Herbicide resistance management and mitigation requires that end users have a means of confirming resistance in weed populations. Traditional methods of confirming resistance in weeds involve collecting seeds of plants surviving treatment in the field, planting them under controlled conditions in a greenhouse, and treating progeny with either PRE or POST herbicides and comparing the plant’s responses to those observed after treating herbicide-susceptible plants in the same manner (Burgos et al. Reference Burgos, Tranel, Streibig, Davis, Shaner, Norsworthy and Ritz2013). A limitation of this type of classical bioassay is that an extended period of time may be required to garner enough putative resistant seed for testing; the space required to conduct such an assay may be limited as well. Typically, classical bioassays to confirm resistance are conducted using 4 to 15 herbicide doses, and are replicated such that up to 200 plants are exposed to each dose (Burgos et al. Reference Burgos, Tranel, Streibig, Davis, Shaner, Norsworthy and Ritz2013).
When resources are available, molecular methods can reduce the time required to confirm herbicide resistance in weeds. Traditionally, DNA is extracted from putative resistant and susceptible weeds of the same species, subjected to polymerase chain reaction (PCR), and analyzed using Sanger sequencing and alignment software (Dèlye et al. Reference Dèlye, Duhoux, Pernin, Riggins and Tranel2015). In several days this process can confirm the presence of target site mutations (i.e., single nucleotide polymorphisms) conferring herbicide resistance. However, this technique has several limitations: 1) the process must be optimized with primers for specific mutation and weed species combinations, 2) the process can only detect resistance via target site mutations that have been elucidated at the DNA level, and 3) the process cannot detect non–target site mechanisms such as enhanced herbicide detoxification and altered absorption and translocation (Burgos et al. Reference Burgos, Tranel, Streibig, Davis, Shaner, Norsworthy and Ritz2013). Improvements in next-generation sequencing technologies, such as RNA sequencing (RNA-Seq) and genotype-by-sequencing (GBS), have helped address these limitations by capturing entire transcriptomes without preexisting genomic or transcriptomic information for a particular species (Duhoux et al. Reference Duhoux, Carrère, Gouzy, Bonin and Dèlye2015). RNA-Seq technology has been used to identify target site mutations conferring resistance to acetolactate synthase (ALS) (WSSA Group 2) and photosystem II (PSII) (WSSA Group 5) inhibitors in annual bluegrass, as well as transcripts upregulating enzymatic systems conferring non–target site resistance to ALS-inhibiting herbicides in Lolium species (Brosnan et al. Reference Brosnan, Vargas, Breeden, Grier, Aponte, Tresch and LaForest2016; Duhoux et al. Reference Duhoux, Carrère, Gouzy, Bonin and Dèlye2015). Although next-generation sequencing technology provides a powerful tool to detect resistance in weeds, this technology is not readily available to agricultural producers at present, reducing the expediency with which results are provided to end users during the growing season.
A rapid in-season quick (RISQ) test has been developed to provide end users an option for screening Lolium and blackgrass (Alopecurus myosuroides Huds.) for resistance to ALS and acetyl co-A carboxylase (ACCase)-inhibiting herbicides during the growing season (Kaundun et al. Reference Kaundun, Hutchings, Dale, Baily and Glanfield2011). The test involves establishing tillers of weeds in petri dishes filled with plant tissue culture agar and a discriminatory rate of herbicide, and evaluating survival in 10 days. Kaundun et al. (Reference Kaundun, Hutchings, Dale, Baily and Glanfield2011) reported that exposing herbicide-susceptible Lolium phenotypes, as well as those with target- and non–target site resistance mechanisms to pinoxaden and clodinafop (both WSSA Group 1) in agar culture at 0.16 and 0.32 μM, respectively, yielded a statistically similar response to treating these phenotypes with the same herbicides via traditional spray application at labeled rates. Additionally, no differences were detected when exposing herbicide-resistant and herbicide-susceptible Lolium phenotypes to mesosulfuron (WSSA Group 2) in agar culture at 0.05 μM and traditional spray application at 14.4 g ha−1. The researchers reported that this method was also successful for detecting ALS and ACCase resistance in blackgrass, wild oat (Avena fatua L.), silky windgrass [Apera spica-venti (L.) Beauv.], hood canarygrass (Phalaris paradoxa L.), and green foxtail [Setaria viridis (L.) Beauv]. Since initial development, this agar-based test has been found to be effective for detecting both target and non–target site resistance in an array of broadleaf and grass weeds of cropping systems (Jackson et al. Reference Jackson, Hutchings, Harris, Dale, Mcindoe and Kaundun2015; Kaundun et al. Reference Kaundun, Hutchings, Harris, Jackson, Shashi-Kiran, Dale and McIndoe2014).
