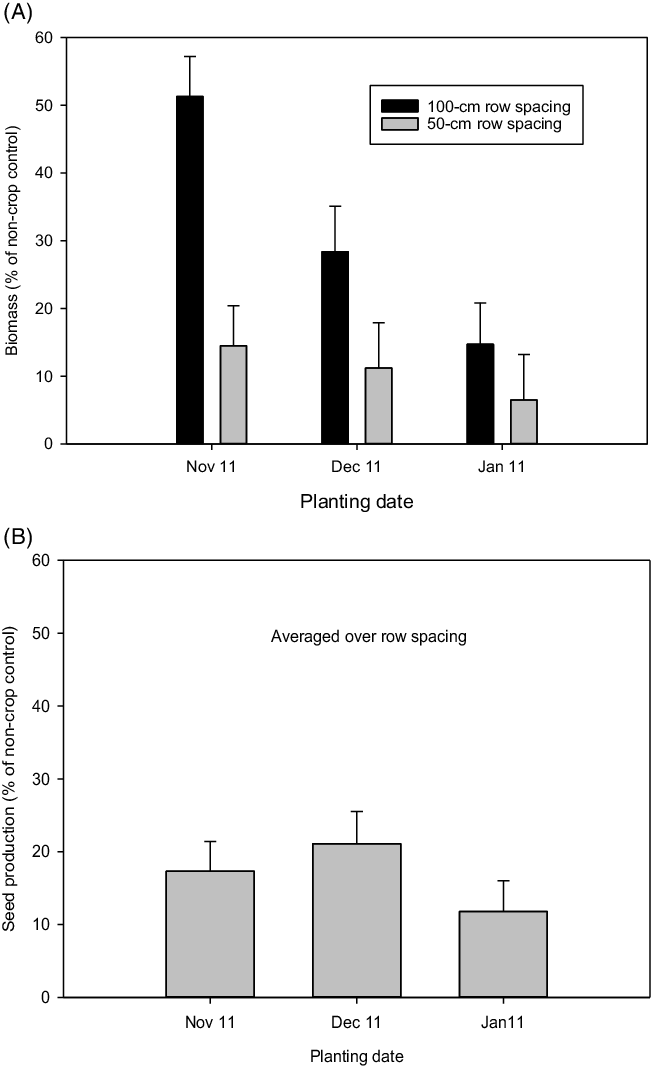Introduction
Junglerice and feather fingergrass are the top two most troublesome weeds in sorghum; combined, they are responsible for annual revenue losses of AU $23 million to Australian crop producers (Llewellyn et al. Reference Llewellyn, Ronning, Ouzman, Walker, Mayfield and Clarke2016). Both species are competitive annual grasses, capable of rapid growth. Aboveground biomass of junglerice and feather fingergrass growing in sorghum has been reported at 16 and 28 g plant–1, respectively (Mahajan et al. Reference Mahajan, Kaur, Thompson and Chauhan2020b). Junglerice is also known to reduce sorghum yield by 3.6% for each week of interference (Smith et al. Reference Smith, Murray, Green, Wanyahaya and Weeks1990). In Australian conditions, junglerice and feather fingergrass plants present in sorghum production fields are reported to produce, on average, 4,060 and 5,740 seeds per plant, respectively (Mahajan et al. Reference Mahajan, Kaur, Thompson and Chauhan2020). Such prodigious seed production can cause rapid population density increases in subsequent crops and fallow phases. However, information on seed production of junglerice and feather fingergrass is lacking in Australia, particularly when they emerge at different times within sorghum crops with different times of planting.
A major challenge to the effective management of junglerice and feather fingergrass in sorghum in Australia is the lack of available herbicides, which is compounded by increasing frequencies of herbicide resistance (GRDC 2017). Control of junglerice and feather fingergrass in sorghum is difficult, as currently only a few herbicides are labeled for preemergence and postemergence application (GRDC 2017). Consequently, their management in conservation cropping systems increasingly relies on nonselective herbicides such as glyphosate during fallow or pre-seeding phases. Increasing cases of glyphosate resistance in both junglerice and feather fingergrass, and resistance of junglerice to atrazine further restrict herbicide options to control these weeds (Heap Reference Heap2021; Mahajan et al. Reference Mahajan, Kaur, Thompson and Chauhan2020). Due to the existing and potential proliferation of herbicide resistance, current and future management decisions must include integrated management techniques, such as crop competition, to reduce the reliance on herbicides.
