Common waterhemp is a small-seeded, summer annual, dioecious, broadleaf weed in the Amaranthus genus and is relatively new to Ontario. Common waterhemp is thought to have been introduced into Ontario via a demonstration combine from Illinois in the early 2000s (Costea et al. Reference Costea, Weaver and Tardif2005). Research conducted on glyphosate-susceptible (GS) populations found that common waterhemp interference in soybean can reduce yield up to 73% (Vyn et al. Reference Vyn, Swanton, Weaver and Sikkema2007). Waterhemp has become a more problematic weed in Ontario following the confirmation of its resistance to glyphosate in 2014 (Heap Reference Heap2017; Schryver et al. Reference Schryver, Soltani, Hooker, Tranel, Robinson and Sikkema2017). The mechanism of glyphosate resistance in the first glyphosate-resistant (GR) common waterhemp population in Ontario is due at least in part to gene amplification, with 6 to 13 copies of the EPSPS gene (PJT, unpublished data). GR common waterhemp has now been confirmed in 40 fields across 3 Ontario counties, including Lambton, Chatham-Kent, and Essex (Schryver et al. Reference Schryver, Soltani, Hooker, Tranel, Robinson and Sikkema2017). In addition, there is resistance to herbicide Groups 2, 5, and 9 in the same field (Schryver et al. Reference Schryver, Soltani, Hooker, Tranel, Robinson and Sikkema2017). This new multiply resistant common waterhemp population presents a significant weed management challenge for Ontario soybean growers.
Waterhemp can be distinguished from other pigweed species by its narrow, lance-shaped leaves, hairless leaves and stems, and potential for growth of up to 3 m in height (Costea et al. Reference Costea, Weaver and Tardif2005; Ontario Ministry of Agriculture and Food [OMAF] 2004). This dioecious species has high genetic diversity due to exchange of genetic traits between separate male and female plants (Costea el al. Reference Costea, Weaver and Tardif2005). This genetic diversity results in high levels of variability in plant morphology, including prostrate to erect growth habit (Costea et al. Reference Costea, Weaver and Tardif2005) and green to red/purple coloration (OMAF 2004). Although closely related to other pigweed species commonly found in Ontario, common waterhemp can be distinguished in the vegetative stage by its entirely hairless stems and leaves and lance-shaped leaves and in the reproductive stage by separate male and female plants.
Because common waterhemp is dioecious, it has the ability to acquire new traits that may confer herbicide resistance more rapidly than in monoecious species (Costea et al. Reference Costea, Weaver and Tardif2005; Wu and Owen Reference Wu and Owen2014). Waterhemp has high fecundity, with one female plant producing up to 4.8 million seeds in a noncompetitive environment (Hartzler et al. Reference Hartzler, Battles and Nordby2004). The seed can remain viable in the soil for up to 14 yr (Burnside et al. Reference Burnside, Wilson, Weisberg and Hubbard1996). Waterhemp has an extended emergence pattern in Ontario, beginning in early spring and continuing through September (Vyn et al. Reference Vyn, Swanton, Weaver and Sikkema2007). Due to a number of biologically advantageous traits, including dioecious reproduction, prolific seed production, and a prolonged emergence pattern, the evolution of herbicide resistance in common waterhemp can occur rapidly.
Glyphosate is a systemic, broad-spectrum, herbicide with a unique mode of action and is widely used globally. Glyphosate is absorbed through the cuticle into the living portion of the plant and moves via the phloem with sugars to the meristematic regions, where it disrupts the shikimate biosynthetic pathway (Duke and Powles Reference Duke and Powles2008; Franz et al. Reference Franz, Mao and Sikorski1997). Glyphosate binds to enolpyruvylshikimate-3-phosphate synthase (EPSPS), displacing phosphoenol pyruvate. This replacement halts the production of 5-enolpyruvylshikamate-3-phosphate, a key precursor in the production of three aromatic amino acids: tyrosine, phenylalanine, and tryptophan (Sikorski and Gruys Reference Sikorski and Gruys1997). Due to its unique mode of action and high efficacy, glyphosate is used extensively around the world.
