There is an increased interest among growers in Virginia, and much of the mid-Atlantic region, in producing strawberries for farm diversification and as a crop for agritourism. From a consumer standpoint, there is growing interest in local and fresh foods. Virginia is among the 14 top strawberry-producing states of the United States (USDA-ERS 2013). The City of Virginia Beach is the most concentrated strawberry-producing area of Virginia, with total annual production value estimated at $750,000 to $1 million (City of Virginia Beach 2011). Soil disinfestation treatments that reduce pathogens, insects, and weed seeds in the soil can greatly improve crop health and fruit yield. The most effective fumigant combination, methyl bromide (MB) plus chloropicrin (Pic), can no longer be used after the ban on the use of MB as a fumigant under the Montreal Protocol.
Many berry growers in the southeastern United States now use 1,3-dichloropropene (1,3-D) plus chloropicrin (Pic) as an alternative to MB plus Pic. Pic is effective for controlling pathogens, but is less effective in controlling weeds and nematodes (Noling and Becker Reference Noling and Becker1994) and can have negative health effects on humans at low doses (CDPR 2010). The chemical 1,3-D provides good control of plant parasitic nematodes, but has been classified as a probable human carcinogen (USEPA 2000). Due to human health issues with these fumigants, stringent regulations are placed on their use. These regulations require that a fumigation management plan be completed prior to application, worker safety precautions be followed as per the fumigant label, and buffer areas must be left untreated, especially for fields adjacent to homes, schools, and hospitals. It is not uncommon in Virginia, as in many geographic areas of the United States, to find urban dwellings close to farms. Many farm managers and growers no longer wish to fumigate because of health concerns, or because they do not see sufficient benefit to justify the cost of soil fumigation (JB Samtani, personal communication). With increasing challenges associated with fumigation, it is important to evaluate nonfumigant approaches. Additionally, with the growing interest in organic production, funding agencies such as the US Department of Agriculture National Institute of Food and Agriculture and producer associations such as the North American Strawberry Growers Association have prioritized research on developing organic pest control tools.
Soil solarization (SS) is a passive approach for heating soil, and is widely used in tropical regions to treat infested cropland. In the United States, SS has been evaluated in Arizona, California, Florida, North Carolina, and Texas, but its efficacy has been variable in these regions, and it has not always been found to be as effective as MB fumigation (Chellemi et al. Reference Chellemi, Olson and Mitchell1994; Hartz et al. Reference Hartz, DeVay and Elmore1993; Ristaino et al. Reference Ristaino, Perry and Lumsden1991). However, to our knowledge, there is no documented work evaluating the performance of SS in the climatic conditions of Virginia. SS is achieved by covering moistened soil with a clear polyethylene tarp for the required time period. It is expected that the soil temperature under the tarp will reach levels much higher than those of the ambient air, and will thus provide sufficient pest control. Exposure time is of importance for thermal death or inactivation of pathogen populations (Katan Reference Katan1983). There is a linear relationship between temperature and time needed to kill most soil-borne pathogens (Pullman, DeVay, and Garber1981). Diseases caused by Verticillium, Rhizoctonia solani, Fusarium oxysporum, and Sclerotium rolfsii can be controlled with a 4- to 6- wk period of SS when 40 to 50 C soil temperatures are achieved (Katan Reference Katan1983, Reference Katan1987; Pullman, DeVay, and Garber Reference Pullman, DeVay and Garber1981; Pullman, DeVay, Garber, and Weinhold Reference Pullman, DeVay, Garber and Weinhold1981). In a study done in Spain, SS for 8 wk effectively reduced Verticillium dahliae populations in the top 20 cm of the soil for a period of 3 yr (Lopez-Escudero and Blanco-Lopez Reference Lopez-Escudero and Blanco-Lopez2001). In California, 4-wk SS did not reduce inoculated V. dahlia populations compared to those of a nontreated control (Samtani et al. Reference Samtani, Gilbert, Weber, Subbarao, Goodhue and Fennimore2012). SS provided disease control and improved crop yields in cotton (Gossypium hirsutum L.) (Fusarium and Verticillium), melons (Cucumis L.) (Fusarium), and potato (Solanum tuberosum L) (Verticillium) (Davis and Sorensen Reference Davis and Sorensen1986; Katan Reference Katan1983). Soil-borne pathogens are less resistant to heat than are many soil saprophytes and antagonists, such as Trichoderma spp. and Bacillus subtilis, and thus more easily killed through SS (Gamliel and Katan Reference Gamliel and Katan1991; Munnecke and Van Gundy Reference Munnecke and Van Gundy1979).
