Introduction
Prosulfocarb (PSC) is a selective PRE or early POST herbicide that has been used over the past three decades against grass and broad-leaved weeds in potatoes (Solanum tuberosum L.), legumes, and cereals (Bailly et al. Reference Bailly, Dale, Archer, Wright and Kaundun2012). PSC is a thiocarbamate herbicide (Herbicide Resistance Action Committee 2009) that inhibits fatty-acid elongation and surface-lipid biosynthesis (Lechelt-Kunze et al. Reference Lechelt-Kunze, Meissner, Drewes and Tietjen2003; Trenkamp et al. Reference Trenkamp, Martin and Tietjen2004). PSC is absorbed primarily via soil (Vera et al. Reference Vera, Gauvrit and Cabanne2001) and is classified as a residual herbicide, providing extended control of germinating or emerged weeds, although it is considered a nonpersistent compound (PPDB 2009). Its efficacy has been reported in several studies in which PSC is a key component of herbicide programs against annual grass weeds such as blackgrass (Alopecurus myosuroides Huds.), silky windgrass, and Italian ryegrass (Bailly et al. Reference Bailly, Dale, Archer, Wright and Kaundun2012; Keshtkar et al. Reference Keshtkar, Mathiassen, Moss and Kudsk2015; Knežević et al. Reference Knežević, Ranogajec and Samota2008).
PSC persistence in the environment is mainly influenced by its bioavailability in the soil (Gennari et al. Reference Gennari, Ambrosoli, Nègre and Minati2002). The period during which a herbicide persists in the soil is the result of a series of transformation and removal processes that are strongly linked to local climate and soil conditions (Alletto et al. Reference Alletto, Coquet, Benoit, Heddadj and Barriuso2010). Studies dealing with the degradation and dissipation of PSC in various environmental compartments generally report biodegradation to be the main process responsible for its dissipation in soil (Gennari et al. Reference Gennari, Ferraris, Nègre and Ambrosoli1998; Rouchaud et al. Reference Rouchaud, Neus, Callens and Bulcke1997). However, information on the dissipation rate of this herbicide is contradictory, especially in field conditions, with half-life estimations varying from 8 to 35 d (Gennari et al. Reference Gennari, Ferraris, Nègre and Ambrosoli1998; PPDB 2009).
Crop management strategies such as tillage practices can also influence the fate of the compound in soil by modifying its bioavailability and degradation rate (Chauhan et al. Reference Chauhan, Gill and Preston2006; Fomsgaard et al. Reference Fomsgaard, Spliid and Felding2003), though information on the effects of tillage on PSC efficacy and dissipation are not currently available. The adoption of conservation tillage (CT) practices, in which soil disturbance is reduced, is being promoted due to its economic and environmental benefits compared with conventional tillage (Alletto et al. Reference Alletto, Coquet, Benoit, Heddadj and Barriuso2010; Melander et al. Reference Melander, Munier-Jolain, Charles, Wirth, Schwarz, van der Weide, Bonin, Jensen and Kudsk2013; Morris et al. Reference Morris, Miller, Orson and Froud-Williams2010).
The reduction of tillage frequency and depth increases the reliance on herbicides, and residual herbicides often show a reduced efficacy under CT (Melander et al. Reference Melander, Munier-Jolain, Charles, Wirth, Schwarz, van der Weide, Bonin, Jensen and Kudsk2013). Noninversion tillage is expected to alter herbicide degradation patterns in soil due to accumulation of crop residues, as has been already reported for metribuzin (Locke and Harper Reference Locke and Harper1991). In the case of PSC, the higher content of soil organic carbon in CT systems has been shown to enhance soil adsorption (Gennari et al. Reference Gennari, Ferraris, Nègre and Ambrosoli1998). Furthermore, stubble residues near or on the soil surface can intercept and absorb a herbicide (Erbach and Lovely Reference Erbach and Lovely1975). On the other hand, microbial activity generally increases when tillage operations are minimized (Biederbeck et al. Reference Biederbeck, Campbell and Hunter1997), which could favor PSC degradation.
Information about herbicide dissipation in soil and how crop management practices influence this process is essential when planning and timing herbicide applications in order to avoid weed control failures. However, no information is available about PSC dissipation in different tillage systems. Therefore, characterizing the effects of soil cultivation on the dissipation of PSC will add valuable information regarding its efficacy against weeds and elucidate the need for subsequent weed control strategies to preserve crop yields, which is of great concern, because PSC has become increasingly important in herbicide resistance management programs in European countries due to the reduced number of herbicides available with modes of action other than acetolactate synthase (ALS) and acetyl-CoA carboxylase (ACCase) inhibition.
Herbicide dissipation can be evaluated with chemical and bioassay techniques, each having advantages and disadvantages. Bioassay is considered a more conventional and time-consuming approach (Rupak et al. Reference Rupak, Sharma, Kulshrestha and Singh2009) but is quite sensitive and detects not only the presence of the parent compound in the soil but also phytotoxic metabolites. On the other hand, high-performance liquid chromatography (HPLC) analysis can accurately quantify residual concentrations of the herbicide in soil, but it is expensive, requiring specialized analytical equipment and laboratory infrastructure (Strachan et al. Reference Strachan, Nanita, Ruggiero, Casini, Heldreth, Hageman, Flanigan, Ferri and Pentz2011). Therefore, the objective of this study was to determine the effects of tillage intensity on the soil dissipation of prosulfocarb using bioassay and HPLC techniques. It was hypothesized that the dissipation of PSC is reduced in conservation tillage systems compared with conventional tillage due to a higher content of organic matter in the upper soil layer. Moreover, it was expectated that HPLC analysis would yield shorter dissipation rates compared with the bioassay, because the latter method reflects not only the effect of PSC but also of any phytotoxic metabolites.