Annual bluegrass is a problematic weed of managed turfgrass systems, including golf courses, athletic fields, and sod farms, as well as residential and commercial lawns. More cases of herbicide resistance have been documented in annual bluegrass than in any other turfgrass weed, encompassing nine different herbicidal sites of action (Heap Reference Heap2016). Cases of multiple resistance in annual bluegrass have recently been confirmed, with phenotypes evolving resistance to ALS- and PSII-inhibiting herbicides (Brosnan et al. Reference Brosnan, Vargas, Breeden, Grier, Aponte, Tresch and LaForest2016), and non–target-site-based resistance has been reported (Svyantek et al. Reference Svyantek, Aldahir, Chen, Flessner, McCullough, Sidhu and McElroy2016).
Currently, turfgrass managers have few readily available means of evaluating herbicide resistance (or susceptibility) in annual bluegrass during the growing season. An enzyme assay to detect resistance to ALS-inhibiting herbicides in annual bluegrass has been developed; however, this technique is specific only to the ALS mode of action (Cross et al. Reference Cross, McCarty, Tharayil, Whitwell and Bridges2013). We hypothesize that the agar-based diagnostic tests developed for grassy weeds of agronomic cropping systems could be used to confirm herbicide resistance in annual bluegrass populations of managed turfgrass in-season. A diagnostic test of this nature would provide turfgrass managers an option to screen for resistance to multiple herbicidal modes of action and deliver timely information to improve the efficiency and effectiveness of herbicide applications for annual bluegrass control. Therefore, our objective was to determine if agar-based diagnostic tests could be used to reliably confirm herbicide resistance in annual bluegrass populations harvested from golf course turf.
Materials and Methods
Research was conducted using a walk-in growth chamber at the University of Tennessee (Knoxville, TN) during the summer and autumn of 2016. This chamber was configured to provide a constant 16 C air temperature, 60% relative humidity, and 16-hour photoperiod. Separate experiments were conducted inside this chamber to evaluate the suitability of agar-based diagnostic tests to confirm resistance to herbicidal inhibitors of ALS, enolpyruvylshikimate-3-phosphate synthase (EPSPS), and PSII. Each experiment was repeated in time during 2016 as follows: EPSPS experiments were initiated on January 15 and February 29, ALS experiments were initiated on May 19 and June 29, and PSII experiments were initiated on October 25 and November 3.
Annual Bluegrass Phenotypes
Annual bluegrass phenotypes with resistance to ALS (R3, R7), EPSPS (R5), and PSII (R3, R4) inhibitors were included in our experiments. Resistance in all of these phenotypes was target-site based and has been described by Brosnan et al. (Reference Brosnan, Vargas, Breeden, Boggess, Staton, Wadl and Trigiano2017). An herbicide susceptible phenotype from University Park, PA was included in all experiments as well. Complete DNA sequences for genes coding for EPSPS (KU382756 – KU382767), the D1 protein (KX258687 – KX258698), and ALS (KY495881 – KY495883, KY495889) in the resistant and susceptible annual bluegrass phenotypes studied herein are available in GenBank (https://www.ncbi.nlm.nih.gov/genbank/). Messenger RNA sequences for homeologs of ALS in the R3 phenotype have been published in GenBank as well (KT346395 – KT346396). All plants were surface-seeded into propagation trays (52 cm by 37 cm by 10 cm; Dillen Products/Myers Industries, Inc., Middlefield, OH), filled with a peat moss growing medium (Growing Mix No. 2, Conrad Fafard, Inc., Agawam, MA) and placed inside the growth chamber. Trays were supplied with complete fertilizer (20 N, 8.7 P, 16.6 K; Southern Ag 20-20-20 Soluble Fertilizer, Boone, NC) such that nitrogen was provided at 49 kg ha−1 wk−1. The trays were irrigated to maximize growth and vigor.