SS can control annual weed seeds up to 5 cm deep in tropical and subtropical climates (Ioannou Reference Ioannou2000; Kumar et al. Reference Kumar, Yaduraju, Ahuja and Prasad1993). In a study done in Turkey, SS provided nearly 100% control of annual bluegrass (Poa annua L.), redroot pigweed (Amaranthus retroflexus L.), wild radish (Raphanus raphanistrum L.), and wild chamomile (Matricaria recutita L.) (Boz Reference Boz2004). Farm profitability increased by $470 per ha when SS was used as a weed control tool (Boz Reference Boz2004). In California, SS provided weed control in strawberries and maintained fruit yield at a much lower cost than did MB (Stapleton et al. Reference Stapleton, Molinar, Lynn-Patterson, McFeeters and Shrestha2005). Weed densities in 8 wk SS-treated plots were reduced 86% to 94% compared to those of a nontreated control, proving that SS is a valuable tool for organic growers and those with limited resources (Stapleton et al. Reference Stapleton, Molinar, Lynn-Patterson, McFeeters and Shrestha2005). SS does not easily control weeds that have a hard seed coat, such as legumes, and it is not effective for the control of perennial weeds (Linke Reference Linke1994). In some cases, SS can cause leaf scorching of emerged weeds and can eventually lead to plant death (Horowitz et al. Reference Horowitz, Regev and Herzlinger1983). Nutsedge (Cyperus spp.) tubers not directly killed by SS can sprout under the clear tarp, but the resulting shoots are trapped under the tarp and killed by foliar scorching (Chase et al. Reference Chase, Sinclair, Shilling, Gilreath and Locascio1998).
In recent years, 1,3-D plus Pic has been a preferred fumigation treatment for strawberry growers in the mid-Atlantic and southeastern regions of the United States. The objectives of this study were to evaluate the potential of SS treatments for weed control in strawberry production in coastal Virginia climatic conditions, and to compare crop yields in plots treated with SS to those of plots treated with 1,3-D plus Pic fumigation.
Materials and Methods
Field studies were established at the Hampton Roads Agricultural Research and Extension Center in the City of Virginia Beach, Virginia (36°9′N, 76°2′W; 3.7 m elevation) during the 2013/2014 and 2014/15 growing seasons in a randomized complete block design with five treatments and four replicates. There were twenty beds, the raised portions of which were 10.6 m long by 0.8 m wide. The beds were oriented north–south in the 2013/2014 growing season, but were changed to an east–west orientation in 2014/15 growing season for practical management reasons. The center 4.6-m length of each bed was used for strawberry plug transplanting and data collection and is hereafter referred to as a plot.