The widespread and repeated use of glyphosate has resulted in the evolution of GR weed populations (Powles Reference Powles2008; Shaner Reference Shaner2009). The increase in glyphosate use is correlated with the introduction of GR crops in Ontario in 1997. In soybean, Young (Reference Young2006) reported that before the introduction of GR soybean, less than 3 million kg year−1 of glyphosate was used annually; this had increased to 30 million kg year−1 by 2002 in the United States. The first confirmed GR weed was rigid ryegrass (Lolium rigidum Gaudin) in Australia in 1996; now there are 37 GR weed species (Heap Reference Heap2017). The overreliance on glyphosate has led to the rapid evolution of GR weeds.
Although new to Ontario, GR common waterhemp was first reported in Missouri in 2005, and has since been reported in 18 U.S. states as of 2016 (Heap Reference Heap2017; Legleiter and Bradley Reference Legleiter and Bradley2008). There are three known mechanisms that confer resistance to glyphosate in GR common waterhemp. The first is gene amplification, first found in closely related Palmer amaranth (Amaranthus palmeri S. Wats.) (Gaines et al. Reference Gaines, Zhang, Wang, Bukun, Chisholm, Shaner, Nissen, Patzoldt, Tranel, Culpepper, Grey, Webster, Vencill, Sammons, Jiang, Preston, Leach and Westra2010). A recent multistate study found that EPSPS gene copy number was correlated with the level of glyphosate resistance, which is consistent with previous findings of a positive relationship between gene copy number and resistance factor (Chatham et al. Reference Chatham, Bradley, Kruger, Martin, Owen, Peterson, Mithila and Tranel2015a; Shaner et al. Reference Shaner, Lindenmeyer and Ostlie2012; Tranel et al. Reference Tranel, Riggins, Bell and Hager2010). This resistance mechanism was found in 91% of 80 common waterhemp populations examined (Chatham et al. Reference Chatham, Wu, Riggins, Hager, Young, Roskamp and Tranel2015b). A second mechanism of glyphosate resistance in common waterhemp is due to an altered target site with a serine to proline substitution at position 106 of the EPSPS enzyme (Pro-106-Ser) (Bell et al. Reference Bell, Hager and Tranel2013). The third mechanism of glyphosate resistance, found in a common waterhemp population in Mississippi, is reduced translocation in conjunction with an altered target site, which conferred a 5-fold level of resistance (Nandula et al. Reference Nandula, Ray, Ribeiro, Pan and Reddy2013). There was a 10% reduction in glyphosate translocated out of the treated leaf in GR compared with GS populations (Nandula et al. Reference Nandula, Ray, Ribeiro, Pan and Reddy2013).
A wide range in the resistance factor to glyphosate exists for common waterhemp. In 2008, a common waterhemp population in Texas, exposed to three glyphosate applications per year in a continuous cotton production field, was found to have a resistance factor of 4 to 60 (Light et al. Reference Light, Mohammed, Dotray, Chandler and Wright2011). Similarly, in 2012, following 8 yr of GR corn and soybean production, a common waterhemp population in Nebraska was found to have a resistance factor of 3 to 39 (Sarangi et al. Reference Sarangi, Sandell, Knezevic, Aulakh, Lindquist, Irmak and Jhala2015a). This vast range in the resistance factor to glyphosate in common waterhemp demonstrates the variability within this species.
Heap (Reference Heap2017) now reports resistance in common waterhemp to six herbicide groups: 2, 4, 5, 9, 14, and 27, making it increasingly difficult to manage, as fewer herbicide options remain to control this weed. Multiple resistance is becoming increasingly common, with a recent discovery of a common waterhemp population in Illinois with resistance to five modes of action, including Groups 2, 4, 5, 14, and 27 (Heap Reference Heap2017). Research on control of common waterhemp in soybean in Canada is limited to GS populations. Vyn et al. (Reference Vyn, Swanton, Weaver and Sikkema2007) reported that acifluorfen or fomesafen (Group 14 herbicides) applied POST provided greater than 80% control.