Materials and Methods
Field Trials and Sample Collection
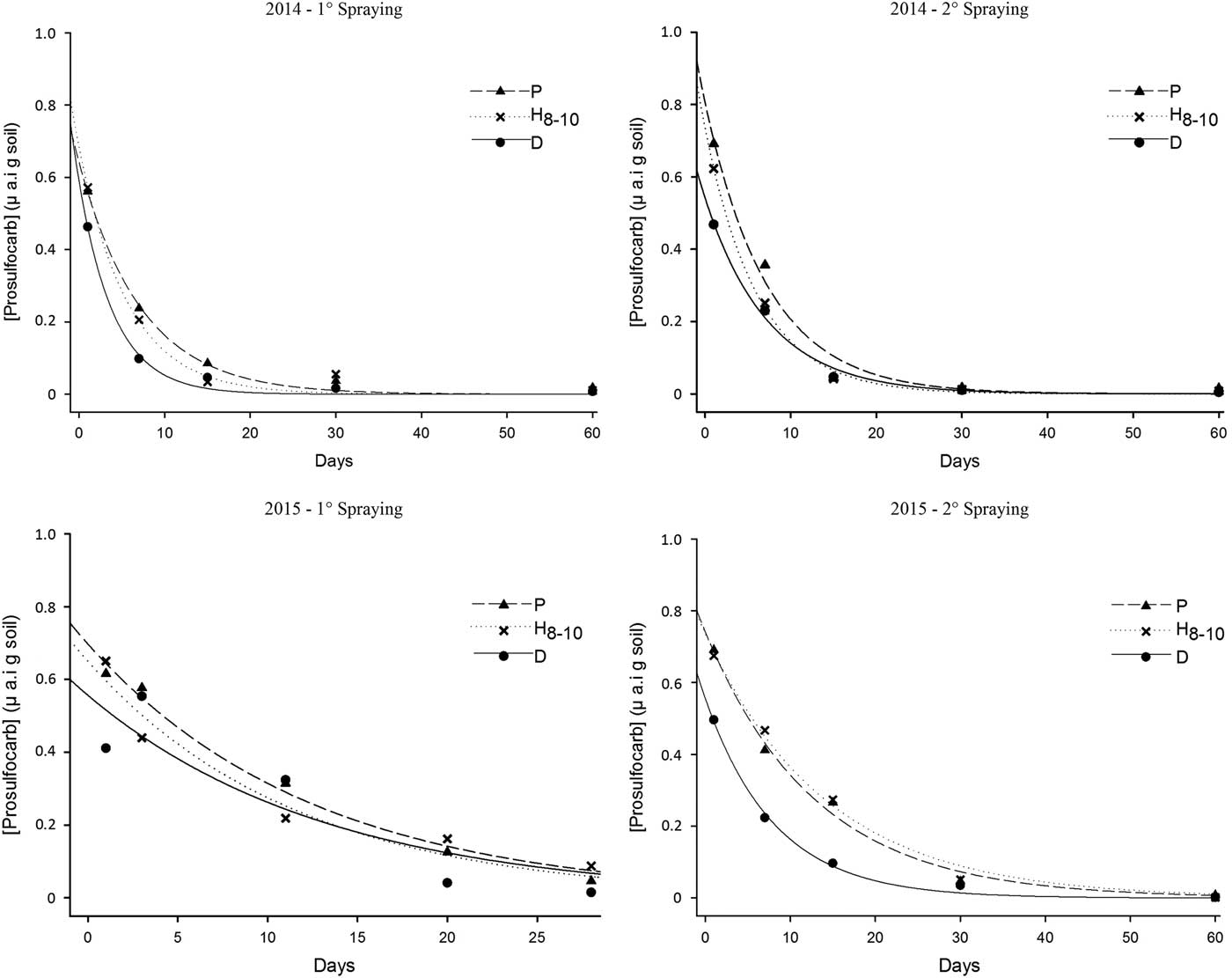
Field trials were established in an ongoing long-term field experiment at the Department of Agroecology, Flakkebjerg, Denmark (55.316°N, 11.383°E) in the autumn of 2014 and repeated in the autumn of 2015. The experimental field had been cropped and cultivated according to normal agricultural practices before the start of the experiment, with oat (Avena sativa L.) being the last preceding crop. The soil is based on ground morainic deposits from the last glaciations and is classified as a Glossic Phaeozem according to the WRB (FAO) system (Krogh and Greve Reference Krogh and Greve1999). The clay (<2 µm), silt (2 to 20 µm), fine sand (20 to 200 µm), coarse sand (200 to 2,000 µm), and organic carbon contents of the soil (0 to 25 cm) were 147, 137, 426, 270, and 12 g kg−1, respectively. The experiment was first established in 2002 to investigate the interactions between crop rotations and tillage methods on various cropping factors and was described in detail in other studies (Hansen et al. Reference Hansen, Munkholm, Olesen and Melander2015; Scherner et al. Reference Scherner, Melander and Kudsk2016).
The experimental design was a split plot with four replicates (blocks). The main plot effect was soil tillage, while the subplots evaluated doses of prosulfocarb. Each tillage plot consisted of two 2.5-m-wide and 25-m-long tillage strips, allowing special treatments and recordings to take place in six subplots, each measuring 3 by 2.5 m net area. A border zone of 1 m was left between the subplots to avoid overlapping of herbicide treatments. The experiment included four crop rotations, R1, R2, R3, and R4, However, only one crop rotation was used per year in this study: R3 in 2014 and R4 in 2015, with the specific crop sequences shown in Table 1. The management of the stubble residues was the only difference between the two rotations: in R4 the straw was chopped and retained after harvest, whereas it was removed in R3. However, in this study the crop residues from the previous crop established in the area were removed mechanically with a round baler (John Deere 449, John Deere GmBH & Co., Mannheim, Germany) in direct-drilled plots in both years with minimum soil disturbance. During the growing seasons, the plots were not cropped to ensure an even herbicide deposition on the soil surface.
Table 1 Crop sequences R3 and R4 and straw management at Flakkebjerg, Denmark.

a Crop residues in non-cropped plots from the previous crop were removed with minimum soil disturbance.
All other crops received the total amount of manure/fertilizer in spring, which were fertilized with100 kg ha−1 NH4-N in pig slurry and the remainder of the fertilizer recommendation was provided by a mineral fertilizer. For winter rape (Brassica napus L.), 30 kg ha−1 N as a mineral fertilizer was applied in autumn. The target fertilizer applications were winter wheat (Triticum aestivum L.) 165, winter rape 171, winter barley (Hordeum vulgare L.) 139, spring barley 117, and oat 92 kgN ha−1. Analyses of slurry were performed before application, and the nitrogen content was used to target an application rate of 100 kg ha−1 NH4-N. At spreading, slurry samples were collected to determine the actual nitrogen content. The slurry was applied with trailing hoses to winter crops and injected after plowing but before sowing to spring crops. The slurry was injected shortly before sowing in plots with spring crops and noninversion tillage.
The tillage systems considered in this study were direct drilling, tine cultivation to 8 to 10 cm (cultivated), and moldboard plowing (plowing) to a 20-cm soil depth. The cultivated treatment was stubble cultivated once after harvest to a depth of 8 to 10 cm using a rotary harrow (Bomford Dyna Drive). Plowing took place 1 mo before the early herbicide application timing. Glyphosate (Roundup Bio®, 360 g ae L−1, Monsanto) at a dose of 540 g ae ha−1 was used in all plots to kill weeds and volunteer crop plants 20 d before herbicide spraying. Furthermore, the soil surface was rolled after soil cultivation in the cultivated and plowed treatments and after removal of crop residues in the direct-drilled plots to flatten the soil surface, ensuring that the herbicide would reach the whole surface.
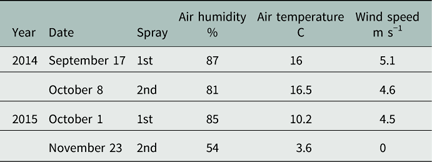
The herbicide treatment consisted of six rates of PSC (0, 500, 1,000, 2,000, 4,000, and 8,000 g ai ha−1, Boxer EC®, 800 g ai L−1, Syngenta Crop Protection A/S) that were sprayed in the subplots at two application timings reflecting early and late sowing time of winter wheat (Table 1). In the second year (2015), the late application was postponed to November to obtain a greater difference in soil temperature between the first and last applications. An experimental field sprayer, Speedy 2500 V/l, equipped with Hardi LD 015-110 nozzles, operating at 2.6 bar pressure at a speed of 4.5 km h−1 and delivering a spray volume of 150 L ha−1, was used. The environmental conditions on all spraying occasions are summarized in Table 2.