Agar Culture
Experiments to determine if diagnostic assays could reliably detect herbicide resistance in annual bluegrass were conducted using autoclavable polycarbonate plant culture boxes (Magenta GA-7, Bioworld, Dublin, OH). These boxes measured 7.6 by 7.6 cm and were 10.2 cm high. Each box was autoclaved at 121 C for 15 minutes prior to being filled with agar growing medium (Plant Agar, Duchefa Biochemie, Haarlem, the Netherlands) similar to that described by Kaundun et al. (Reference Kaundun, Hutchings, Dale, Baily and Glanfield2011). Agar was prepared by dissolving 5.5 g agar powder in 1 L distilled water. Murashige-skoog basal medium with vitamins (10MS) (PhytoTechLab, Shawnee Mission, KS) was added to this solution at a rate of 4.43 g L−1, and pH was adjusted to 6.5 by adding sodium hydroxide solutions prepared at 0.1, 1, or 10 N. This amended solution was sterilized at 121 C and 103 kPa for 20 minutes using a tabletop autoclave (SterilElite, Fisher Scientific, Waltham, MA). To limit fungal and bacterial growth, 7 mL of a premixed rifampicin–dimethyl sulfoxide solution (1000 ppm) with 32 μL azoxystrobin (Heritage TL, Syngenta Professional Products, Greensboro, NC) was added to the autoclaved agar medium using a syringe equipped with a sterilization filter tip. Herbicides used to determine the suitability of this agar assay to detect annual bluegrass resistance to ALS, EPSPS, and PSII inhibitors were trifloxysulfuron (Monument, Syngenta Professional Products), glyphosate (Touchdown Hi-Tech, Syngenta Professional Products), and simazine (Princep, Syngenta Professional Products), respectively. Similar to the study described by Kaundun et al. (Reference Kaundun, Hutchings, Dale, Baily and Glanfield2011), eight concentrations of each herbicide were evaluated: trifloxysulfuron (0, 6.25, 12.5, 25, 50, 75, 100, and 150 μM), glyphosate (0, 6, 12, 25, 50, 100, 200, and 400 μM), and simazine (0, 6, 12, 25, 50, 100, 200, and 400 μM). Every herbicide concentration was replicated 50 times in each experiment.
Each polycarbonate plant culture box received 65 mL of plant tissue culture agar with 10MS solution, and was amended with one of the eight herbicide concentrations. The solution in each plant culture box was allowed to cool and solidify into a gel under ambient conditions in a laboratory. Mature annual bluegrass plants (>5 tillers) cultured in the growth chamber were washed free of growing medium using distilled water, and were dissected into single tillers with <7 cm of aboveground tissue and 5 cm of root tissue. These cleaned tillers were then added to the gel inside each plant culture box using a scalpel and forceps that had been sterilized by autoclaving as previously described (Figure 1). Care was taken to ensure that all root tissue and the apical meristem of each plant were placed inside the gel while simultaneously minimizing leaf tissue contact with the sides of the gel or the plant culture box itself. Phenotypes included in experiments were as follows: ALS (R3, R7, S), EPSPS (R5, S), and PSII (R3, R4, S). In each experiment, all phenotypes were transplanted into each plant culture box. After transplanting, all plant culture boxes were placed back inside the growth chamber. Survival of each phenotype was assessed seven days after transplanting during our ALS experiments, and 10 days after transplanting in our studies with EPSPS and PSII inhibitors. Survival was determined by lack of discoloration or necrosis on new tissue growth from the apical meristem of each phenotype.

Figure 1 Individual annual bluegrass (Poa annua L.) tiller after being inserted into agar gel inside a polycarbonate plant culture box.
Whole Plant Comparisons
Suitability of the agar-based assay for detecting target-site resistance to ALS-, EPSPS-, and PSII-inhibiting herbicides was determined by comparing susceptibility in agar to that observed after treating whole plants (R3, R4, R5, R7, and S) with traditional spray applications of each herbicide. In order to do so, greenhouse propagation trays containing 98 cells (21 cm3) were filled with Sequatchie silt loam soil (fine-loamy, siliceous, semiactive, thermic humic Hapludult) that measured 6.2 in soil pH and 2.1% in organic matter content. These trays were surface-seeded with herbicide-resistant and herbicide-susceptible phenotypes of annual bluegrass. Trays were stored inside a previously described growth chamber where they were subirrigated and supplied with complete fertilizer at a nitrogen rate of 49 kg ha−1 wk−1.