Competitive crops reduce weed growth by competing for light, water, and nutrients. Sorghum is vulnerable to weed interference during the early stages of the season, which is exacerbated by typically wide (100-cm) row spacing (GRDC 2017). However, simple management choices of narrow row spacing may drastically increase sorghum crop competition to reduce weed biomass and seed production. Selecting sorghum cultivars with greater height, leaf area ratio, and leaf area index resulted in 34% to 55% higher yield in the presence of velvetleaf (Abutilon theophrasti Medik) (Traoré et al. Reference Traoré, Mason, Martin, Mortensen and Spotanski2003). Increasing densities of sorghum from 4.5 to 7.5 plants m–2 decreased density of Japanese millet [Echinochloa esculenta (A. Braun) H. Scholz.], biomass, and seed production by 22%, 27%, and 38%, respectively (Wu et al. Reference Wu, Walker, Osten and Robinson2010).
Previous studies revealed that reducing the row spacing in sorghum can assist weed control with a minimal effect on yield. In Nebraska, under low weed pressure from velvetleaf and foxtail grass (Setaria faberi Herrm), sorghum yield was reduced less in 38-cm rows vs 76-cm rows (Grichar et al. Reference Grichar, Besler and Brewer2004). The effect of row spacing on weed biomass has been reported (Burnside et al. Reference Burnside and Colville1964), but little information is available on junglerice and feather fingergrass seed production (GRDC 2020). Further, adjusting the date of crop planting can minimize weed pressure in some crops (Chauhan and Mahajan Reference Chauhan and Mahajan2012; Mahajan et al. Reference Mahajan, Brar and Walia2002), and weed growth has been reported to differ based on planting date in rice (Oryza sativa L.), wheat (Triticum aestivum L.) and wheat (Matloob et al. Reference Matloob, Khaliq, Tanveer, Hussain, Aslam and Chauhan2015; Rasmussen, Reference Rasmussen2004). Growth and seed production of junglerice and feather fingergrass could therefore possibly be influenced by adjusting the planting time of sorghum. The objectives of this study were to determine the effect of sorghum planted in narrow row spacing on junglerice and feather fingergrass growth and seed production as well as to determine if the time of planting has any effect.
Materials and Methods
Site Description
Field experiments were conducted during the summer growing seasons of 2017–2018 and 2018–2019 (November to March) at the Gatton research farm of the University of Queensland, Queensland, Australia (27.5514 °S, 152.3428 °E). In the summer growing season of 2018–2019, the same experiment was also conducted at Narrabri, The University of Sydney, New South Wales, Australia (30.3065 °S, 149.8114 °E). The soil at the Gatton site was medium clay (33% sand, 46% silt, and 21% clay) and had a pH of 7.1, with an organic matter content of 2.6%. The soil at the Narrabri site was brown clay (22% sand, 20% silt, and 58% clay) with a pH of 6.4 and an organic matter content of 1.8%.
Experimental Design and Observations
In each year, two separate experiments were conducted in sorghum crops––one with junglerice and the other with feather fingergrass. In each experiment, the growth and reproduction of junglerice and feather fingergrass were studied by growing weed plants alone or in competition with sorghum. The experiments were arranged in a factorial randomized complete block design with a two-way treatment structure of planting time (November 11, December 11, and January 11) and row spacing (fallow, 50 cm, and 100 cm). Each experiment had four replications per treatment.