Prior to initiation of the 2013 experiment, the site had not been under strawberry cultivation and was primarily grassy vegetation that was maintained by mowing. Soil at the site was a Tetotum loam (sandy loam, deep, moderately well drained, parent material: loamy fluvial and marine sediments), with 0% to 2% slope, and a pH of 5.6. Soil tests were conducted in both seasons by sending representative soil samples to the Virginia Polytechnic Institute and State University Soil Testing Lab in late June. Limestone was broadcast on July 9, 2013 at 3,706 kg ha−1 and on July 18, 2014 at 3,089 kg ha−1 to bring the soil pH to the desired level of 6.2. Preplant fertilizers were applied according to soil test recommendations at bed formation in each growing season. For preplant SS treatments, beds were covered with 1 mil clear embossed polyethylene tarp (Robert Marvel Plastic Mulch, LLC, Annville, PA 17003) and non-SS treated beds were covered with 1.25 mil virtually impermeable film (VIF) (TriEst Ag Group, Inc, Greenville, NC 27835). Preplant treatments included 1) 1,3-D plus Pic (39% 1,3-dichloropropene plus 59.6% chloropicrin; TriEst Ag Group, Inc., Greenville, NC 27835) shank-fumigated in beds at 157 kg ha−1 on August 30 in both seasons with the beds covered with VIF at time of fumigation; 2) SS for a 6-wk duration initiated on August 15, 2013 and August 21, 2014; 3) SS for a 4-wk duration initiated on September 6, 2013 and September 3, 2014; 4) SS 4-wk treatment initiated September 6, 2013 and September 3, 2014 and replaced with black VIF on October 4, 2013 and October 1, 2014, and 5) a nontreated control covered with black VIF on October 4, 2013 and October 1, 2014.
Moisture content in the SS beds during the treatment period was maintained at a minimum of 70% field capacity (Elmore et al. Reference Elmore, Stapleton, Bell and DeVay1997) and was measured occasionally using a FieldScout TDR 100 soil moisture meter with a 20-cm rod length (Spectrum Technologies, Inc., Aurora, IL 60504). Temperature probes (U12- 015, Onset Hobo Data Loggers, Onset Computer Corporation, Bourne, MA 02532) were inserted at 5-, 15-, and 30-cm depths within a single replicate of SS beds at the time of treatment initiation and in a nontreated control bed that was left bare until treatment initiation. Temperature probes collected data at 10 minute intervals during the treatment period. Italian ryegrass [Lolium perenne ssp. multiflorum (Lam.) Husnot] was seeded at 280 kgha−1 as a cover crop in furrows between strawberry beds on October 4, 2013 and October 3, 2014, prior to punching holes for transplanting strawberries. This practice is commonly used by many annual plasticulture strawberry growers in the mid-Atlantic region. Italian ryegrass was mowed as needed throughout the strawberry growing season. Following completion of all preplant treatments, ‘Chandler’ strawberry plugs (Aaron’s Creek Farms Plant Nursery, Buffalo Junction, VA 24529) were transplanted in all plots on October 4, 2013 and October 3, 2014, in two rows at a 36 cm in-row spacing. A 15 mil single drip line with a 30.5-cm emitter spacing was used to irrigate and fertigate the beds (Berry Hill Irrigation, Inc., Buffalo Junction, VA 24529). Steps involved in plot establishment for each treatment to enable weed and yield data collection are listed in Table 1. In the 2014/15 study, plants were treated with captan (Captan 50W, Drexel Chemical Co., Memphis, TN 38113) at 3.36 kgaiha−1 and pyraclostrobin (Cabrio EG fungicide, BASF corporation, Florham Park, NJ 07932) at 0.98 kg ha−1 on October 9 and October 30, 2014 to manage leaf spot [Mycosphaerella fragariae (Tul.)].
Table 1 Steps that were involved to initiate the different preplant treatments and to collect weed count and fruit yield data in annual plasticulture strawberry production in the 2013/2014 and 2014/2015 growing seasons in the City of Virginia Beach, VA.

Winter protection of the strawberry plants was done as needed by covering plants with a 40 gm−2 floating row cover (Atmore Industries, Inc., Atmore, AL 36502). Fertigation was done on a weekly basis in both seasons starting April 2, 2014 and April 1, 2015, alternating calcium nitrate (15.5-0-0; YaraLiva Calcinit, Yara North America Inc., Tampa, FL 33602) at 7.9 kgha−1wk−1 of nitrogen, and potassium nitrate (13.5-0-46.2; Multi-K GG, Haifa North America, Inc., Altamonte Springs, FL 32701) at 3.9 kgha−1wk−1 of nitrogen using a 53Lmin−1 Dosatron® injector (Berry Hill Irrigation, Inc., Buffalo Junction, VA 24529).