Herbicides were applied to these trays using an enclosed spray chamber (Generation III track sprayer, DeVries Manufacturing, Hollandale, MN) at 215 L ha−1 via an 8004 EVS nozzle (TeeJet, Wheaton, IL). Herbicides were mixed with nonionic surfactant (Activator 90, Loveland Products, Greeley, CO) at 0.25% (v/v) and applied with a water carrier at recommended label rates as follows: trifloxysulfuron, 28 g ha−1; glyphosate, 1,120 g ha−1; and simazine, 1,120 g ha−1. Plants treated with trifloxysulfuron (R3, R7, and S) and glyphosate (R5 and S) were at a minimum of a one-tiller state at application. Because simazine is labeled for PRE and early POST control of annual bluegrass (Anonymous 2014), plants treated with simazine in our experiments (R3, R4, and S) had formed true leaves but had not yet begun to tiller. As in the agar assay, survival of each phenotype was determined by the absence of any discoloration or necrosis at 35 days after application.
Statistical Analysis
Data were analyzed by herbicide target; experiments using ALS-, EPSPS-, and PSII-inhibiting herbicides were analyzed separately. Each 50-replication experiment in agar and whole plant comparison was repeated in time under controlled conditions during 2016. Fisher’s exact test (α = 0.05) was used to determine herbicide concentrations in agar that yielded responses that were not significantly different from those generated after treating whole plants via traditional spray application. This test was performed using the fisher.test function in R (version 3.2.3. Vienna, Austria). Results of Fisher’s exact test from each experimental run are presented to show the lowest herbicide concentration in agar that would consistently yield responses not different from a traditional spray application in that run. Percent survival data for each phenotype in the second experimental run are presented in tables to provide an indication of plant sensitivity to each herbicide under our experimental conditions. All data and analysis files from this research are publically available at https://doi.org/10.6084/m9.figshare.4551520.
Results and Discussion
Detecting ALS Resistance
Whole plant responses to trifloxysulfuron were as expected, with 92% to 100% of the resistant phenotypes surviving treatment at 28 g ha−1, compared to only 4% for the susceptible phenotype (Table 1). Survival for all phenotypes was 100% when established in gel devoid of trifloxysulfuron; however, when trifloxysulfuron concentration increased to 6.25 μM, only 2% of susceptible plants survived treatment compared to 94% to 96% for plants of phenotypes resistant to trifloxysulfuron via target-site mutation. Seven days after exposure, survival of the three annual bluegrass phenotypes at the 12.5 μM concentration of trifloxysulfuron in agar medium was not significantly different than that 35 days after whole plant treatment at 28 g ha−1. This was the lowest concentration of trifloxysulfuron in agar that resulted in survival similar to whole plant treatment in both runs of our ALS experiments. A similar statistical relationship was detected in survival data from whole plant application and exposure to 25 to 50 μM trifloxysulfuron in agar; however, survival percentages in agar for were much lower than those observed for whole plant treatment, suggesting that these increased concentrations may be too high to consistently detect differences among certain annual bluegrass phenotypes resistant and susceptible to ALS-inhibiting herbicides such as trifloxysulfuron.
Table 1 Percent survival of herbicide-resistant (R3, R7) and herbicide-susceptible (S) annual bluegrass phenotypes surviving exposure to trifloxysulfuron in agar culture or whole plant treatment. Survival percentages from the second experimental run are presented below, along with Fisher’s exact test results from both runs.

a R3 and R7 were acetolactate synthase (ALS)-resistant annual bluegrass phenotypes collected from different golf course locations in Memphis, TN. Resistance to ALS-inhibiting herbicides in each was due in part to target-site mutation. S was an herbicide-susceptible phenotype from University Park, PA, that had no history of exposure to ALS-inhibiting herbicides.
b Plants with a minimum of one tiller were treated with trifloxysulfuron using 215 L ha−1 carrier via an 8004 EVS nozzle inside an enclosed spray chamber. Spray solution contained nonionic surfactant at 0.25% (v/v).
c Exposure to trifloxysulfuron in polycarbonate plant culture boxes filled with agar amended with different concentrations of trifloxysulfuron. Each phenotype was replicated 50 times at each herbicide concentration and repeated in time.
d Fisher’s exact test was used to determine herbicide concentrations in agar that yielded responses that were not significantly different from those generated after treating whole plants via traditional spray application. ***, **, *, and NS represent P≤0.0001, P≤0.001, P≤0.05, and nonsignificant, respectively.