A number of studies found that the use of a PRE herbicide is an important component of a successful control strategy for GR common waterhemp in soybean. Although the Group 15 herbicides (pyroxasulfone, dimethenamid-P, and S-metolachlor) provide excellent control in some studies, control has been variable. Legleiter et al. (Reference Legleiter, Bradley and Massey2009) found that S-metolachlor/metribuzin applied PRE compared with glyphosate applied POST for the control of GR common waterhemp resulted in greater net returns. In that research, densities of GR common waterhemp were reduced from up to 70 plants m−2 with glyphosate applied POST to less than 5 plants m−2 when a PRE herbicide was applied. Similarly, research conducted in Ontario reported that S-metolachlor/metribuzin applied PRE controlled GS common waterhemp by 94% (Vyn et al. Reference Vyn, Swanton, Weaver and Sikkema2007). Sarangi et al. (Reference Sarangi, Sandell, Knezevic, Irmak and Jhala2015b) reported higher soybean yields with a full rate of a Group 15 applied PRE compared with a sequential application, with yields of 2,100 and 1,800 kg ha−1, respectively. In contrast, Behnken et al. (Reference Behnken, Breitenbach, Gunsolus and Bongard2015) reported that layered applications of a PRE herbicide controlled GR common waterhemp 90% to 95% compared with 62% to 81% with a single PRE application, resulting in an increase in soybean yield of 0.40-0.94 T ha−1. Finally, acetochlor, an additional Group 15 herbicide, provided GR common waterhemp control equivalent to the weed-free control, but this herbicide is not available in Ontario (Jhala et al. Reference Jhala, Malik and Willis2015).
The objectives of this research were: 1) to determine the glyphosate resistance factor of GR common waterhemp populations in Ontario in controlled and field environments, 2) to ascertain the efficacy of soil-applied residual herbicides for the control of GR common waterhemp, and 3) to evaluate two-pass weed control programs for the control of GR common waterhemp in soybean. This is the first research on the control of GR common waterhemp in soybean in Canada.
Materials and Methods
Biologically Effective Rate (BER)
These experiments were conducted in both the greenhouse and the field.
For the BER greenhouse study, seeds from two populations of common waterhemp representing a GS population (collected near Petrolia, ON) and a GR population (collected near Walpole, ON) were stratified by refrigeration at 4 C for 8 wk. The GS population was from a known GS site (Vyn et al. Reference Vyn, Swanton, Weaver and Sikkema2007). The GR seed was collected in 2014 from a grower’s field with the first confirmed case of GR common waterhemp in Ontario (Schryver et al. Reference Schryver, Soltani, Hooker, Tranel, Robinson and Sikkema2017). Waterhemp seeds were germinated in trays filled with soilless mixture (Pro-Mix® PGX, Premier Tech Horticulture, Québec, Canada). When the seedlings were at the cotyledon to first true-leaf stage, they were transplanted into individual pots (10-cm diameter) containing the same soilless mixture. When the common waterhemp seedlings were 10 cm in height, glyphosate was applied at 14, 28, 56, 113, 225, 450, 900, 1,800, or 3,600 g ae ha−1 for the GS population, and 56, 113, 225, 450, 900, 1,800, 3,600, 7,200, or 14,400 g ae ha−1 for the GR population. Glyphosate was applied in a spray chamber with a flat-fan nozzle calibrated to apply 200 L ha−1 at 280 kPa while moving at 2.15 km h−1. Plants were then arranged in a randomized complete block design with 10 replicates. An untreated control was included in each replicate. Common waterhemp control was estimated visually at 1, 2, 3, and 4 wk after application (WAA) on a scale of 0% to 100%. A rating of 0% represented a healthy plant, and 100% represented complete mortality. Dry weight was determined at 4 WAA by cutting any remaining plant material at the soil line, placing it in a paper bag, and drying it in a kiln at 60 C to a constant moisture. The experiment was repeated three times.