Data Collection
Weed count data were collected by establishing a 1.5 m long by 0.8 m wide viewing window on bed tops, soon after strawberry plugs were transplanted. For treatments with a black tarp, a weed viewing window covered with clear tarp was installed by replacing the black tarp in the 1.5 m long window area. In the 2013/14 growing season, emerged weeds in the viewing window area were counted 6 wk after transplanting (WAT) on November 15, 2013, 18 WAT on February 6, 2014, and 28 WAT on April 21, 2014. In the 2014/15 growing season, weeds were counted 4 WAT on November 4, 2014, 9 WAT on December 10, 2014, and 22 WAT on March 9, 2015. These evaluation dates were chosen as weeds in many plots appeared to be competing with crop plant in the window area, but before the weeds became excessive and difficult to count. After each evaluation, emerged weeds in strawberry beds were carefully removed by hand or with a sickle through the strawberry planting holes. Weeds were counted by species and counts for all species were summed to determine cumulative total weed density. Weeds emerging from the bed shoulders were not included in the weed count, but were hand-weeded at each weed evaluation date.
In both seasons, crop stand was recorded on a monthly basis starting in November, and continued throughout the growing season. Crop stand was taken separately for plants inside the weed viewing window and outside of the viewing window where crop yield data was taken. The vigor and health of all plants in each plot were evaluated using a scale of 0 (dead plant) to 10 (extremely vigorous). Disease incidences were monitored on a similar scale, including crown rots caused by Phytophthora cactorum or Colletotrichum gloeosporioides, and black root rot caused by Rhizoctonia and Fusarium. Strawberry fruits were harvested twice per week beginning May 2, 2014 and May 4, 2015 and ending June 13, 2014 and June 16, 2015, respectively, for the two growing seasons. Fruits were harvested from 16 plants per plot over a 3.1-m bed length. This harvested area was outside of the previously described weed viewing window in each plot. There were a total of 13 harvests for the 2013/2014 growing season and 12 harvests for the 2014/2015 growing season. Fruits from each harvest were categorized as marketable or nonmarketable and then weighed. Nonmarketable fruits included those that were small (less than 10 g), diseased, misshapen or deformed, or overripe or rotten. The weights of marketable and nonmarketable fruits were added to determine total yield. Cumulative berry yield data from each plot was totaled for all harvests, divided by the number of plants in each plot, and expressed as marketable and total yield per plant. Fruit size was recorded once a week on five randomly selected fruits per replicate for each harvest date using a digital Vernier caliper (Neiko® 01407A, Taiwan). The maximum fruit width was measured below the proximal end of the fruit below the fruit cap or calyx.
Data Analysis
Data were analyzed using PROC MIXED in SAS with growing seasons and treatments as fixed effects (release 9.1; SAS Institute Inc., Cary, NC, USA). Prior to running the ANOVA, data were checked for normality of residuals. A two-way growing season by treatment interaction was analyzed for dominant weed species and cumulative weed count. Crop stand counts and health ratings from crop transplant to final harvest were averaged and the mean data were subjected to ANOVA. For some of the weed species counts, log(x+1) transformation was used, and for cumulative marketable yield data, sqrt(x) transformation was used to achieve normality of residuals. In such cases, the original least-squares means are presented but the separation letter is of the transformed least-squares mean value. Fruit size data were averaged for the season and the average fruit size was subjected to ANOVA. Least-squares means were compared using the PDIFF option (P=0.05). The LSD option was used to adjust for multiple comparisons, and letters separating the least-square means were assigned using a macro “PDMIX 800” (Saxton Reference Saxton1998).
