Introduction
The United States produces 2,707 million pounds of fresh-market tomato and 29,509 million pounds of processed tomato annually (USDA 2016). In Mississippi, tomato is grown on more than 444 acres across 627 farms, with gross sales of more than $6 million annually (USDA 2012). A tomato crop favors the occurrence of weeds during its growing cycle due to wide plant spacing and slow development in the initial weeks after transplanting or seeding (Ronchi et al. Reference Ronchi, Serrano, Silva and Guimarães2010). Moreover, it is sensitive to many herbicides, and weed management is a significant constraint in tomato production (Ormeño et al. Reference Ormeño, Fuentes and Soffia2003). There are reports of reduced fruit yield due to interference caused by weeds (Ronchi et al. Reference Ronchi, Serrano, Silva and Guimarães2010). Yellow nutsedge can cause a 28% reduction in tomato dry weight (Morales-Payan et al. Reference Morales-Payan, Stall, Shilling, Charudattan, Dusky and Bewick2003), whereas yield reductions of up to 77% were observed with Chenopodium album, Ambrosia artemisiifolia, Amaranthus spp., and Digitaria sanguinalis (Weaver and Tan Reference Weaver and Tan1983). Stall and Morales-Payan (Reference Stall and Morales-Payan2003) observed a 50% and 81% decrease in the yield of transplanted tomato due to the infestation of Cyperus esculentus (50 plants m−2) and Cyperus rotundus (100 plants m−2), respectively. The physiological similarity between species is an essential factor to be considered in competition with weeds; the more similar their physiological characteristics, the more intense the competition suffered by the crop (Ronchi et al. Reference Ronchi, Serrano, Silva and Guimarães2010).
Therefore, a critical need exists to improve current weed management techniques. One of the potential methods is the use of safeners to provide selective weed control. The use of safeners improves herbicide selectivity on some crops, thus providing an additional weed control strategy to farmers. Safeners are compounds used to protect seeds of crop species from an otherwise phytotoxic herbicide (Galon et al. Reference Galon, Maciel, Agostinetto, Concenço and Moraes2011). Safeners have been widely used to achieve herbicide selectivity in dicotyledonous plants; for example, 1,8-naphthalic anhydride protected beans in field experiments from EPTC damage (Blair Reference Blair1979); soybean was protected by growth regulators, fungicides, and other agrochemicals from metribuzin injury (Phatak and Varvina Reference Phatak, Varvina, Hatzios and Hoagland1989); cucumber pretreated with naptalam protected against chloramben injury (Knerr and Hopen Reference Knerr and Hopen1989); and cotton seeds treated with disulfoton or dietholate were protected from clomazone damage (Yazbek and Foloni Reference Yazbek and Foloni2004). In Arabidopsis, all tested safeners increased the activity of GST, but the response was dependent on the type of protector to which the seedlings were exposed and the substrate used for GST activity assays (DeRidder et al. Reference DeRidder, Dixon, Beussman, Edwards and Goldsbrough2002). The GSTs and P450s enhance the defense system of plants, and upon being induced by the presence of safeners, they reduce or eliminate the phytotoxic effects of the herbicide (Riechers et al. Reference Riechers, Kreuz and Zhang2010).
In tomato plants the GST genes are present in a higher number than in other plants such as Arabidopsis (1.6 times), sweet orange (3.8 times), lettuce (3.2 times), cotton (1.5 times), and Capsella rubella (1.8 times; Dong et al. Reference Dong, Li, Zhang, He, Daud, Chen and Zhu2016; He et al. Reference He, Guan, Chen, Gou, Liu, Zeng and Lan2016; Rezaei et al. Reference Rezaei, Shobbar, Shahbazi, Abedini and Zare2013; Sappl et al. Reference Sappl, Carroll, Clifton, Lister, Whelan, Harvey Millar and Singh2009; Yang et al. Reference Yang, Liu and Zeng2014). The fact that GST from Arabidopsis is induced by herbicide safening agents suggests that the process of recognition, signaling, and activation of genes required for the protective mechanism is present in dicotyledonous plants as well as in cereals (DeRidder et al. Reference DeRidder, Dixon, Beussman, Edwards and Goldsbrough2002). In maize (Zea mays), the expression of a GST gene protects the plant from the chloroacetanilide herbicides (i.e., alachlor, metolachlor) by encoding an enzyme lacking peroxidase activity (Karavangeli et al. Reference Karavangeli, Labrou, Clonis and Tsaftaris2005).