For the BER field study, eight field experiments (four with GS and four with GR common waterhemp) were conducted over a 2-yr period (2015 and 2016) in commercial soybean fields with known GS or GR common waterhemp. For the GR common waterhemp studies, DeKalb® ‘30-61RY’ soybean was seeded in rows spaced 75-cm apart at approximately 400,000 seeds ha−1 at a depth of 3.75 cm. Field location and year, soil characteristics, seeding date, herbicide application date, and common waterhemp size and density at application are presented in Table 1. The GS population research was set up in established soybean fields. The experiments followed a randomized completed block design with four replications. Each replicate included a weedy and a weed-free control. The weed-free plots were maintained weed-free primarily with S-metolachlor/metribuzin (1,943 g ai ha−1) applied PRE, but in one instance, due to crop emergence, glyphosate (900 g ae ha−1) plus imazethapyr (75 ai ha−1) was applied POST. Following the initial herbicide application, subsequent hand hoeing was done as required. Plots were 2.25-m wide (three soybean rows spaced 75-cm apart) by 8-m long, and glyphosate was applied at 14, 28, 56, 113, 225, 450, 900, 1,800, 2,700, or 5,400 g ae ha−1 for the GS populations and 113, 225, 450, 900, 1,800, 2,700, 5,400, 10,800, 21,600, or 43,200 g ae ha−1 for the GR populations. Glyphosate was applied when the majority of common waterhemp reached 10 cm in height using a CO2-pressurized backpack sprayer and 1.5-m handheld boom with four ULD 120-02 nozzles (Hypro, New Brighton, MN) spaced 50-cm apart and calibrated to deliver 200 L ha−1 at 280 kPa. Common waterhemp control was estimated visually at 1, 2, 4, 8, and 12 WAA on a scale of 0% to 100%. Zero percent indicated no common waterhemp control, and 100% was complete common waterhemp death. Density and dry weight were determined at 4 WAA. Waterhemp plants were counted in two 0.5-m2 quadrats, cut at the soil surface, placed in paper bags, and dried in a kiln at 60 C to a constant moisture, and the dry weight was recorded. Soybean yield from the weedy and weed-free controls was determined at maturity by hand cutting the soybean from two 1-m sections of a row taken from the center row of each plot and threshed in a stationary thresher. Soybean yield was adjusted to 13% moisture.
Table 1 Field site and herbicide application information for biologically effective rate and one- and two-pass common waterhemp control studies conducted on glyphosate-susceptible (GS) and -resistant (GR) common waterhemp in 2015 and 2016 in Ontario.Footnote a
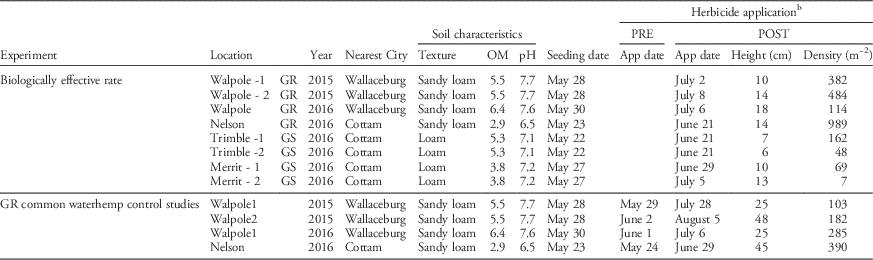
a Abbreviations: App, application; OM, percent organic matter.
b Size and density assessments were conducted in the untreated check plots.
GR Common Waterhemp Control in PRE and PRE followed by POST Programs
Eight field experiments were conducted over a 2-yr period (2015 and 2016) to evaluate 14 herbicides applied PRE for the control of GR common waterhemp and to evaluate a PRE followed by POST program.
Four field experiments were completed to address each of the above objectives, with two studies at Walpole Island in 2015 (separated in time) and one at Walpole Island and one near Cottam, ON, in 2016. These experiments included a PRE experiment and a PRE followed by POST experiment conducted in four different environments.
Glyphosate (1,800 g ae ha−1) was applied before seedbed preparation to remove the confounding effect of other weed species. Field location and year, soil characteristics, seeding date, herbicide application date, and common waterhemp size and density at application are presented in Table 1. DeKalb® 30-61RY soybean was seeded in rows spaced 75-cm apart at approximately 400,000 seeds ha−1 at a depth of 3.75 cm. All field experiments were established as a randomized complete block design with four replicates. A weedy and weed-free control was included in each replicate. The weed-free check was maintained weed-free with S-metolachor/metribuzin (1,943 g ai ha−1) applied PRE followed by hand hoeing as required. The plots were 2.25 m in width (three soybean rows spaced 75-cm apart) and 8 m in length. Herbicides were applied with a CO2-pressurized backpack sprayer and a 1.5-m handheld boom with four ULD 120-02 nozzles (Hypro) spaced 50-cm apart and calibrated to deliver 200 L ha−1 at 280 kPa. PRE herbicides were applied within 5 d after seeding (Table 1) and POST applications were applied when common waterhemp escapes reached approximately 10 cm in height in the PRE treatments.