Detecting EPSPS Resistance
Whole plant responses to glyphosate were as expected, with 94% of resistant plants surviving treatment at 1,120 g ha−1 compared to only 6% of plants susceptible to glyphosate (Table 2). As was true in our ALS experiments, nearly all plants survived in agar medium devoid of glyphosate, indicating that our testing conditions provided a growing environment suitable for annual bluegrass. Survival of the resistant and susceptible phenotypes in glyphosate-amended agar gel did not match whole plant results at concentrations of 6 to 50 μM in both experimental runs, likely due to high percentages of the S phenotype surviving exposure (Table 2). When glyphosate concentration was increased to 100 μM, survival data from whole plant and agar testing were not significantly different from one another. A total of 100% and 8% of the resistant and susceptible phenotypes, respectively, survived 10 days of exposure to 100 μM glyphosate in agar, compared to 94% and 6% survival 35 days after whole plant treatment at 1,120 g ha−1. Similar to what was observed with our ALS assays, increasing glyphosate concentration in agar media above 100 μM was not beneficial in detecting differences among resistant and susceptible phenotypes of annual bluegrass.
Table 2 Percentage of herbicide-resistant (R5) and herbicide-susceptible (S) annual bluegrass plants surviving exposure to glyphosate in agar culture or whole plant treatment. Survival percentages from the second experimental run are presented below, along with Fisher’s exact test results from both runs.

a R5 was a glyphosate-resistant annual bluegrass phenotype collected from a golf course in Bean Station, TN. Resistance in R5 was due in part to target-site mutation. S was an herbicide-susceptible phenotype from University Park, PA, that had no history of exposure to glyphosate.
b Plants with a minimum of one tiller were treated with glyphosate using 215 L ha−1 carrier via an 8004 EVS nozzle inside an enclosed spray chamber. Spray solution contained nonionic surfactant at 0.25% (v/v).
c Exposure to glyphosate in polycarbonate plant culture boxes filled with agar amended with different concentrations of glyphosate. Each phenotype was tested 50 times at each herbicide concentration in each of two experiments, for a total of 100 replications each.
d Fisher’s exact test was used to determine herbicide concentrations in agar that yielded responses that were not significantly different from those generated after treating whole plants via traditional spray application. ***, **, *, and NS represent P≤0.0001, P≤0.001, P≤0.05, and nonsignificant, respectively.
Detecting PSII Resistance
Similar to our ALS and EPSPS experiments, whole plant responses to simazine were as expected, with 100% of resistant plants surviving treatment at 1,120 g ha−1, compared to only 2% of plants susceptible to PSII-inhibiting herbicides (Table 3). Nearly all resistant and susceptible plants (96% to 100%) survived in agar medium devoid of simazine, indicating that the growing environment was suitable for annual bluegrass. However, survival of plants in agar gel amended with simazine at 6 to 400 μM did not match whole plant responses; significant differences were detected for each concentration in both experimental runs. Reasons for this response are not clear, but could be related to the maturity of plants used in our agar-based assay. Simazine is typically applied prior to or shortly after annual bluegrass seedling emergence in autumn (Anonymous 2014). Use of mature, tillered plants in our agar assay may have limited simazine activity, even on phenotypes known to be susceptible to PSII-inhibiting herbicides. This could explain why survival of the susceptible phenotype was ≥92% at all herbicide concentrations. Increasing the concentration of simazine in agar may improve efficacy of this assay for diagnosing PSII resistance in annual bluegrass. Additionally, an alternative PSII inhibiting herbicide with efficacy for annual bluegrass control, such as amicarbazone, may be better suited for this diagnostic assay than simazine. Amicarbazone, a triazolinone herbicide, is labeled for POST control of mature annual bluegrass and has been found to act at the same target site as do triazine herbicides (Anonymous 2016; Dayan et al. Reference Dayan, Trindade and Velini2009). Moreover, cross-resistance to both simazine and amicarbazone has been reported in annual bluegrass (Perry et al. Reference Perry, McElroy, Dane, van Santen and Walker2012).