Table 2 Dates and environmental conditions for each herbicide application made in 2014 and 2015.
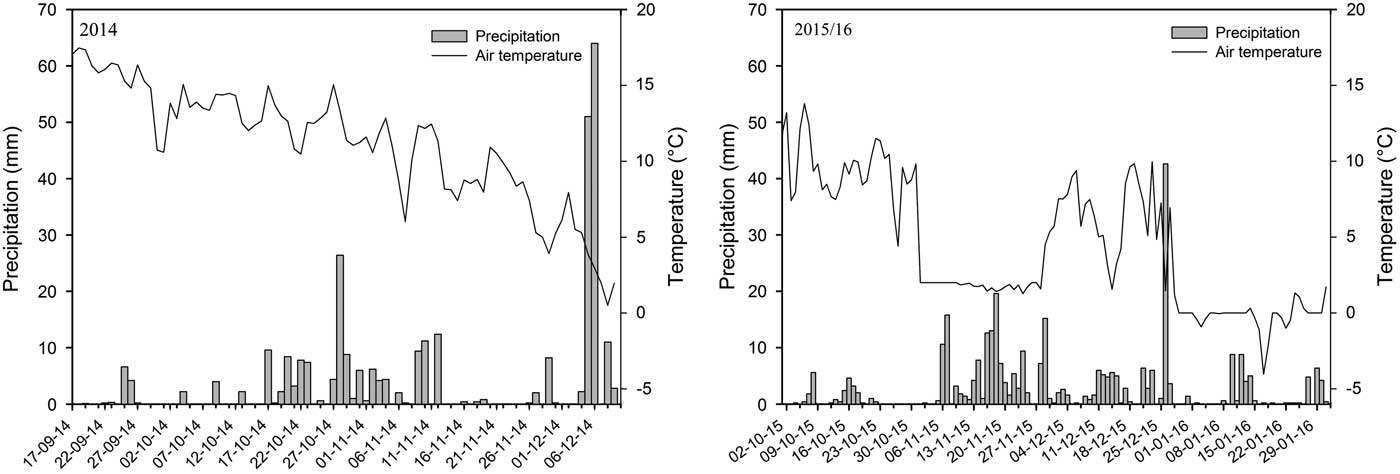
Soil temperature was recorded throughout the experimental period using data loggers (HOBO Pendant®), with temperature probes placed at 3-cm soil depths. Precipitation was also recorded from a automatic weather station (high-performance system: WS-HP1 from Delta T®) placed 200 m from the experimental area. Soil samples from the 0- to 10-cm soil layer were collected in each tillage system and from all four replicates to evaluate soil carbon concentration with the elemental analysis (CHNS/O) method proposed by Segnini et al. (Reference Segnini, Santos, Silva, Martin, Borato, Melo and Bolonhezi2008).
Sampling
PSC is a very lipophilic compound (Kow=3.02×1004 at 20 C) and is therefore considered an immobile herbicide, remaining in the upper soil layer (PPDB 2009). Hence, to avoid mixing the herbicide into the soil matrix, intact soil-column samples were collected from each subplot using stainless-steel cylinders (10-cm height by 5-cm diameter). In 2014, samples were collected 1, 7, 15, 30, and 60 d after herbicide application (DAA). In 2015, due to the fast dissipation rate observed in the previous year, samples were collected 1, 3, 11, 20, and 28 DAA for the early spray date. Sampling for the second spraying was performed using the same intervals as in 2014. The collected samples were stored for approximately 30 d in darkness at −18 C to prevent herbicide degradation. In total, 720 samples were collected per year (5 dates after sowing by 2 spraying times by 6 herbicide doses by 3 tillage systems by 4 replicates).
Additional soil samples were collected from each subplot for HPLC analysis on the same dates as for the bioassays. These samples were collected from the subplots sprayed with 1,000 g ai ha−1 PSC. The samples comprised eight subsamples collected with an auger (10-cm height by 2-cm diameter) in each subplot. The subsamples from each subplot were mixed in a plastic bag and stored in darkness at −18 C to stop herbicide degradation. In total, 120 samples were collected per year. Sampling was concluded at the beginning of December in the first year of the experiment (2014), and by the end of January 2016 in the second year (2015–16).
Bioassay
Two bioassays were conducted in February 2015 and February 2016 to determine PSC dissipation rates, using the intact soil samples collected in the field experiments conducted in 2014 and 2015, respectively. Both trials were carried out in a climate chamber using a constant temperature of 10 C, a 16:8 h (light:dark) photoperiod, and air humidity of 70%. The soil samples were removed from the freezer and placed in the chamber 24 h before seeds were sown.
Seeds of silky windgrass were collected at the end of July 2014 from mature plants growing in untreated plots in the same area in which the field experiment was located. The seeds were dried for 3 wk at room temperature and cleaned by being passed through mesh sieves of different sizes. The cleaned seeds were stored in paper bags at 4 C in the dark until bioassays were initiated. Germination tests were performed before the bioassays to check seed viability. Thirty seeds were sown in each unit. The seeds were placed on the soil surface and covered with a thin layer of gravel to maintain high soil moisture in the topsoil. To promote germination, 25 ml of deionized water was added to the top of each pot on the day of sowing. Subsequently, the pots were subirrigated twice a week, applying the same amount of water per pot. The aboveground portions of the plants were harvested 40 d after sowing, and fresh biomass weight was measured. Dry biomass weight was recorded following drying for 24 h at 80 C.
HPLC Analysis
HPLC analysis of samples from the field experiments established in 2014 and 2015 was performed in March 2015 and 2016, respectively. Samples were removed from the freezer and kept at room temperature for 3h, allowing them to thaw. Afterward, samples were thoroughly mixed and placed in plastic pots in which the thickness of the soil layer was approximately 5 cm. Mass reduction was performed using a metal auger (10-cm height by 0.6-cm diameter), with 10 subsamples taken in each sample. Thereafter, these subsamples were mixed, and 10 g of this soil was used for the analyses.
The reduced soil samples (10 g) were transferred to 50-ml Falcon tubes, to which 5 g of Ottawa sand and 10 ml of 100% methanol were added. These samples underwent sonication for 10 min and then were placed in an Intelli-Mixer to rotate at 40 rpm for 1 h. After this, samples were centrifuged at 4,000 rpm for 10 min at 20 C, and the clear supernatant was recovered. To extract any remaining residues completely, the same samples were subsequently extracted again using the same procedure. The clear supernatants from the two extraction cycles were combined and placed in a brown flask (100 ml), and the weight of the extracted volume was recorded. Extracts were stored at −18 C until chromatographic analysis was performed using an Agilent 1200 HPLC system (Santa Clara, CA, USA) coupled with a Sciex (Forest City, CA, USA) 3200 QTRAP triple-quadrupole mass spectrometer (MS/MS). The MS was equipped with electrospray ionization. Aliquots of 250 μl from each of the extracts were diluted 1:1 with Milli-Q water and filtered (PTFE filters, 0.22 μM, Titan Filtration Systems, Sun SRi, Wilmington, NC, USA) directly into HPLC vials using a syringe. The analytical selectivity of the method was tested by analyzing blank samples collected from the untreated control plots. No interfering peaks were detected. Analytical calibration curves presented good linearity, with R2 values >0.99. Extraction recoveries ranged from 79.5% to 104.1% with relative standard deviation ≤15.7% obtained for PSC at 5 and 500 ng g−1 fortification levels.