The PRE herbicides were applied at the highest labeled rate in the province of Ontario. Herbicides used in the PRE followed by POST weed control study included pyroxasulfone/sulfentrazone (300 g ai ha−1) or S-metolachlor/metribuzin (1,943 g ai ha−1) applied PRE and glyphosate (900 g ae ha−1), glyphosate (900 g ae ha−1) plus acifluorfen (600 g ai−1 ha), or glyphosate (900 g ae ha−1) plus fomesafen (240 g ai ha−1)+Turbocharge® 0.5% v/v applied POST. All herbicides were evaluated alone and in a combination of a PRE followed by a POST herbicide.
Waterhemp control was assessed visually at 1, 2, 4, 8, and 12 WAA after the POST application on a scale of 0% to 100%, with a rating of 0% indicating no control and 100% indicating complete common waterhemp control. Waterhemp density and dry weight were determined 4 WAA from a 0.5 m−2 subsample of each plot. Waterhemp was counted, cut at the soil surface, placed in paper bags, and dried in a kiln at 60 C, and the dry weight was recorded. Soybean seed yield and moisture was recorded from each plot at crop maturity, and yield was adjusted to 13% moisture.
Statistical Analysis
For BER studies (greenhouse and field), statistical analysis was conducted in SAS v. 9.4 PROC NLIN (SAS Institute 2012). For the greenhouse study, regression analysis was completed using an ascending dose–response curve (Equation 1) for all control assessments, density, and dry weight; this model provided the best fit (Bowley Reference Bowley2008):
where variable Y is the percent control of common waterhemp, C is the lower asymptote, D is the upper asymptote, b is the slope of the line (negative for all control ratings, positive for density and dry weight) and I50 is the rate to achieve 50% response between the upper and lower asymptotes (Bowley Reference Bowley2008). For the field study, the common waterhemp density and percent dry weight predicted values were modeled using an inverse exponential equation (Equation 2):
where the variable Y is the percent control of common waterhemp, a represents the lower asymptote, b the reduction in Y from the initial value to a, and c denotes the slope from the initial point to a.
Using the predicted values from each respective variable equation, ED50, ED80, and ED95 values (representing effective dose required to achieve 50%, 80%, and 95% control, respectively) were determined.
For the common waterhemp control study, data analysis was completed using the PROC MIXED procedure in SAS. The variance was partitioned into fixed effects of treatment and random effects of block, environment, and environment by treatment interaction. To confirm that the assumptions of variance analysis were met, including errors being independent, homogeneous, and normally distributed, residuals were plotted by predicted, treatment, and block for each variable. Normality was tested by Shapiro-Wilk test (Shapiro and Wilk Reference Shapiro and Wilk1965) in PROC UNIVARIATE in SAS. Data transformations included an arcsine square-root transformation, square-root transformation, and log transformation to meet the above assumptions.
For statistical analysis for the PRE herbicide study, transformed data underwent a Tukey’s test, and the pdmix800 macro was used to separate the means (Saxton Reference Saxton1998). Least-squares means comparisons were performed with a type I error rate set at P=0.05. Treatment means from transformed data were back-transformed for presentation purposes; the standard error was calculated from untransformed data.
In the PRE followed by POST weed control study, nonorthogonal contrasts using PROC MIXED COVTEST for the two PRE herbicides, the three POST herbicides, and the herbicide timings of PRE vs. POST, PRE vs. PRE followed by POST, and POST vs. PRE followed by POST were completed. Tukey’s test was used with significance level of P=0.05.
Results and Discussion
Biologically Effective Rate of Glyphosate
In greenhouse studies, based on the ED50 at 4 WAA, the resistance factor was 4.5 when comparing the amount of glyphosate required to achieve 50% control of common waterhemp in the GR and GS populations (Table 2). Interestingly, the resistance factor was similar for the ED80, ED90, and ED95, reflecting a consistent response across populations over time when compared with the GS populations. Based on the ED80, ED90, and ED95 at 4 WAA, the resistance factor was 4.6, 4.7, and 4.9, respectively (Table 2). This study suggests that any of these four parameters can be used to determine the resistance factor for the tested populations.
