, 2,4-D, dicamba + atrazine, and saflufenacil (Table 3). Dicamba mixed with glyphosate improved Palmer amaranth control compared to glyphosate applied alone (Inman et al. Reference Inman, Jordan, York, Jennings, Monks, Everman, Bollman, Fowler, Cole and Soteres2016). Among herbicide treatments tested, bromoxynil + pyrasulfotole + atrazine and 2,4-D + bromoxynil + fluroxypyr provided the least control of Palmer amaranth (63% to 72%) 8 WAT.
Table 3. Efficacy of various herbicides for Palmer amaranth control, shoot biomass, and seed production in postharvest wheat stubble averaged across 2019 and 2020 growing seasons at the Kansas State University Agricultural Research Center near Hays, KS.
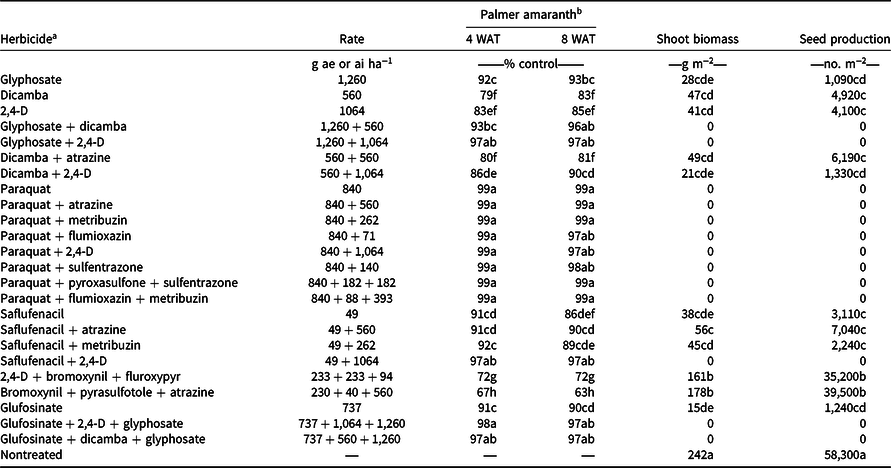
a Herbicides were applied on August 2, 2019, and August 14, 2020, when Palmer amaranth plants were 60 to 90 cm tall and inflorescence initiation had started.
b Means within a column with similar letters are not significantly different based on Fisher’s protected LSD test (α = 0.05).
Shoot Dry Biomass and Seed Production
Averaged across years, the nontreated control had the highest Palmer amaranth shoot biomass (242 g m−2) and seed production (58,300 seeds m−2) compared with herbicide programs, suggesting that the most of herbicides tested were effective for controlling Palmer amaranth and reducing seed production. Consistent with Palmer amaranth control, the most of tested herbicides prevented shoot biomass and seed production of Palmer amaranth (Table 3). In contrast, Palmer amaranth plants treated with glyphosate, dicamba, 2,4-D, dicamba + atrazine, dicamba + 2,4-D, saflufenacil, saflufenacil + atrazine, saflufenacil + metribuzin, and glufosinate produced shoot biomass ranging from 15 to 56 g m−2 and produced 1,090 to 7,040 seeds m−2 (Table 3). Averaged over the2 yr, 2,4-D + bromoxynil + fluroxypyr and bromoxynil + pyrasulfotole + atrazine were least effective in reducing Palmer amaranth shoot biomass (161 to 178 g m−2) and seed production (35,200 to 39,500 seeds m−2) in postharvest wheat stubble (Table 3). These results are consistent with previous reports, in which late-season applications of glufosinate, dicamba, and 2,4-D reduced seed production of GR Palmer amaranth by 95% and seed viability by 39% to 51% in Arkansas (Jha and Norsworthy Reference Jha and Norsworthy2012). Late-season applications of glufosinate, dicamba, 2,4-D, and glyphosate also reduced up to 99% seed production of other weed species such as redroot pigweed (Amaranthus retroflexus L.), velvetleaf (Abutilon theophrasti L.), sicklepod (Senna obtusifolia L.), and common lambsquarters (Chenopodium album L.; Biniak and Aldrich Reference Biniak and Aldrich1986; Fawcett and Slife Reference Fawcett and Slife1978; Taylor and Oliver Reference Taylor and Oliver1997). Similarly, a single or sequential late-season applications of 2,4-D or dicamba provided >96% inflorescence injury and seed reduction of GR giant ragweed (Ganie et al. Reference Ganie, Kaur, Jha, Kumar and Jhala2018).
Practical Implications
If left uncontrolled, Palmer amaranth cohorts emerging after wheat harvest can contribute >50,000 seeds m−2. Late-season applications of paraquat alone or in a mixture with atrazine, metribuzin, flumioxazin, 2,4-D, sulfentrazone, pyroxasulfone + sulfentrazone, or flumioxazin + metribuzin; and glyphosate + dicamba, glyphosate + 2,4-D, saflufenacil + 2,4-D, glufosinate + dicamba + glyphosate, and glufosinate + 2,4-D + glyphosate provided 96% to 99% control and prevented seed production of Palmer amaranth in postharvest wheat stubble. These results highlight that aforementioned herbicide programs were quite effective even when applied to 60- to 90-cm-tall Palmer amaranth plants. Among all tested late-season herbicide programs, 2,4-D + bromoxynil + fluroxypyr and bromoxynil + pyrasulfotole + atrazine failed to provide adequate control (as low as 63% to 72% at 8 WAT) and had the lowest reductions of Palmer amaranth biomass and seed production. Late-season control of Palmer amaranth in wheat stubble would be a crucial component of integrated Palmer amaranth management programs to manage the soil seed bank of herbicide-resistant Palmer amaranth in the CGP region. Besides future infestations and soil seedbank replenishments, Palmer amaranth control after wheat harvest is also important to conserve available soil water for subsequent crops in the CGP region (Haag and Schlegel Reference Haag and Schlegel2018). Although late-season applications of soil residual herbicides such as atrazine, flumioxazin, metribuzin, pyroxasulfone, and sulfentrazone with burndown chemistries were not evaluated in the current study, they would also help in controlling winter annual weeds in winter months and Palmer amaranth during early spring in the subsequent year. However, it is important to understand the plant-back restrictions and carryover effects of these soil residual herbicides in NT dryland environment when making decisions on crop selection in the following season.
Although the late-season application of paraquat alone provided excellent control of Palmer amaranth in this study, the occurrence of resistance to multiple herbicide SOAs evident in Kansas and some neighboring states makes the use of a single SOA inadvisable. Palmer amaranth seedlings can emerge after late-season herbicide applications under favorable conditions (high temperature and available surface soil moisture), which can lead to seed production and soil seedbank additions for future infestation. For effective control of late-emerging cohorts, mixes of paraquat with soil residual herbicides investigated in this study should be used for controlling Palmer amaranth in postharvest wheat stubble. Future studies should investigate the use of other tactics, including alternative crops such as summer cover crops, double cropping sequence, improved crop rotations, and strategic tillage for managing Palmer amaranth seedbanks in NT dryland production systems of the CGP region.
Acknowledgments
We thank Mr. Taylor Lambert and Ms. Natalie Aquilina at Kansas State University Agricultural Research Center in Hays, KS, for providing technical assistance in conducting the field experiments. No specific funding was sought to support this research. No conflicts of interest have been declared. This publication is contribution no. 21-328-J from the Kansas Agricultural Experiment Station, Manhattan, KS.