Three soil samples of 5 g were also collected from each field sample to determine the soil water content by weight. The samples were weighed before and after drying for 24 h at 105 C, allowing the PSC concentrations to be expressed on the basis of grams of dry soil.
Data Analyses
A three-parameter log-logistic model (Streibig et al. Reference Streibig, Rudemo and Jensen1993) was fit to the fresh biomass of silky windgrass for each combination of treatments (tillage systems by doses), as described by Equation 1:
where y is the fresh weight of silky windgrass, x is the herbicide dose (g ai ha−1), d (g of fresh weight) is the upper limit or mean response when the herbicide dose is zero, E (or LD50) is the dosage (g ai ha−1) that reduces fresh weight by 50%, and b is the slope of the dose–response curve around LD50. The data were fit to this equation for all sampling dates (DAA) and combinations of year, soil cultivation, and application timing.
The estimated LD50 values from Equation 1 were used to estimate the percent of the prosulfocarb remaining at different sampling dates. These estimates were used to calculate herbicide half-lives for prosulfocarb in three different tillage systems. The ED50 value at 1 DAA was set to 100% (C 0). The LD50 doses of the sampling dates (e.g. 7, 15, 30, and 60 DAA) were converted to the percentage of PSC remaining, using the following equation:
where x is the LD50 dose at 7, 15, 30, or 60 DAA from Equation 1 and y is the LD50 dose at 1 DAA (C 0). The PSC (%) estimates were used to calculate PSC half-lives for the tillage systems using three different models to describe PSC transformation kinetics: single first-order (SFO), first-order multicompartment (FOMC), and double first-order in parallel (DFOP) models (Boesten et al. 2006; Etzerodt et al. Reference Etzerodt, Mortensen and Fomsgaard2008; Schreiber et al. Reference Schreiber, Scherner, Massey, Zanella and Avila2017). The chi-square (χ2) test with α=0.05 was used to estimate whether the model was appropriate or not and to assess the test’s lack of fit. The model producing the smallest error percentage provided the best fit. Based on the data fit to the curve (dg-adj R2) and error distribution, the SFO model, represented by Equation 3, was chosen:
where C is the concentration of prosulfocarb at time t, C 0 is the initial concentration of prosulfocarb, and k is the degradation rate (slope). Subsequently, their half-lives (LD50) were calculated by Equation 4 as proposed in Lynch (Reference Lynch1995):
where LD50 is the required time to reduce the herbicide concentration by 50%. Confidence intervals (95%) for the estimated parameters C 0 and k were generated to allow comparisons between treatments.
Herbicide dissipation based on the HPLC analyses was also fit by tillage system using the same three kinetic models. Based on the model fit, the SFO model was chosen, and the analyses therefore were also performed using Equations 3 and 4.
Tillage effects on carbon concentration in soil were analyzed using a linear model for each crop rotation (R3 and R4). Means were calculated as least-squares means and adjusted according to the Tukey test (P<0.05).
All statistical analyses were performed using R statistical software (R Core Team 2013) with the add-on package ‘drc’ (Ritz and Streibig Reference Ritz and Streibig2005) for dose–response analyses and the package ‘kinfit’ (Ranke and Lindenberger Reference Ranke and Lindenberger2015) for herbicide dissipation.
Results and Discussion
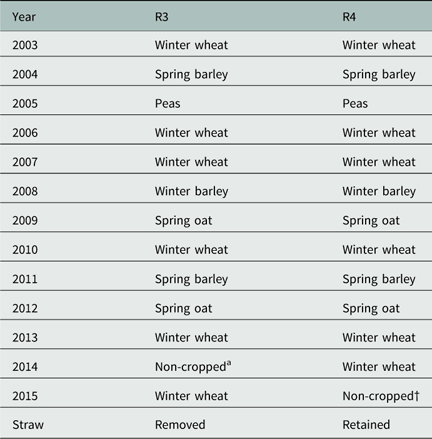
The three-parameter log-logistic model (Equation 1) provided a good fit to the data, with R2 values >0.98. Therefore, ED50, b, and d values could be estimated with relatively low standard errors for all combination of treatments (Supplementary Tables S1 and S2, respectively). The estimated parameters from Equation 3 and herbicide half-life (LD50) calculated with Equation 4 are shown in Table 3. The reduction of silky windgrass biomass as a function of herbicide dose and the corresponding herbicide dissipation over time (DAT) are shown in Figure 1. As expected, the LD50 doses of PSC increased over sampling dates.
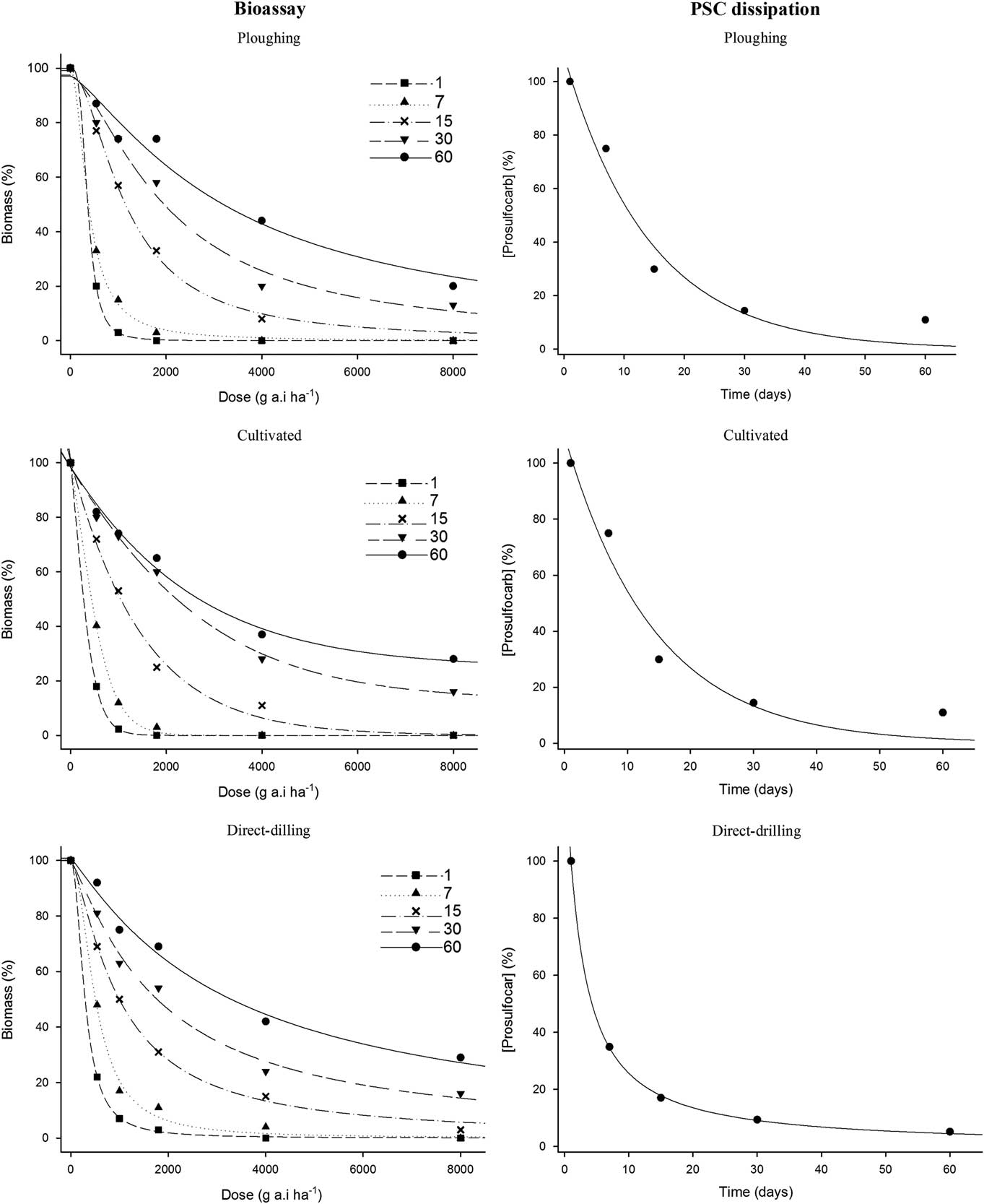
Figure 1 Bioassay results (left) showing the reduction of silky windgrass biomass (%) as a function of 6 doses of prosulfocarb and herbicide dissipation (right) in soil (%) over time (days after spraying) in three tillage systems (plowing, cultivated, and direct drilling). Curves show data from the first spraying time in 2014, for which bioassay results (left) were fit to a three-parameter log-logistic model and herbicide dissipation results (right), calculated based on LD50 values from the bioassay, were fit to the single first-order model.