Table 2 Rate response of glyphosate on the control of resistant and susceptible common waterhemp populations 1, 2, 3, and 4 wk after application (WAA) and the dry weight at 4 WAA in greenhouse experiments conducted in 2015.Footnote a
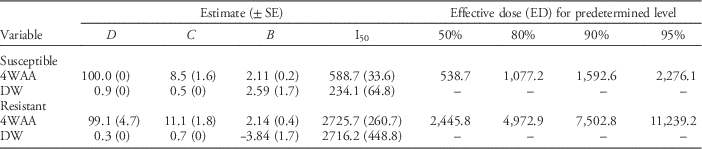
a Average of three experimental runs of each population. D is the upper limit and C is the lower limit. B is the slope of the line. I 50 is the rate to achieve 50% response between the upper and lower asymptotes.
In field study, at 1 WAA, there was little response, because glyphosate is a slow-acting herbicide, while beyond 4 WAA, new common waterhemp plants were emerging, because glyphosate does not provide residual control, which confounded the results in the field studies. Thus, the ratings completed at 2 and 4 WAA are presented and compared with the GS population (Table 3). Based on the ED50 at 2 and 4 WAA, the resistance factors were 14.2 and 27.9, respectively, which were much higher than in the greenhouse study. Based on the ED50, the resistance factors based on common waterhemp density and dry weight were 2.6 and 8.6, respectively; while based on the ED80, the resistance factors based on common waterhemp density and dry weight were 3.9 and 10.7, respectively (Table 3). In the field studies, GR common waterhemp interference reduced soybean yield 50% (unpublished data). In the study in which common waterhemp density was the highest, soybean yield was reduced 98% due to GR common waterhemp interference, with the highest yield of 3.50 T ha−1 found in the weed-free check and the lowest of 0.06 T ha−1 found in the nontreated control (unpublished data).
Table 3 Rate response of glyphosate on the control of resistant and susceptible common waterhemp populations at 2 and 4 wk after application (WAA) and the percent dry weight at 4 WAA in field experiments conducted in 2015 and 2016.Footnote a
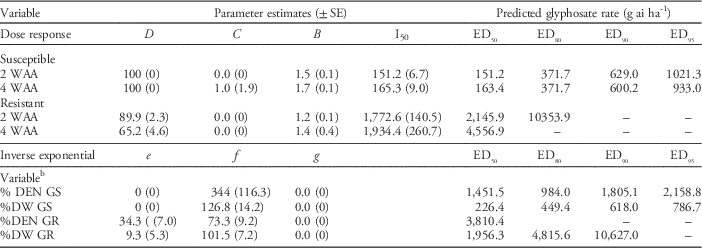
a Average of four experimental runs for each population. Dose–response parameters (Equation 1): B, slope; C, lower asymptote; D, upper asymptote; I50, rate required for 50% response.
Inverse exponential (Equation 2): e, lower asymptote; f, magnitude of response; g, slope of response.
b Abbreviations: DEN, percent density of nontreated control; DW, percent dry weight of nontreated control; GS, glyphosate susceptible, GR, glyphosate resistant.
Although there were similarities in common waterhemp response to glyphosate in the greenhouse and field studies, there was more variability in the field study. In the field, common waterhemp continued to emerge following the application of glyphosate, because glyphosate does not provide residual weed control. The later-emerging common waterhemp made control assessments difficult, because it was difficult to know which plants were present at the time of glyphosate application. In addition, the greenhouse experiments were conducted on a single GR population from Walpole, discovered in 2014, and did not include GR common waterhemp from near Cottam, which was discovered in 2015 (Schryver et al. Reference Schryver, Soltani, Hooker, Tranel, Robinson and Sikkema2017), after the greenhouse experiments were completed. Due to the pooling of field BER results, there was more variability in the regression analysis, as populations exhibited slightly different responses to glyphosate. The GR common waterhemp population from near Cottam was more resistant to glyphosate than the Walpole population. In general, the greenhouse experiments provided a better determination of the resistance factor, because the results are not confounded by the late-emerging common waterhemp cohort and were taken from one population, resulting in more uniform results. Despite these differences, the objective of this research was to determine the resistance factor of GR common waterhemp in Ontario. Future research could explore the response of specific populations in a greenhouse setting to gain insight into the differing resistance factors of certain populations and how they relate to grower management practices.