The fields were selected with a history of low infestation of junglerice and feather fingergrass at each site. The field sites were prepared for planting by two passes with a cultivator. Sorghum was planted in 50- and 100-cm spaced rows at a density of 10 plants m–2 with respective planting dates. At Gatton, sorghum varieties used in the experiments were Buster with Concep II (a crop safener; Syngenta Crop Protection Pty Ltd., Macquarie Park, NSW, Australia) in 2017 and imidazolinone-tolerant Elite Sentinel IG in 2018. At Narrabri, the sorghum variety used in the experiments was MR43. At each planting date of sorghum, weed species were planted in trays filled with a potting mix and kept in a screenhouse. Weed seedlings were transplanted when they reached the two- to three-leaf stage as a single plant in each treatment replicate, centered between crop rows. The individual junglerice and feather fingergrass replicates were spaced 2 m apart.
Experimental plots were irrigated, whenever necessary, depending upon rainfall to keep the crop free from water stress. At Gatton, other weeds were controlled with S-metolachlor applied preemergence at 0.96 kg ai ha–1 in 2017, and imazamox applied postemergence at 0.025 kg ai ha–1 plus imazapyr 0.011 kg ha–1 (plus 1% Hasten oil) 2 d before the transplanting of junglerice and feather fingergrass in 2018. The spots where junglerice and feather fingergrass were to be transplanted were covered with plastic trays prior to spraying to keep these areas herbicide free. Herbicides were sprayed with a CO2 pressurized backpack sprayer equipped with flat-fan nozzles delivering a water volume of 100 L ha–1. At Narrabri, other weeds were controlled by hand-weeding.
Observations were taken on the number of days to seedhead emergence for junglerice and feather fingergrass. To estimate seed numbers of junglerice and feather fingergrass, lobes in each seedhead and rachilla segment (pedicel base) in each lobe were counted. It was observed that each rachilla segment in the lobe had one seed. The total number of seedheads per plant was counted separately. To estimate seed production per head, two intact seedheads were chosen randomly from each plant. To estimate the total number of seeds, each rachilla segment (pedicel base) was counted and the resulting sums averaged to calculate seeds per head. At harvest, aboveground biomass of weed plants from each plot was bagged separately, dried in an oven at 70 C for 72 h, and weighed. The corresponding cumulative growing degree days (GDD) at each day after emergence were calculated as:
where base temperature (Tb) was considered as 10 C for summer weeds (Norsworthy et al. Reference Norsworthy, Oliveira, Jha, Malik, Buckelew, Jennings and Monks2008; Mahajan et al Reference Mahajan, Mutti, Walsh and Chauhan2019a).
Statistical Analyses
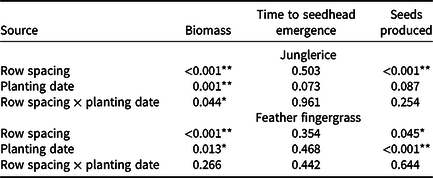
Data for all locations were normalized as a percent of the noncompetitive control and then subjected to a two-way linear mixed-model ANOVA with Genstat (Version 18.2; https://www.vsni.co.uk/software/genstat) (Table 1). The two factors were sorghum row spacing and planting date, with experimental runs modeled as random effects. Where no interaction was found, the main effects are reported (feather fingergrass); otherwise, interactions are reported (junglerice). No significant interactions were found in three experimental runs; therefore, data were pooled for experimental runs. Means were compared using Fisher’s protected LSD at 5%.
Table 1. ANOVA of weed biomass, time to seedhead emergence, and number of seeds produced, as influenced by sorghum row spacing and planting date, and their interaction. a
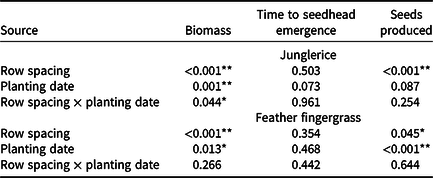
a Data pooled over three experimental runs. Asterisks denote significance of P values: * P < 0.05; ** P < 0.001.