Table 3 Percentage of herbicide-resistant (R3, R4) and herbicide-susceptible (S) annual bluegrass plants surviving exposure to simazine in agar culture or whole plant treatment. Survival percentages from the second experimental run are presented below, along with Fisher’s exact test results from both runs.
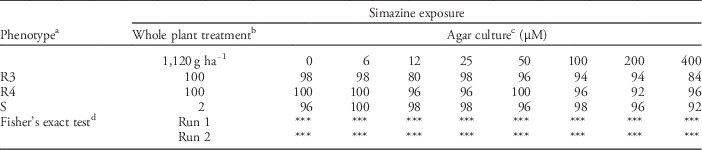
a R3 was a simazine-resistant annual bluegrass phenotype collected from a golf course in Memphis, TN. R4 was a simazine-resistant phenotype collected from a sod farm in Arlington, TN. Resistance in each phenotype was due in part to target-site mutation. S was an herbicide-susceptible phenotype from University Park, PA, that had no history of exposure to simazine.
b Non-tillered plants with a minimum of two leaves were treated with simazine using 215 L ha−1 carrier via an 8004 EVS nozzle inside an enclosed spray chamber. Spray solution contained nonionic surfactant at 0.25% (v/v).
c Exposure to simazine in polycarbonate plant culture boxes filled with agar amended with different concentrations of simazine. Each phenotype was tested 50 times at each herbicide concentration in each of two experiments, for a total of 100 replications each.
d Fisher’s exact test was used to determine herbicide concentrations in agar that yielded responses that were not significantly different from those generated after treating whole plants via traditional spray application. ***, **, *, NS represent P ≤ 0.0001, 0.001, 0.05 or non-significant.
Our findings indicate that an agar-based diagnostic assay conducted with individual annual bluegrass tillers can be used to consistently detect resistance to ALS- or EPSPS-inhibiting herbicides in less than 10 days. Discriminatory concentrations to screen future annual bluegrass samples for resistance to trifloxysulfuron and glyphosate using the assay methods described herein should be 12.5 and 100 μM, respectively. These concentrations are greater than those identified by Kaundun et al. (Reference Kaundun, Hutchings, Dale, Baily and Glanfield2011, Reference Kaundun, Hutchings, Harris, Jackson, Shashi-Kiran, Dale and McIndoe2014) using a similar agar-based assay to detect for resistance in Lolium. The researchers reported discriminatory concentrations of 0.05 and 50 μM for mesosulfuron and glyphosate, respectively. This difference may be related to weed species, herbicide (in the case of the ALS assay), or minor differences in experimental methodology. The RISQ test outlined by Kaundun et al. (Reference Kaundun, Hutchings, Dale, Baily and Glanfield2011) used seedling weeds (one- to three-leaf) and involved placing both root and shoot tissue in contact with gel in a petri dish. In our experiments, we cloned individual tillered plants and placed only root and apical meristem tissue in contact with gel inside a polycarbonate plant culture box. Rationale for this decision was that turfgrass managers were more likely to submit a tillered annual bluegrass plant for testing than a seedling plant with less than three leaves. A diagnostic assay using tillered plants would remove the need for diagnosticians to collect and germinate seed to produce seedling plants for use in a standard RISQ test.
Although the diagnostic assay outlined herein can be used to detect annual bluegrass resistance to ALS- and EPSPS-inhibiting herbicides, future research is needed to expand the test for use with other modes of action used to control annual bluegrass, particularly microtubule-disrupting herbicides such as pronamide and cellulose biosynthesis–inhibiting herbicides such as indaziflam. Moreover, future research exploring the efficacy of this testing system for detecting herbicide resistance in other turfgrass weeds, particularly goosegrass [Eleusine indica (L.) Gaertn.), is well warranted.
Acknowledgements
The authors would like to thank Tyler Campbell, Daniel Farnsworth, Trevor Hill, Cory Wright, Mitchell Riffey, and Dallas Taylor for their efforts in this research. This work was supported by the Tennessee Agricultural Experiment Station and the United States Golf Association. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the University of Tennessee Institute of Agriculture.