Results and Discussion
Soil and Air Temperature
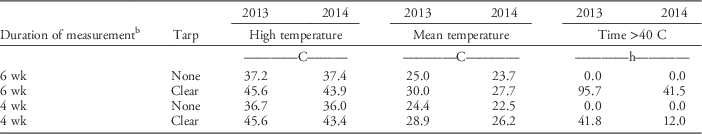
Soil temperature at 5 cm under clear tarp (SS treatments) was slightly higher in 2013/2014 than it was in the 2014/2015 growing season (Table 2). Many soil organisms begin to be negatively affected at 40 C; thus, the accrued time ≥40 C is presented in Table 2 (Stapleton and DeVay Reference Stapleton and DeVay1995). During 2013/2014, time ≥40 C was greater than it was during 2014/2015 for the 6-wk and 4-wk SS treatments. In both growing seasons, the highest soil temperatures achieved were the same for both the 6 wk and 4 wk SS periods, but the total time ≥40 C was greater in the 6-wk SS than it was in the 4-wk SS period. The highest soil temperature under clear tarp was >8 C higher than it was in an untarped bed in the 2013/2014 growing season. For the 2014/2015 growing season, there was a 6.5 C temperature difference between clear tarped and untarped beds for the 6 wk SS period, and a 7.4 C difference for the 4-wk SS period. In both growing seasons, mean soil temperature under clear tarp was 3.7 to 5 C higher than the soil temperature in the untarped bed. Air temperature data retrieved from the US Department of Agriculture Natural Resources Conservation Service, National Water and Climate Center (USDA-NRCS 2016) for the nearest station, which was 6.6 km from the research site, was in slight contrast to soil temperature recorded under clear tarp. In 2014, higher ambient air temperature (37.2 C) was recorded during the 6-wk SS period than that recorded in 2013 (33.3 C).
Table 2 Soil temperature collected at a 5-cm depth during the 6-wk and 4-wk soil solarization (SS) treatment periods, in beds with no tarpFootnote a or a clear tarp in annual plasticulture strawberry production in the City of Virginia Beach, VA.
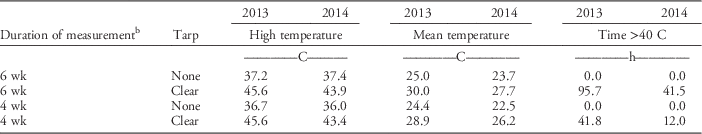
a Bed with no tarp was a nontreated control replicate that was left bare until treatment initiation to record soil temperature.
b In 2013, data are from August 16 through October 3, 2013 for the 6-wk SS and from September 6 through October 3, 2013 for the 4-wk SS treatments. For 2014, data are from August 21 through October 1, 2014, and from September 3 through October 1, 2014, for the 6-wk and the 4-wk SS treatments, respectively.