Results and Discussion
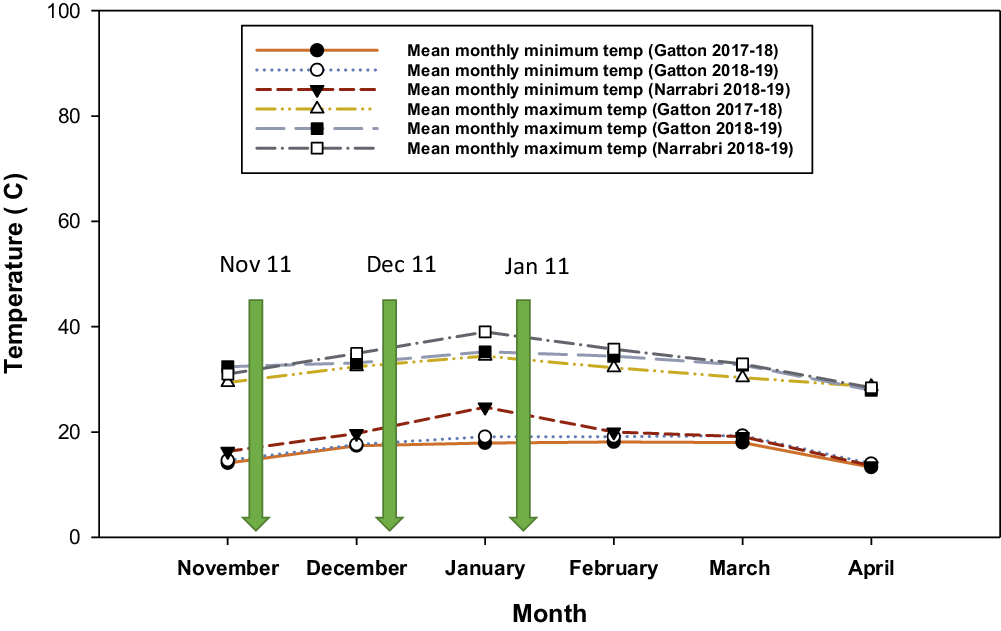
During the summer growing seasons the monthly mean minimum temperature at Gatton varied from 13.3 to 18.1 C in 2017–2018 and 14 to 19.1 C in 2018–2019––lowest in April and highest in February (Figure 1). At Narrabri, the monthly mean minimum temperature in 2018–2019 varied from 13.6 to 24.7 C––lowest in April and highest in January (Figure 1). Monthly mean maximum temperatures at Gatton varied from 29.4 to 34.4 C in 2017–2018 and 27.9 to 35.2 C in 2018–2019––lowest in April and highest in January. At Narrabri, the monthly mean maximum temperature in 2018–2019 varied from 28.4 to 35.7 C––lowest in April and highest in January (Figure 1). Consequently, warmer growing-season conditions following the November and December plantings were optimum for the growth of junglerice and feather fingergrass, whereas plants emerging from the January planting underwent cooler conditions during the reproductive phase.
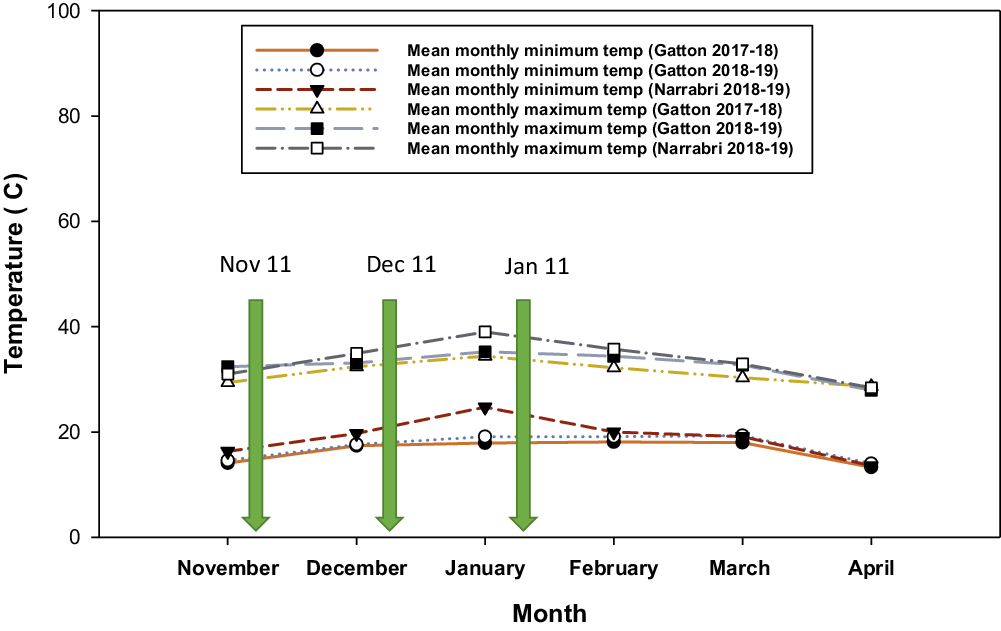
Figure 1. Mean monthly minimum and maximum temperature during growth of junglerice, feather fingergrass, and sorghum crop in field studies conducted at Gatton and Narrabri, Australia in 2017–2018 and 2018–2019.