GR Common Waterhemp Control in a PRE followed by POST Programs
In the PRE herbicide control study, common waterhemp had an extended emergence pattern. In these studies, common waterhemp began emerging shortly after seedbed preparation and continued to emerge until the last assessment late in October (Figure 1), as was also observed in studies by Vyn et al. (Reference Vyn, Swanton, Weaver and Sikkema2007) in Ontario. The most efficacious herbicides applied PRE were pyroxasulfone, flumioxazin+imazethapyr+metribuzin, metribuzin, S-metolachlor/metribuzin, pyroxasulfone/sulfentrazone, and pyroxasulfone/flumioxazin. Those herbicides provided between 87% and 97% control of GR common waterhemp at 8 WAA (Table 4). In general, these herbicides were the most efficacious at all assessment timings. Of the 14 herbicide treatments evaluated, only 2 provided greater than 90% full-season residual control of GR common waterhemp. The control of GR common waterhemp with most PRE herbicides did not provide acceptable season-long control. For instance, the control of GR common waterhemp with dimethenamid-P/saflufenacil, saflufenacil, imazethapyr, dimethenamid-P, and S-metolachlor decreased by 23% to 36% from 2 to 12 WAA. In contrast, there was no decrease in GR common waterhemp control from week 2 to 12 with pyroxasulfone/flumioxazin. At 12 WAA, some herbicides such as imazethapyr, pendimethalin, and saflufenacil provided equivalent control to the nontreated control with 4%, 11%, and 26% GR common waterhemp control respectively. The poor control with imazethapyr can be attributed to common waterhemp at all sites having Group 2 resistance (Schryver et al. Reference Schryver, Soltani, Hooker, Tranel, Robinson and Sikkema2017; PJT, unpublished data).
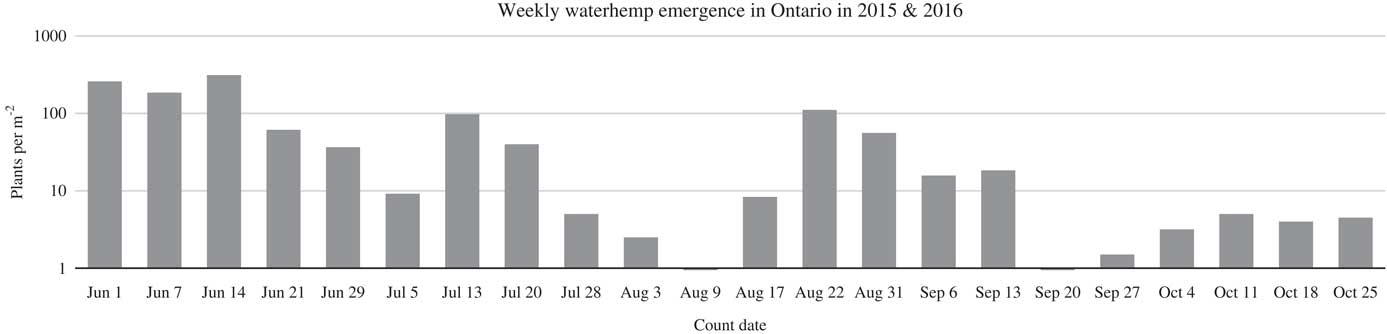
Figure 1 Weekly common waterhemp emergence counts conducted from June to late October in Ontario in 2015 and 2016 represented as average plants per m2.
Table 4 Means for percent common waterhemp control, density, biomass, and soybean yield with PRE herbicides at Walpole and Cottam, ON, in 2015 and 2016.Footnote a
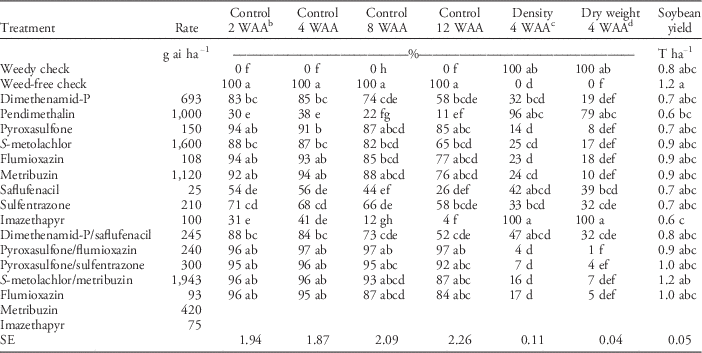
a Means followed by the same letter within a column are not significantly different according to Fisher’s protected LSD (P=0.05).
b Abbreviation: WAA, weeks after application.
c Percentage of untreated.
d Visual control estimates based on comparisons made to weedy and weed-free check.