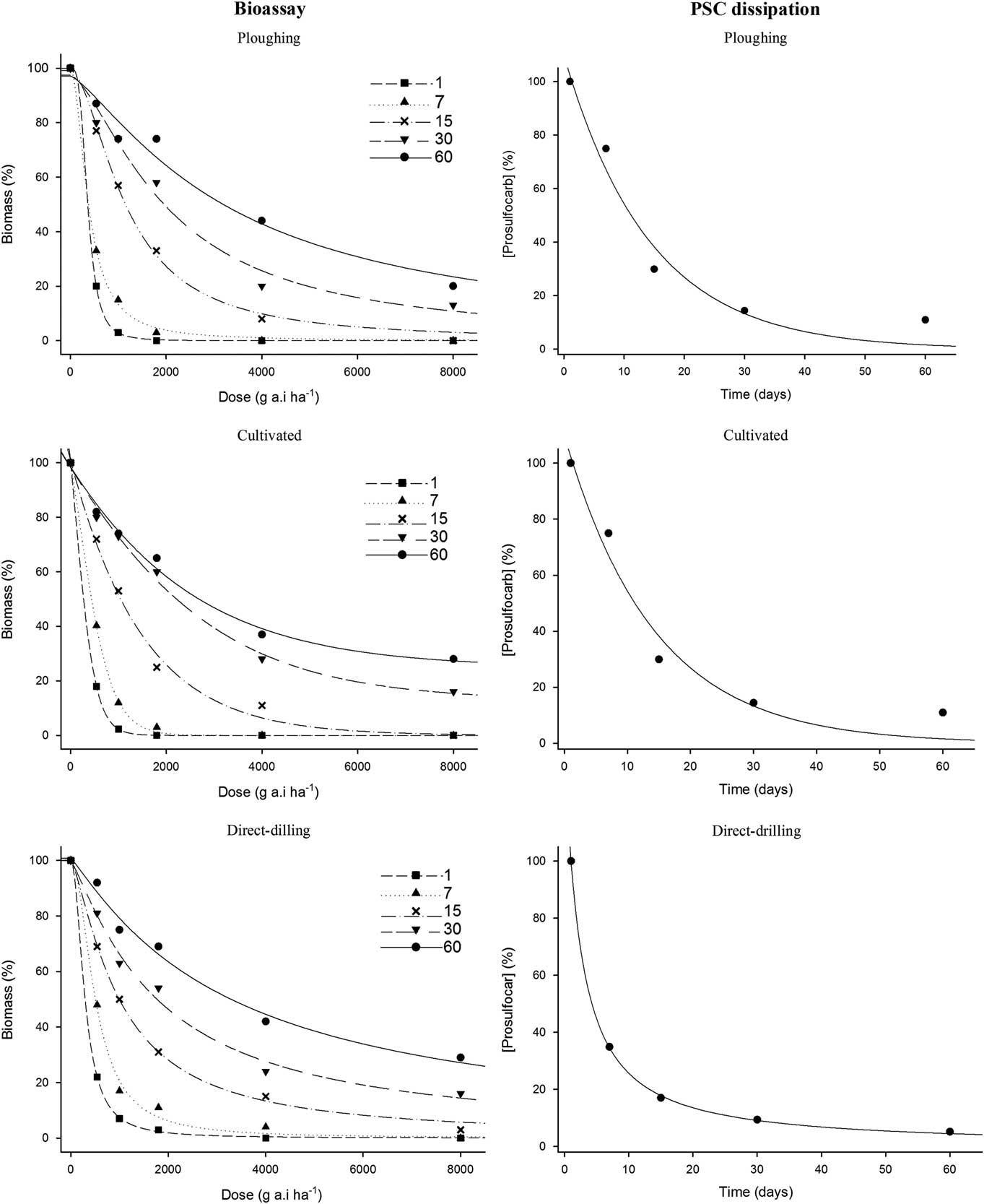
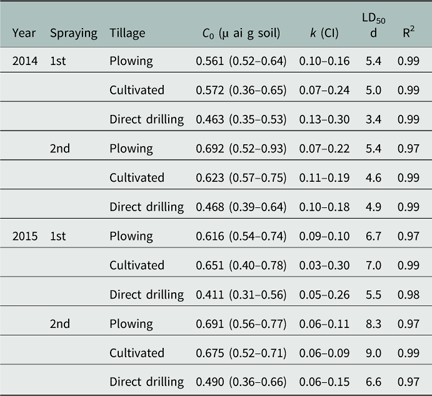
Table 3 Prosulfocarb dissipation rate constant (k) and half-life (LD50) as a function of tillage system, spraying time, and year based on bioassay data.Footnote a
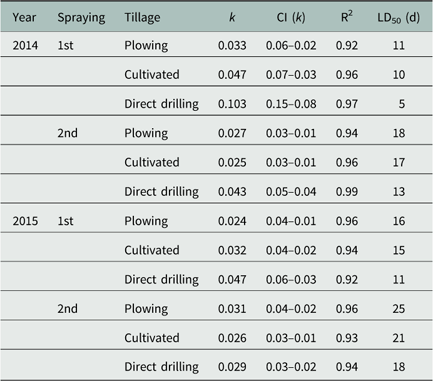
a Parameter k and 95% confidence intervals (CI) were estimated using Equation 3. LD50 values were calculated by dividing the natural log (2) for k values estimated with the SFO model.