Junglerice
Averaged over experimental runs, biomass of junglerice in fallow conditions without sorghum crop competition was 217 ± 19, 215 ± 10, and 166 ± 11 g plant–1, respectively, for November 11, December 11, and January 11 planting times. When grown in competition with sorghum, decreasing the row spacing from 100 to 50 cm reduced junglerice grass biomass by 36% for the November 11 planting time (Figure 2A). The reduction of junglerice biomass for the December 11 sorghum planting was 17%. For the January 11 sorghum planting, row spacing of sorghum did not influence junglerice biomass; however, in each row spacing, the reduction was >85% compared to crop-free fallow conditions (Figure 2A).
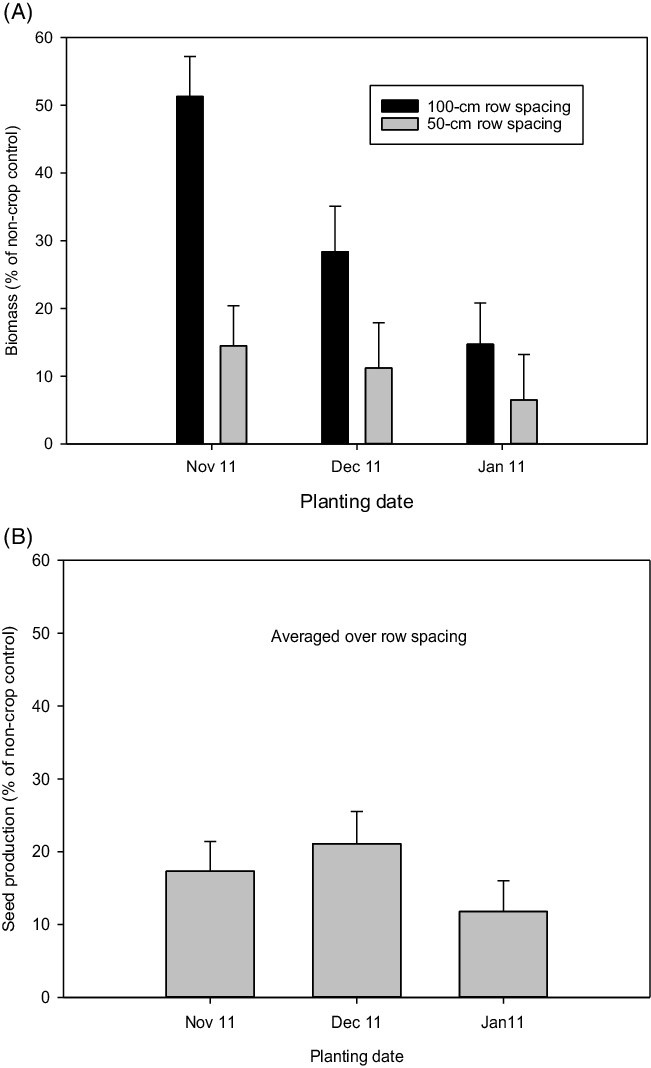
Figure 2. (A) Junglerice biomass in relation to sorghum row spacing and planting date, and (B) junglerice seed production (averaged over row spacing) as a percentage of plants grown in crop-free fallow conditions. Error bars indicate standard error for the mean of four replicates in field studies conducted at Gatton and Narrabri, Australia in 2017–2018 and 2018–2019.