Weed Density
Common chickweed [Stellaria media (L.) Vill.], cudweed (Gnaphalium spp.), henbit (Lamium amplexicaule L.), purple deadnettle (Lamium purpureum L.), wild garlic (Allium vineale L.), and white clover (Trifolium repens L.) were the dominant weed species in both growing seasons. The growing season by treatment interaction was significant for common chickweed, cudweed, and wild garlic (Table 3). In the 2013/2014 growing season, all plots receiving a SS treatment had lower common chickweed density than nontreated and 1,3-D plus Pic plots. In the 2014/2015 growing season, common chickweed density was lower in the 6-wk SS and 1,3-D plus Pic plots compared to that of the nontreated and 4-wk SS treatments. The greater reductions seen in the 4-wk SS treatments in 2013/2014 compared to 2014/2015 growing season may have been caused by the longer time above 40 C in the soil for the 2013/2014, 4-wk SS treatments (Table 2). In the 2013/2014 growing season, cudweed density in the 6-wk SS plots was not significantly different than that of either 4-wk SS treatment, but was significantly lower than that of the nontreated control and 1,3-D plus Pic treatments. In the 2014/2015 growing season, only the 6-wk SS treatment had lower cudweed density than did the nontreated control. Lamium spp. density compared to the nontreated control was only suppressed by the 6-wk SS treatment. Elmore et al. (Reference Elmore, Stapleton, Bell and DeVay1997) and Katan (Reference Katan1981) reported that many weed species, including common chickweed and Lamium spp., can be controlled with SS. For white clover, only the main effect of the growing season was significant (P>0.0001; data not shown), with higher clover density in the 2013/2014 season than there was in the 2014/2015 season. White clover is a common weed species of turfgrass and landscapes in many regions and tolerates low mowing (Uva et al. Reference Uva, Neal and DiTomaso1997). Because the study site was under naturalized lawn cover prior initiating of these strawberry experiments, higher white clover density in the first growing season is not surprising. Neither the 1,3-D plus Pic nor the SS treatments effectively suppressed wild garlic in either growing seasons (Table 3). For cumulative weed counts, the growing season by treatment interaction was significant. In the 2013/2014 growing season, weed density was numerically highest in nontreated plots and not different from 1,3-D plus Pic-treated plots (Table 3). Six-week SS plots had the lowest cumulative weed count of all the treatments in the 2013/2014 growing season, but the count was not significantly different from that of the 4-wk SS treatments. In the 2014/2015 growing season, the 4-wk SS treatment had the highest weed density, while the lowest weed density was observed in the 6-wk SS treatment.
Table 3 Cumulative weed counts in 1.5 m lengths of bed for the 2013/2014 and 2014/2015 growing seasons in the City of Virginia Beach, VA, as affected by preplant treatments in annual plasticulture strawberry production.

a 1,3-dichloropropene plus chloropicrin (39:60 by weight) was shank fumigated at 157 kg ha−1.
b Growing season by treatment interaction was significant for common chickweed, cudweed, wild garlic, and cumulative weed count for the season. For Lamium spp., only the treatment main effect was significant and data are pooled over the two growing seasons.
c Means with the same letter within a column are not significantly different using least significance difference at P≤0.05. Data are from 1.5 by 0.8 m−2 window.
The 1,3-D plus Pic treatment did not effectively control weeds in these studies. This fumigant combination can provide good pathogen control, but limited weed control (Duniway Reference Duniway2002; Sande et al. Reference Sande, Mullen, Wetzstein and Houston2011). In this study, weed evaluations were done in a ‘window’ area. In grower fields, the black tarp likely provides an additive effect with the fumigant to provide more effective weed control than was observed in this study.
Crop Stand and Health
Strawberry stand counts did not differ among treatments for either growing season (data not shown). For the plant health rating, the growing season by treatment interaction was significant (P=0.0156). In the 2013/2014 growing season, there was no difference in health ratings (8.1 to 8.4) among treatments (data not shown). In the 2014/2015 growing season, average ratings ranged from 6.6 for plants in the 1,3-D plus Pic-treated plots and 4-wk SS treatments, to 7.3 for plants in the nontreated and 6-wk SS plots (data not shown). Overall for the 2014/2015 growing season, many strawberry plugs had symptoms of leaf spot at planting, and thus these rating differences are probably caused by differences in initial plant quality rather than preplant soil treatments.
Crop Yield and Fruit Size
Fruit size did not differ among the treatments (data not shown).