In 2014, bioassay results showed a faster dissipation of PSC at the early spraying timing than at the late timing, with mean LD50 values of 8.6 and 16 d, respectively (Table 3). However, significant differences in the dissipation rate (k) between tillage systems and between spraying times were only observed for direct drilling, with values ranging from 0.15 to 0.08 and 0.05 to 0.04 on the first and second spraying, respectively. Comparisons between the tillage systems within application timings showed that herbicide dissipation in plowing and cultivated systems was significantly different from direct drilling in both spraying times. For example, in the first spraying timing, k values ranged from 0.07 to 0.02 in plowing and cultivated systems and from 0.15 to 0.08 in direct drilling (Table 3). As observed in 2014, herbicide dissipation in 2015 was more rapid at the early than at the late timing, with mean LD50 values of 14 and 21.3 d, respectively (Table 3). However, there were no significant differences between tillage systems at any of the spraying times, with LD50 values varying between 11 to 16 and 18 to 25 d among the tillage systems at the first and second spraying times, respectively.
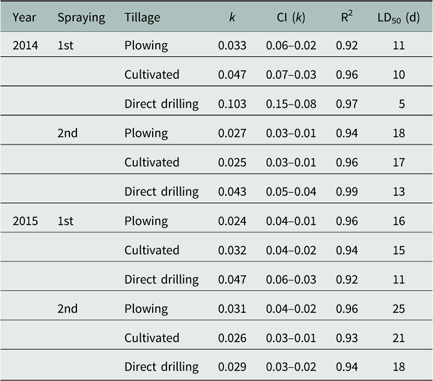
In general, R2 adj was high, and the errors were <15% for HPLC analysis. The estimated parameters from Equation 3 and the corresponding degradation endpoints (LD50) calculated using Equation 4 are shown in Table 4. The dissipation behavior of PSC in three tillage systems over time (days after spraying) in 2014 and 2015 is shown in Figure 2.
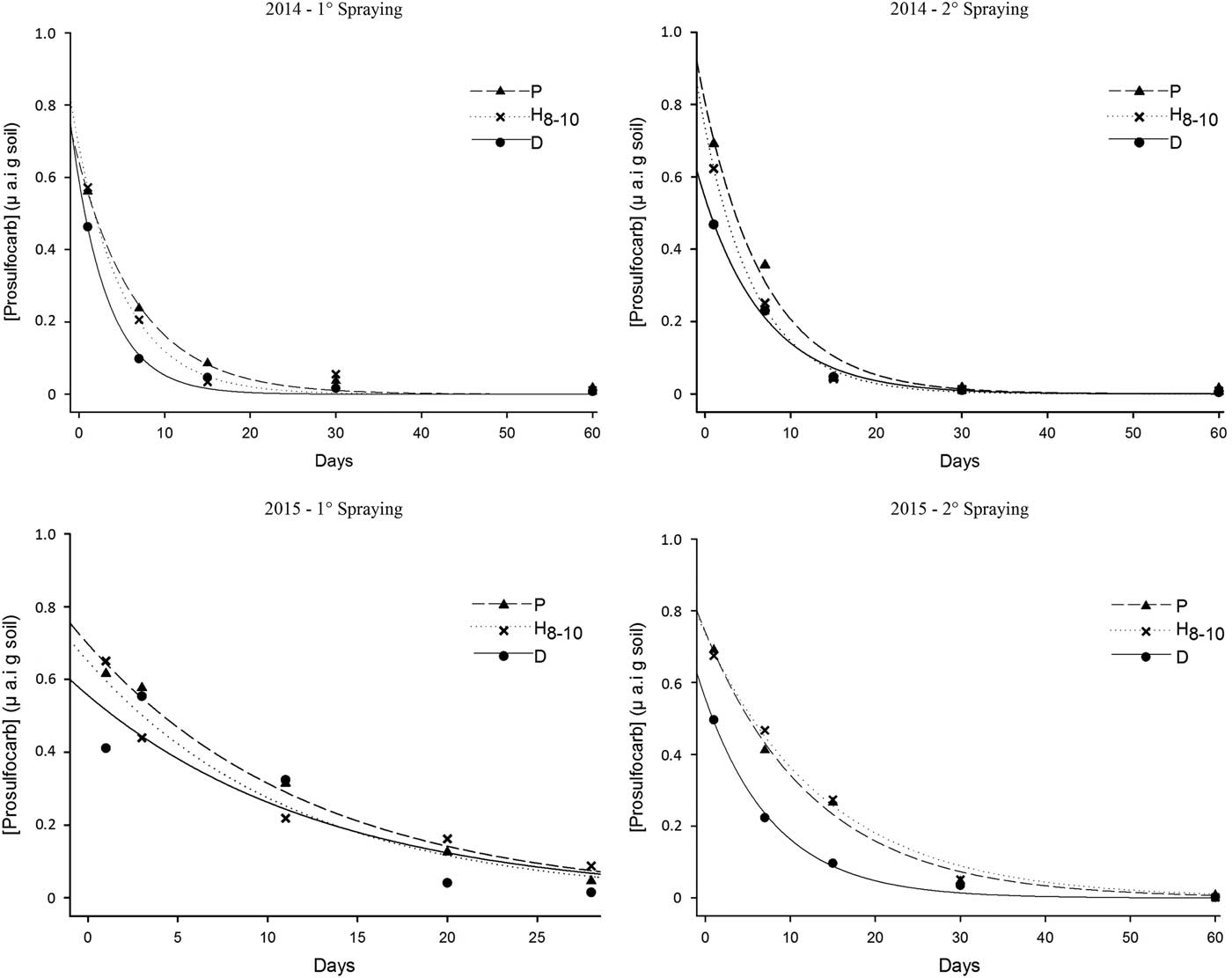
Figure 2 Dissipation of prosulfocarb in soil over time (days after spraying) as a function of tillage system (P, plowing; C, cultivated; D, direct drilling), spraying time, and year. Curves show data fit to the observed values from HPLC analyses using the single first-order model.
Table 4 Prosulfocarb, initial concentration in soil (C 0), dissipation rate constant (k), and half-life (LD50) as a function of tillage systems, spraying time, and years based on the chemical analyses.Footnote a
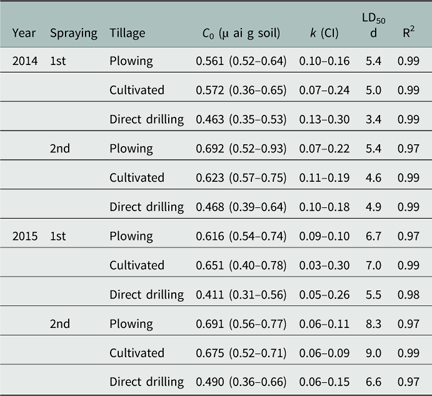
a Parameter k and 95% confidence intervals (CI) were estimated using Equation 3. LD50 values were calculated by dividing the natural log (2) for k values estimated with the SFO model.
In agreement with the dissipation results observed in the bioassays (Table 3), PSC dissipation as determined with HPLC analysis was not statistically different in either of the years within or between application timings for all tillage systems (Table 4). Nevertheless, PSC half-life values were generally lower under direct drilling compared with the other tillage systems. Thus, the hypothesis that the dissipation of PSC is reduced in conservation tillage systems compared with conventional tillage due to a higher content of organic matter in the upper soil layer and that dissipation rates estimated with HPLC would be faster compared with the bioassay results (because the latter method reflects not only the effect of PSC but also of any phytotoxic metabolites) was only partly supported. Bioassay and HPLC analyses did not show clear significant effects of tillage practices on PSC dissipation rates, although some half-life values were lower in direct drilling than in plowing. However, PSC dissipation rates estimated via HPLC analyses were indeed faster than those estimated with bioassays.