Under crop-free fallow conditions, junglerice took 58 ± 2, 57 ± 4, and 51 ± 1 d for seedhead emergence, following planting on November 11, December 11, and January 11 planting, respectively (Figure 1). Duration of junglerice seedhead emergence was the same (P > 0.05) in narrow- and wide-row spacing sorghum at each planting date (Table 1). Under fallow conditions, junglerice took 847, 987, and 622 GDD for seedhead emergence, respectively, for November 11, December 11, and January 11 planting.
In the absence of sorghum crop competition, seed production of junglerice in fallow conditions was 16,254 ± 538, 20,281 ± 1,364, and 12,380 ± 1,710 seeds plant–1, respectively for November 11, December 11, and January 11 plantings. Seed production of junglerice remained similar at both row spacings. However, crop competition (averaged over row spacing) reduced seed production of junglerice by 83%, 79%, and 88% for November 11, December 11, and January 11 planting, respectively, compared to fallow conditions (Figure 2B).
Feather Fingergrass
The biomass of feather fingergrass under crop-free fallow conditions was 1,099 ± 109, 563 ± 88, and 541 ± 64 g plant–1, respectively, for the November 11, December 11, and January 11 plantings. Averaged over row spacing, crop competition for the November 11, December 11, and January 11 plantings reduced the biomass of feather fingergrass by 87%, 87%, and 92%, respectively, compared to crop-free fallow conditions (Figure 3A). Averaged over planting date, crop competition at 100-cm and 50-cm row spacing reduced the biomass of feather fingergrass by 85% and 93%, respectively, compared to crop-free fallow conditions (Figure 3B).

Figure 3. Biomass of feather fingergrass in relation to (A) planting date, (B) row spacing, and (C) seed production as a percentage of crop-free fallow conditions. Error bars indicate standard error for the mean of four replicates in field studies conducted at Gatton and Narrabri, Australia in 2017–2018 and 2018–2019.
Under crop-free fallow conditions, feather fingergrass took 71 ± 2, 75 ± 1, and 68 ± 1 d for seedhead emergence, respectively, for the November 11, December 11, and January 11 plantings (Figure 1). Duration for seedhead emergence of feather fingergrass was similar in narrow- and wide-row spacings of sorghum (Table 1). Under crop-free fallow conditions, feather fingergrass took 1,117, 1,354, and 1,129 GDD for seedhead emergence, respectively, for the November 11, December 11, and January 11 plantings.
Seed production of feather fingergrass under fallow conditions was 143,175 ± 12,582, 141,250 ± 1,339, and 90,030 ± 19,179 seeds plant–1, respectively, for the November 11, December 11, and January 11 plantings. For each sorghum planting, seed production of feather fingergrass was similar at both row spacings (Table 1). Averaged over row spacing, crop competition for the November 11, December 11, and January 11 plantings reduced the seed production of feather fingergrass by 86%, 91%, and 93%, respectively, compared to crop-free fallow conditions (Figure 3C).
This study revealed that biomass of junglerice was affected by the interaction of row spacing and planting dates. Under fallow conditions, seed production of junglerice was highest with the December planting compared to the November and January plantings. Junglerice planted in December accumulated higher GDD for seedhead emergence compared to the November and January planting; that caused higher seed production for December planting compared with November and January plantings.
The accumulation of GDDs determines the maturity and seed production of plants. For example, in sunflower (Helianthus annuus L.), seed production decreased with late planting because the total GDDs decreased from 1,731 to 1,621, where the delayed planting resulted in lower temperatures during the seed filling period (Sur and Sharma,Reference Sur and Sharma1999). However, another study concluded that lower yields associated with late planting in sunflower were due to warmer temperatures during the early growth period, which promoted excessive early stem growth (Beard and Geng, Reference Beard and Geng1982). Similarly, under fallow conditions, early- and late-planted junglerice in this study produced a lower number of seeds compared to mid-planted junglerice, as the GDDs decreased in early and late planting.