Numerous compounds have been screened as potential safeners, including fenclorim, benoxacor, flurazole, dichlormid, oxabetrinil, and fluxofenim (Galon et al. Reference Galon, Maciel, Agostinetto, Concenço and Moraes2011). Of these six safeners, benoxacor and fenclorim can effectively increase GST activity in tomato when treated with herbicides (Islam et al. Reference Islam, Rahman, Islam and Ghosh2017). There has also been reports of increased GST activity, up to five times, with the use of benoxacor and fenclorim safeners, with the model substrate 1-chloro-2,4-dinitrobenzene (DeRidder et al. Reference DeRidder, Dixon, Beussman, Edwards and Goldsbrough2002).
For successful weed management, it is crucial to use herbicides with different modes of action to achieve broad-spectrum weed control. Herbicides registered or recommended for use on tomato with different modes of action include pyroxasulfone, flumioxazin, metribuzin, fomesafen, imazamox, sulfentrazone, bicyclopyrone, and linuron (Chaudhari et al. Reference Chaudhari, Jennings, Monks, Jordan, Gunter and Louws2015; Ronchi et al. Reference Ronchi, Serrano, Silva and Guimarães2010). For C. rotundus control, sulfentrazone, a protoporphyrinogen oxidase (PPO) inhibitor, is shown to drastically reduce tuber viability (Silva et al. Reference Silva, Almeida, Salgado and Alves2014). Sulfentrazone provides 63% to 95% and 45% to 97% control when applied PRE or POST, respectively (Grichar et al. Reference Grichar, Besler and Brewer2003). Fomesafen, another PPO inhibitor, when applied PRE, can control Cyperus spp. up to 50% at 28 days after application (DAA; Miller and Dittmar Reference Miller and Dittmar2014). In addition, fomesafen can control several biotypes of Palmer amaranth [Amaranthus palmeri (S.) Wats.] by up to 96% at 21 days after treatment (Bond et al. Reference Bond, Oliver and Stephenson2006). Metribuzin, a photosystem II inhibitor, controls several broadleaf weeds, including up to 75% control of Palmer amaranth when applied PRE (Meyers et al. Reference Meyers, Jennings and Monks2017). Steele et al. (Reference Steele, Porpiglia and Chandler2005), on the other hand, reported greater than 97% control of Palmer amaranth plants using pyroxasulfone PRE, whereas Geier et al. (Reference Geier, Stahlman and Frihauf2006) reported 85% and 99% control of Palmer amaranth at rates of 125 to 332 g ha−1, respectively. Flumioxazin, a chlorophyll biosynthesis inhibitor, has been used to control several species of broadleaf weeds when applied PRE (Cranmer et al. Reference Cranmer, Altom, Braun and Pawlak2000), while imazamox, an acetohydroxyacid synthase inhibitor, is used to control some broadleaf weeds and many grasses (Blackshaw Reference Blackshaw1998; Nelson and Renner Reference Nelson and Renner1998). Bicyclopyrone is currently registered for PRE control of broadleaf and annual grass weed species with a hydroxyphenylpyruvate dioxygenase inhibitor (Bertucci et al. Reference Bertucci, Jennings, Monks, Jordan, Schultheis, Louws and Waldschmidt2018), while linuron, a photosystem II inhibitor, is used widely to control grasses and broadleaf weeds in some broadleaf crops (Grenni et al. Reference Grenni, Caracciolo, Rodríguez-Cruz and Sánchez-Martín2009). The aim of the present study was to evaluate the safening effect of benoxacor and fenclorim on tomato against herbicides such as pyroxasulfone, flumioxazin, metribuzin, imazamox, fomesafen, sulfentrazone, bicyclopyrone, and linuron applied POST.
Materials and Methods
Experiments were conducted in 2018 in a greenhouse at the R. R. Foil Plant Science Research Center at Mississippi State University, Starkville, MS (32.46936111°N, 88.78333333°E). The minimum and maximum temperatures were set at 20 C and 30 C, respectively, with 70% relative humidity. All studies were conducted with ‘Better Boy’, a tomato cultivar commonly grown in the southern United States. The experimental design was completely randomized with four replications, repeated twice, and arranged in a 9 × 3 factorial design, where Factor A represented herbicide treatments (eight herbicides and a nontreated control), and Factor B consisted of three safener treatments (benoxacor, fenclorim, and a nontreated safener control).