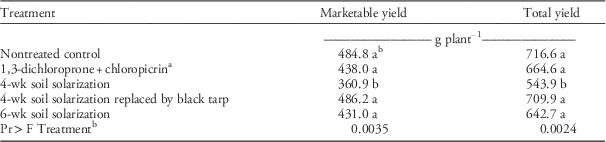
The growing season by treatment interaction was not significant for cumulative marketable or total yield data, therefore the main effect data are presented. All treatments except for the 4-wk SS treatment had similar crop yields. The yield with the 4-wk SS was lower than that with other treatments (Table 4). It is possible that the weeds in the 4-wk SS may have competed with the crop plant for nutrients, affecting berry yield, particularly in the 2014/2015 growing season. Strawberries transplanted into fumigated plots do not always achieve higher yields over nontreated control plots, as has been observed in earlier studies (Samtani et al. Reference Samtani, Ajwa, Weber, Browne, Klose, Hunzie and Fennimore2011, Reference Samtani, Gilbert, Weber, Subbarao, Goodhue and Fennimore2012). Compared with nontreated plots, the 4-wk SS treatments did not improve yield in strawberries (Samtani et al. Reference Samtani, Gilbert, Weber, Subbarao, Goodhue and Fennimore2012) or in pickling cucumber (Cucumis sativus L.) compared to 6-wk SS (Keinath 1995). Strawberry yield increased by 12% over a nontreated control with a 10-wk SS treatment period (Hartz et al. Reference Hartz, DeVay and Elmore1993).
Table 4 Cumulative marketable and total strawberry yield in soil solarization experiments conducted during 2013/2014 and 2014/2015 in the City of Virginia Beach, VA. Data were averaged over two growing seasons.
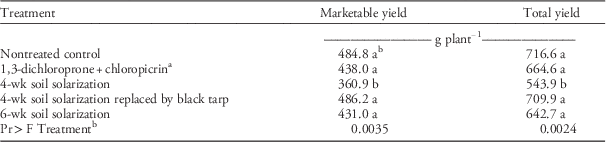
a 1,3-dichloropropene plus chloropicrin (39:60 by weight) was shank fumigated at 157 kg ha−1.
b Means with the same letter within a column are not significantly different using least significance difference at P≤0.05. P-value presented is of treatment main effect.
Several factors can influence the efficacy of SS, including geographic location, soil conditions, weather, tarp thickness, treatment duration, and bed orientation (Elmore et al. Reference Elmore, Stapleton, Bell and DeVay1997). It is prudent for each grower to understand the pest type and density at their sites before determining whether preplant treatment is needed. A longer duration of SS may provide better efficacy at greater depths (Katan Reference Katan1981). In our study, 6-wk SS was more effective at providing weed control than was the 4-wk SS. Implementation of SS treatment in Virginia can be a challenge as wet soils and rain events can delay initiating SS in a timely manner. In our study, in the first season, our intent was to conduct an 8-wk SS period instead of a 6-wk SS period, but wet soils and rainfall during initiation time delayed bed formation. Canada geese (Branta canadensis L.) also disturbed the process of SS by pecking on clear tarp in these studies. To remedy this initial damage, clear tape was used to seal any holes caused by geese in clear tarp. Fencing of the study site and use of scare-eye balloons (Bird-X Chicago, IL 60612) soon after study beds were made, kept geese away from beds in subsequent months of the growing season.
Virginia growers hand pull weeds that emerge in the planting holes to prevent competitive suppression of strawberry plant growth and yield. This was done at planting holes in our site on plants on which yield data was collected. We did not record weed biomass in our study. It is likely that weed biomass may have a more direct correlation with observed yield than do the weed count data. For SS treatments, having a tarp with black shoulders and a clear top would further reduce hand weeding costs. SS could be a useful pest control tool for organic growers, small farms, limited resource growers, or growers that need to address pest issues in buffer areas prior to strawberry transplanting.
Acknowledgments
Thanks to Adam Sleeper, Alfred Smith, Jillian Rajevich, Sanghamitra Das, Kelly Jacobson, Sydney Covey, Andrew Hall, Simon McPhearson, and Samridhi Vashisth for their assistance with study establishment, maintenance, and data collection. Thanks to Dr. Brij Gupta for reviewing the manuscript prior to submission. This study was funded in part by a Virginia USDA Specialty Crop Block Grant under Agreement Numbers FFY 2013-4B1 and FFY 2014-567.