The seedhead emergence time in late-planted (January) junglerice and feather fingergrass was induced with considerable shortening of the vegetative phase (by ∼7 d) as a result of enhancement of daylight and temperature in January and February. Rapid seedhead emergence or shortening of the vegetative phase in junglerice and feather fingergrass with late planting can be correlated with the photoperiod-sensitive nature of these weeds (Mahajan et al. Reference Mahajan, Sharma, Kaur and Chauhan2015).
Temperature and photoperiod have been shown to play a major role in weed growth and development. For example, days to panicle emergence in wild oats (Avena fatua L. and Avena sterilis ssp. ludoviciana Durieu Gillet & Magne) has been reported to decrease as temperature increases and the photoperiod decreases (Mahajan and Chauhan Reference Mahajan and Chauhan2021; Sharma et al. Reference Sharma, McBeath and Vanden Born1977). In another study, late cohorts of junglerice were found smaller in height than early cohorts because of variation in temperature and photoperiod (Mahajan et al. Reference Mahajan, Walsh and Chauhan2019b). Feather fingergrass took fewer days for seedhead emergence in January planting compared to December planting; however, seed production was similar for the November 11 and December 11 planting dates. This suggests that in the summer season, early cohorts of feather fingergrass could produce a high number of seeds; this situation therefore requires early weed management to restrict further reinfestation.
This study demonstrated that the presence of a sorghum crop reduced seed production of junglerice and feather fingergrass through crop competition, and the reduction was greater when the crop was sown late. Narrow-row spacing of sorghum reduced the biomass of junglerice and feather fingergrass to a greater extent than wide-row spacing. These results are similar to other studies that suggest narrow crop row spacing reduces weed biomass (Chauhan and Johnson Reference Chauhan and Johnson2010; Clay et al. Reference Clay, Kleinjan, Clay, Forcella and Batchelor2005; Mulugeta and Boerboom Reference Mulugeta and Boerboom2000). The improved competitive effect of narrow crop rows in this study and previous studies could be the result of fast or early canopy closure compared to crops grown with wide rows, which might have entailed low light penetration to the soil surface and consequent reduced weed growth (Knezevic et al. Reference Knezevic, Horak and Vanderlip1999; Scott et al. Reference Scott, Martin and Riethmuller2013).
Practical Implications
Our results suggest that, compared to wide rows, narrow sorghum row spacings can suppress weed growth and development in both junglerice and feather fingergrass. These results may be helpful for sorghum growers who are struggling to control these weeds in current wide-row configurations. In Australian sorghum production, the typical practice is to grow sorghum in 100-cm row spacings because of the perception of increased access to soil moisture for the crop; however, this practice may lead to proliferation of these weeds in inter-rows. Feather fingergrass is particularly adapted to expansion in unoccupied space, as it can produce roots from stem nodes (GRDC 2020). Both weeds are highly prolific in seed production, and if not controlled, can easily produce enough seeds to re-infest the fields. Therefore, with the use of narrow-row spacings in sorghum and manipulating planting time, weed growth and seed production of junglerice and feather fingergrass could be reduced.
Our results also suggest that vigorous growth of junglerice and feather fingergrass may occur in sorghum fields planted at wide 100-cm row spacings and could rapidly eventually lead to a dense weed seedbank in subsequent years. This vigorous growth of weeds in terms of biomass could be reduced by planting sorghum in narrow-row spacing and adjusting the planting time of sorghum. Reducing the weed seedbank through narrow-row spacings and late planting of sorghum could be a valuable tool in an integrated weed management program and could help reduce the pressure to use herbicides. These results are in line with our previous study, in which late cohorts of junglerice and feather fingergrass in sorghum produced fewer seeds than early cohorts (Mahajan et al. Reference Mahajan, Walsh and Chauhan2019b). However, there is a need to quantify the yield penalty if sorghum is to be planted late in the season for developing a viable weed management program. Results are also a reminder that junglerice and feather fingergrass have prolific seed production in crop-free fallow conditions, regardless of when the plants emerge.
Acknowledgments
The authors would like to thank the Grains Research and Development Corporation (GRDC) for investing in this research. No conflicts of interest have been declared.