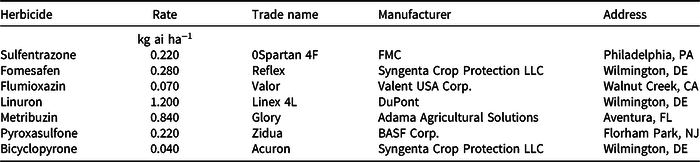
Safeners were applied to seeds after the dissolution of the safener in methanol, due to its low solubility. Tomato seeds were immersed in the safener solution for 1 h before sowing. After 1 h of treatment, seeds were air-dried for 10 min at room temperature and seeded. The rate of fenclorim and benoxacor used were 0.67 g L−1 (18 g ai kg−1 seeds) and 10 µM (70.5 g ai kg−1 seeds), respectively (Fuerst et al. Reference Fuerst, Irzyk and Miller1993; Shen et al. Reference Shen, Gao, Eneji and Chen2013). Seeds incubated in methanol-only solution were used as control treatments. Each experimental unit consisted of trays with six cells (50-ml cell) filled with Sunshine Professional Growing Mix (SunGro® Horticulture Sunshine Mix No. 2 Basic, Colombier, Quebec, Canada) soil, and was composed of 75% to 85% of Canadian sphagnum peat moss. Each tray was sown with 18 tomato seeds and irrigated with a surface drip system. Plants were thinned to one plant per cell before herbicide application. Herbicides were applied at the 3-leaf stage, or 25 days after sowing, using a spray chamber calibrated to deliver 200 L ha−1, fitted with an AIXR 11002 nozzle (TeeJet Technologies, Wheaton, IL) and maintained at a spray pressure of 275.8 KPa. The herbicide treatments evaluated are listed in Table 1.
Table 1. Herbicide treatments applied at R. R. Foil Plant Science Research Center at Mississippi State University, Starkville, MS.
Crop injury was evaluated visually at 3, 7, 14, and 21 DAA, using the scale of 0% to 100%, where 0% is without injury symptoms, and 100% is complete desiccation of shoot tissues. At 21 DAA, the shoot tissues were harvested, stored in paper bags, oven-dried at 60 C for 72 h, and weighed on a precision scale.
Statistical Analysis
Data were subjected to ANOVA using the Proc Mixed Method=Type3 process in version 9.4 of SAS (SAS Institute Inc., Cary, NC 27513). Treatment means were separated using the Tukey’s test at an alpha level of ≤ 0.05. The safener and herbicide treatments were considered as fixed effects, whereas replications and runs were random effects (Yang Reference Yang2010). The normality of the data was investigated by plotting q-q plots for residuals of visual injury and biomass in JMP13 (SAS Institute Inc.). The q-q plots showed normal distribution of data.
Results and Discussion
Visible injury on tomato plants at 7, 14, and 21 DAA was significant (P < 0.05) for the interaction between herbicide and safener (Table 2). At 3 DAA, metribuzin, imazamox, and bicyclopyrone caused less than 5% injury (Table 2), whereas the most noticeable injuries were observed with flumioxazin, sulfentrazone, and linuron treatments. Pretreatment with safeners did not result in any change in injury compared with control plants, except with flumioxazin, with injury higher than 1.2 times and 1.1 times, and pyroxasulfone with injury higher than 1.9 times and 2.1 times with benoxacor and fenclorim, respectively, compared with the control (Figure 1A). At 7 DAA, flumioxazin and linuron caused greater than 77% injury regardless of safener treatment. Similar to the findings at 3 DAA, metribuzin, imazamox, fomesafen, and bicyclopyrone caused minimal lesions on tomato plants at 7 DAA. Pretreatment with fenclorim and benoxacor reduced the injury caused by sulfentrazone 50% and 44%, respectively. Benoxacor-safened plants also showed 20.6% less injury with linuron treatment compared with the control plants. On the other hand, benoxacor and fenclorim increased the injury caused by pyroxasulfone by 1.63 times and 1.6 times, respectively, whereas benoxacor increased the injury from imazamox treatment by 4.5 times (Figure 1B).
Table 2. Interaction of herbicide and safener on visual injury at 3, 7, 14, and 21 days after application in tomato plants. a

a Abbreviations: DAA, days after application.
b Significant at 5% probability by the Tukey’s test.
c Nonsignificant at 5% probability by the Tukey’s test.

Figure 1. Visible estimates of injury at 3 (A), 7 (B), 14 (C), and 21 (D) days after application of herbicides in tomato plants treated with safeners before sowing. Means were separated within assessments using Tukey’s test at P < 0.05. Means followed by the same lowercase letter do not differ.
At 14 DAA, pyroxasulfone, flumiozaxin, and linuron caused >86% necrosis in tomato plants (Figure 1C). The effect of sulfentrazone and bicyclopyrone was intermediate with damage greater than 35% regardless of the safener treatment. Metribuzin, imazamox, and fomesafen caused less than 24% injury across all safener treatments. Pretreatment with benoxacor reduced the injury on tomato from sulfentrazone (30%) and bicyclopyrone (40%) compared with plants without safener treatment. Fenclorim safener was able to reduce the injury caused by bicyclopyrone by 26% at 14 DAA (Figure 1C). Greater than 76% necrosis was observed at 21 DAA with pyroxasulfone, flumioxazin, and linuron treatments. Therefore, these herbicides are not suitable for POST application on tomato (Figure 1D). However, less injury by metribuzin (27%), bicyclopyrone (25%), and fomesafen (25%) to tomato plants occurred from benoxacor-treated seeds. Fenclorim PRE resulted in 82%, 40%, and 23% reduction in tomato injury caused by imazamox, metribuzin, and bicyclopyrone, respectively.