The estimated LD50 values based on the bioassay results were markedly longer than the corresponding values from the HPLC analyses. For example, in 2014, the mean LD50 was 12 d in the bioassay compared with less than 5 d based on the chemical analyses. Several comparative studies between bioassays and chemical analysis have been performed to clarify herbicide dissipation in the soil (Eberle and Gerber Reference Eberle and Gerber1976; Hollaway et al. Reference Ritz and Streibig1999; Rupak et al. Reference Rupak, Sharma, Kulshrestha and Singh2009). When comparing both methods, the authors showed that there is a correlation between them for some herbicides such as 2,4-D, indicating that the methods provide almost identical results (Eberle and Gerber Reference Eberle and Gerber1976). However, this correlation is not achieved for all herbicides, and the results presented here are in accordance with the ones reported by Rupak et al. (Reference Rupak, Sharma, Kulshrestha and Singh2009), in which the bioassay was found to be a more sensitive technique than HPLC analysis for evaluation of metsulfuron-methyl dissipation in soil.
One possible explanation for the observed discrepancy between the two methods in the present study could be that one or more of the PSC degradation products exert an effect on plant growth. Whereas the HPLC analysis only detected PSC, the bioassays reflected the activity of the parent compound, PSC, as well as any degradation product with herbicidal properties. The metabolic pathway for the degradation of PSC in soil has been proposed, with prosulfocarb sulfoxide being the dominant metabolite (European Food Safety Authority [EFSA] 2007). The estimated LD50 value for this metabolite was from 1.6 to 3.9 d, indicating low persistence in soil (EFSA 2007). Moreover, prosulfocarb sulfoxide shows higher mobility in soil when compared with PSC, but no information is available to date regarding the pesticide activity and toxicological relevance of the metabolite (EFSA 2007).
PSC is registered as a PRE herbicide with some residual activity. However, the period in which the herbicide remains active in the soil is influenced by its dissipation rate. The decline of PSC concentration in soil is expected to be mainly the result of biodegradation (Gennari et al. Reference Melander, Munier-Jolain, Charles, Wirth, Schwarz, van der Weide, Bonin, Jensen and Kudsk1998; Rouchaud et al. Reference Rouchaud, Neus, Callens and Bulcke1997), which depends on the microbial activity in the soil. The PSC dissipation rate is therefore expected to be higher under warmer conditions due to increased microbial activity (Gavrilescu Reference Lal2005), and the differences observed in herbicide dissipation between spraying occasions can likely be explained by the higher temperatures at the early timing.
Soil temperature and precipitation recorded in both years showed a clear seasonal difference within and between years (Figure 3). In general, lower temperatures and more precipitation were observed at the late timing in both years, as expected. However, in 2014, the differences in temperature between application timings were small (12.8 and 9.8 C, respectively). Moreover, the precipitation records in both periods were quite similar, with 157.4 and 182.1 mm in the first and second sampling periods. This lack of difference prompted the postponement of the late timing in 2015 in order to explore the effects of more extreme environmental conditions on the interaction between tillage and herbicide dissipation. In 2015, the average temperature and precipitation recorded at the early and late sampling periods were 9.3 and 3.7 C and 23.3 and 174.4 mm, respectively. The lower precipitation in the early sampling period was partly a result of the shorter sampling period.
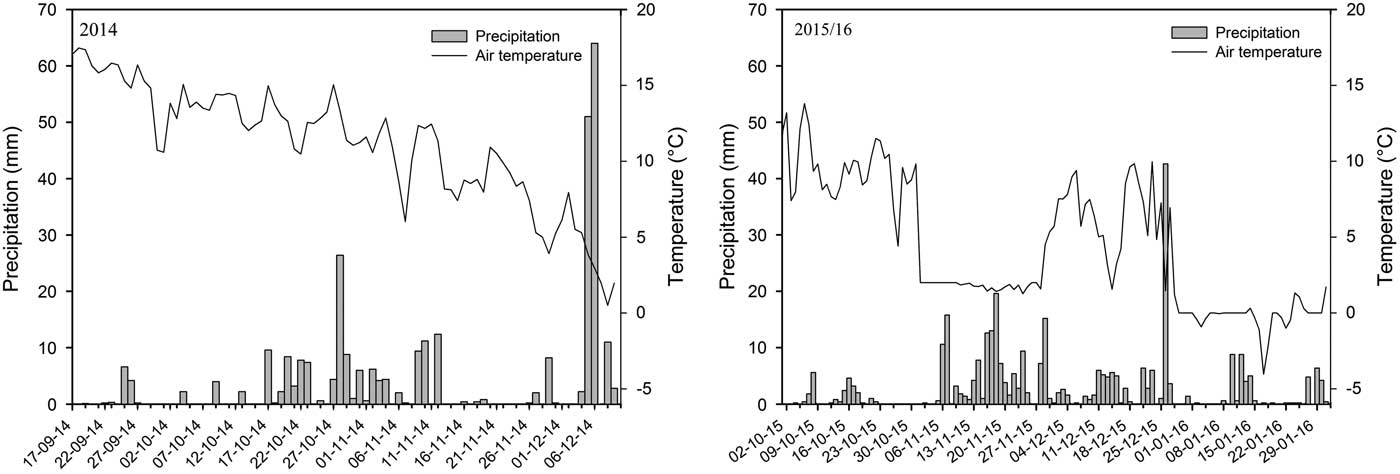
Figure 3 Soil temperature and precipitation data at Flakkebjerg, Denmark, during the whole experimental period of 2014 and 2015–16.
Moreover, the physicochemical characteristics of a herbicide can also influence its dissipation pathway. PSC has a moderate to high vapor pressure (6.9 mPa at 25 C), and it has been detected in rainwater samples in Sweden (Kreuger et al. Reference Kreuger, Törnquist and Kylin2004). This indicates PSC movement from the sprayed field to the atmosphere via volatilization. Nevertheless, PSC volatilization is expected to be an issue primarily from plant surfaces, with reported losses reaching up to 80% in 48 h. From the soil surface, the PSC volatilization rate is markedly lower, less than 5%, due to higher adsorption (Jensen et al. Reference Jensen, Spliid and Svensmark2007). In the field experiment, PSC was applied to the soil surface. In the direct-drilled plots, the herbicide could have been intercepted by the crop residues located near the soil surface, from which it could evaporate. However, the majority of the crop residues were removed from the plots before spraying, and crop residues therefore did not influence the fate of PSC in this study. Furthermore, the initial concentrations (C 0) recovered from the soil samples from the direct-drilled plots were similar to the application rate (0.67 g–1 soil). Although C 0 values were always lower under direct drilling than in plowed and cultivated systems, no significant differences were observed. Therefore, even though PSC is prone to volatilization, this was not expected to be a cause of dissipation in the present study.
When applied to the soil, PSC is adsorbed strongly, which is attributed to its high Kow and low water solubility (PPDB 2009), and its adsorption is expected to increase with soil organic carbon content (Gennari et al. Reference Melander, Munier-Jolain, Charles, Wirth, Schwarz, van der Weide, Bonin, Jensen and Kudsk1998). Soil carbon concentrations in this study differed between tillage practices, with direct-drilling and cultivated systems having significantly higher carbon content in comparison with plowing in both crop rotations (Table 5). This was expected, because with reduced soil-tillage intensity, the decomposition of soil organic matter is reduced, resulting in higher soil carbon content over time (Lal Reference Lal2002). Furthermore, microbial activity also increases when the soil carbon content is higher (Gavrilescu Reference Gavrilescu2005), which may lead to a more rapid degradation of the herbicide (Fomsgaard and Kristensen Reference Fomsgaard and Kristensen1999).
Table 5 Mean carbon concentration (g C kg−1 dry soil) determined with elemental analysis (CHNS/O) method in 2016 as affected by crop rotations (R3 and R4) and tillage (plowing, cultivated, and direct drilling) for 0- to 10-cm soil layer.Footnote a
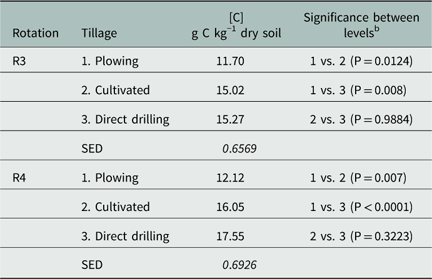
a Maximum standard errors of differences (SED) between least-squares means are given in italics.
b Compared by Tukey tests at the 5% level of significance.
A residual herbicide is expected to persist in the soil for an appropriate time to suppress weed emergence. However, in both years, the LD50 values estimated using HPLC analysis were not within the range of values (8 to 35 d) based on chemical analyses reported in the literature (Gennari et al. Reference Melander, Munier-Jolain, Charles, Wirth, Schwarz, van der Weide, Bonin, Jensen and Kudsk1998; PPDB 2009). One possible reason for the shorter half-lives of PSC in this study could be enhanced soil biodegradation due to a history of repeated applications of the herbicide (Alletto et al. Reference Alletto, Coquet, Benoit, Heddadj and Barriuso2010). When degradation is enhanced, herbicide efficacy is lessened, possibly resulting in reduced crop yields (Chung Reference Chung2000).
Enhanced degradation of herbicides was first observed in soil treated with a phenoxyalkanoic acid herbicide (2,4-D and MCPA), and later for EPTC, another thiocarbamate herbicide (Audus Reference Audus1951; Rahman et al. Reference Rahman, Atkinson, Douglas and Sinclair1979). The presence of adapted soil microflora has been reported to reduce the recovery of PSC by 35% in comparison with a sterilized soil (Gennari et al. Reference Locke and Harper2002). In our experiments, PSC was applied in a field in which PSC had been used frequently and most recently 2 yr before the initiation of the study. Therefore, it is likely that the soil microflora were adapted to PSC, and that degradation began immediately after spraying with no lag phase. This would explain the low LD50 values estimated and would be one of the main limitations and weakness of our study, because it was not our intention to investigate enhanced degradation of the herbicide. Bioassay and HPLC techniques are well accepted by the scientific community, provided that some principles regarding site selection, experimental design, sampling collection, storage, and analyses are strictly applied (Mueller and Senseman Reference Mueller and Senseman2015).
PSC is recommended as an alternative to ALS and ACCase inhibitors to delay the evolution of resistance in grass weeds such as silky windgrass and ryegrass (Keshtkar et al. Reference Keshtkar, Mathiassen, Moss and Kudsk2015). Using information from the decision support system Crop Protection Online (Department of Agroecology, Aarhus University, and SEGES 2016), grass species can be ranked according to their susceptibility to PSC. Silky windgrass is the most susceptible species, followed by annual bluegrass (Poa annua L.), ryegrass, and blackgrass. Thus, different doses of PSC are required to control these grass species. For example, the dose required to reduce a population of blackgrass by 50% is approximately four times greater than the one required for the same level of control of silky windgrass. Therefore, a more rapid dissipation of PSC can lead to control failures against the least susceptible grass weed species, highlighting that both spray timing and dose should reflect the composition of the weed flora. Only one case of resistance to PSC has been reported so far (Heap Reference Heap2016), and like other thiocarbamate herbicides, PSC is not considered to be prone to resistance, and its relatively fast degradation rate may explain this (Somerville et al. Reference Somerville, Powles, Walsh and Renton2017). PSC is recommended for use in rotation or in sequence with ALS and ACCase inhibitors to delay the evolution of resistance to these more resistance-prone sites of actions. Rapid degradation may, however, jeopardize the potential benefit of PSC as an antiresistance management tool, because only the first flush of weeds will be exposed to lethal PSC doses. This may particularly apply to reduced-tillage systems, in which annual grass weeds germinate over a longer period than in plowed soils (Scherner et al. Reference Scherner, Melander and Kudsk2016).
PSC is also recommended for control of problematic grass weeds such as rattail fescue [Vulpia myuros (L.) K. C. Gmel]. Noninversion tillage systems, especially direct drilling, were found to affect the emergence dynamics of silky windgrass and rattail fescue by prolonging the period during which emergence occurs by more than 10 d in comparison to plowing systems (Scherner et al. Reference Scherner, Melander, Jensen, Kudsk and Avila2017). Considering the results of Scherner et al. (Reference Scherner, Melander, Jensen, Kudsk and Avila2017), PSC should persist in soil at least a month after sowing the crop to provide satisfactory control under a direct-drilling system. However, in the present study, we found that the residual activity of PSC is generally short, especially when PSC is applied in the early autumn, a time when the majority of winter cereals are sown and treated with PSC. Although the tillage system did not significantly affect the PSC dissipation rate, tillage systems do affect the emergence period of the grass weeds. Thus, it is clear that management interventions for silky windgrass and rattail fescue cannot follow the same strategy in noninversion tillage systems and plowing. Therefore, not only timing of PSC spraying should be reconsidered, but its spraying frequency; otherwise, PSC is likely to provide unsatisfactory control.
In summary, the present study showed that PSC soil degradation was relatively fast, but the estimated half-life of the herbicide varied between the bioassay and HPLC analysis. The bioassays revealed longer LD50 values than estimated by the HPLC analyses, emphasizing the added value of bioassays for evaluating herbicide effects. Furthermore, the study revealed that tillage effects on PSC dissipation were not as evident as the effects of environmental conditions/application timing. Given the rapid dissipation of PSC, it is suggested that current spraying strategies based on one application should be reconsidered, particularly on farms practicing reduced tillage, in which grass weeds tend to emerge over a longer period. In these tillage systems, split application strategies should be considered to ensure maximum control with and benefit from PSC as an antiresistance management tool.
Acknowledgments
We would like to thank the Danish Ministry of Food, Agriculture and Fisheries and the Science without Borders Programme in Brazil for funding this research. Technicians Eugene Driessen, Karen Bjørn Heinager, and Bente Birgitte Laursen are acknowledged for their skillful technical assistance.
Supplementary materials
To view supplementary material for this article, please visit https://doi.org/10.1017/wet.2017.103