The dry biomass of tomato plants in this study showed significant interaction (P < 0.05) between herbicides and safeners (Table 3). Pretreatment with benoxacor caused a lesser reduction in tomato biomass with pyroxasulfone (2.3 times) and metribuzin (1.7 times) compared with plants without a safener. Plants that were pretreated with fenclorim had lesser biomass reduction with metribuzin (1.9 times), imazamox (2.1 times), and fomesafen (2.2 times) than plants without safener pretreatment. Across all herbicide treatments, the highest reduction in tomato biomass was caused by linuron without a safener pretreatment (Figure 2).
Table 3. Dry biomass at 21 days after application of herbicides in tomato plants treated with safeners before sowing.

a Control: without safener.
b Control: without herbicide.
c Significant at 5% probability by the Tukey’s test. Means followed by the same lowercase letter in the column and uppercase in the row do not differ from each other.

Figure 2. Dry biomass (%) of the nontreated without safener at 21 days after application of herbicides in tomato plants treated with safeners before sowing. Means were separated within assessments using Tukey’s test at P < 0.05. Means followed by the same lowercase letter do not differ from each other.
The safeners tested in this study reduced the injury caused by certain herbicides. Benoxacor and fenclorim have the potential to reduce injury caused by bicyclopyrone and imazamox. In a related study, fenclorim was shown to protect rice (Oryza sativa L.) against injuries caused by pretilachlor (Wu et al. Reference Wu, Omokawa and Hatzios1996). However, due to the lack of studies on the modes of action of these safeners in crops, the mechanism of how safeners protect plants from herbicide injury is not clear. We can, however, hypothesize that safeners promote rapid metabolization of the herbicide or interact with them at the sites of action (Galon et al. Reference Galon, Maciel, Agostinetto, Concenço and Moraes2011), thus reducing the crop injury. Safeners reduce, but not completely reverse, the herbicide damage to sensitive crops.
It is likely that the rapid herbicide metabolism occurs mainly due to herbicide-glutathione conjugation and increased activity of the GST enzyme. Among the herbicides conjugated by GSTs are sulfonylureas, imidazolinones, aryloxyphenoxypropionates, triazines, and chloroacetanilides (Galon et al. Reference Galon, Maciel, Agostinetto, Concenço and Moraes2011). The GST activity is induced by the pretreatment of corn, rice, and grain sorghum with different safeners such as dichlormid, benoxacor, fenclorim, and flurazole, improving their tolerance to thiocarbamate and chloroacetanilide herbicides (Riechers et al. Reference Riechers, Kreuz and Zhang2010).
Using herbicides is the most effective method of controlling weeds in commercial tomato production; however, the tolerance of tomato to most herbicides is low. Safeners may alleviate this problem. The effectiveness of a safener depends on the mode of action of the herbicide, the crop to be safened, and the weeds to be controlled, because the mechanism of action of these chemical protectants is very specific (Galon et al. Reference Galon, Maciel, Agostinetto, Concenço and Moraes2011).
Pretreatment of tomato with fenclorim reduced visual injury caused by imazamox and bicyclopyrone, whereas benoxacor reduced visual injury from bicyclopyrone. Additionally, tomato plants showed less biomass reduction when treated with fenclorim after application of imazamox, fomesafen, and metribuzin, while pretreatment with benoxacor resulted in less biomass reduction after metribuzin application compared with plants that did not receive any safener treatment. Weeds cause significant yield losses in tomato and the use of a single method of control is not sufficient. Findings from this study show the potential use of benoxacor and fenclorim as safeners for crop protection. To expand the utility of this potential tool, studies are needed to understand how these safeners work in tomato.
Acknowledgments
Funding for this project was provided by the Specialty Crop Block Grant sponsored by the Mississippi Department of Agriculture and Commerce/U.S. Department of Agriculture–Agriculture Marketing Service and is based upon work that is supported by the U.S. Department of Agriculture–National Institute of Food and Agriculture Hatch project, accession number 230060. We thank Swati Shrestha, Brooklyn Schumaker, Bruna Martins, Pedro Ferreira, and Ziming Yue for their assistance throughout the research.
Conflicts of Interest
The authors declare that there are no conflicts of interest.