In the two-pass weed control study, GR common waterhemp control was influenced by herbicide application timing and herbicide choice. Pyroxasulfone/sulfentrazone and S-metolachlor/metribuzin applied PRE provided equivalent control of GR common waterhemp with estimates of 79% and 67% control, respectively, at 12 WAA (Table 5). Fomesafen+glyphosate was more efficacious than acifluorfen+glyphosate for the control of GR common waterhemp, although differences were not always statistically significant. Across all control assessments, glyphosate with fomesafen provided 14% to 20% better control of GR common waterhemp than did acifluorfen. Both fomesafen and acifluorfen combined with glyphosate applied POST following a PRE herbicide provided better control of GR common waterhemp than glyphosate alone applied POST for all assessments, with the exception of common waterhemp density. Following the application of PRE herbicide, there was no difference in GR common waterhemp control between either fomesafen or acifluorfen when combined with glyphosate applied POST. The PRE herbicides provided better control of GR common waterhemp than the POST-only herbicides evaluated for all control estimates, with the exception of common waterhemp dry weight. At 4 and 12 WAA, herbicides applied PRE provided 45% and 53% better control of GR common waterhemp than herbicides applied POST, respectively. In general, control of GR common waterhemp was better when a POST herbicide followed a PRE herbicide in contrast to PRE only. At 4 and 12 WAA, the addition of a herbicide applied POST following a PRE herbicide improved GR common waterhemp control 17% and 21%, respectively, while the addition of a PRE herbicide to a POST herbicide improved control 62% and 74%, respectively. Soybean yield was greater in PRE followed by POST when compared with POST alone with 1.54 and 1.19 T ha−1, respectively (Table 5).
Table 5 Contrasts of herbicides and herbicide timing PRE, POST, and PRE followed by POST in the control of glyphosate-resistant (GR) common waterhemp in GR soybean summarizing four experiments in 2015 and 2016 in Ontario.Footnote a

a Abbreviations: fb, followed by; WAA, weeks after application.
b Visual control estimates based on comparisons made to weedy and weed-free check.
c Herbicide always included addition of Turbocharge® surfactant at 0.5% v/v.
*Significance at P<0.05.
**Significance at P<0.0001.
A limitation of the methodology used in this experiment was that the POST application timing was when GH common waterhemp escapes in the PRE herbicides reached 10 cm in height. Consequently, the GR common waterhemp was much taller where no PRE herbicide was applied, which resulted in reduced weed control when glyphosate was combined with fomesafen and acifluorfen applied POST without a PRE herbicide. Although this research was conducted with GR soybean, the findings presented could also be adopted for GR common waterhemp control in identity-preserved soybean with the removal of glyphosate from the POST tank mixes tested.
In conclusion, glyphosate is no longer an efficacious POST option for the control of common waterhemp in many fields in Ontario. The glyphosate resistance factor at 4 WAA, based on greenhouse studies, was 4.5, while it was up to 27.9 based on field studies. This study concludes that pyroxasulfone/flumioxazin, pyroxasulfone/sulfentrazone, and S-metolachlor/metribuzin are the most efficacious soil-applied herbicides for the control of GR common waterhemp in soybean in Ontario. A two-pass weed control program of a PRE followed by a POST herbicide provided ≥94% GR common waterhemp control. Although use of a PRE residual herbicide provides a basis for acceptable control, weed escapes should also be controlled with a POST herbicide in a two-pass weed control program. The POST herbicides acifluorfen and fomesafen are effective for the control of GR common waterhemp, but use should be monitored due to the existence of widespread protoporphyrinogen oxidase resistance in common waterhemp in the United States and could be argued to be a short-term solution in Ontario (Heap Reference Heap2017). Moving forward, there is a vital need for an increased awareness of herbicide resistance. Growers need to diversify their crop production/weed management systems. Research such as the comprehensive study published by Meyer et al. (Reference Meyer, Norsworthy, Young, Steckel, Bradley, Johnson, Loux, Davis, Kruger, Bararpour and Ikley2015) finds that future technologies show more effective common waterhemp and Palmer amaranth control than current technologies. Specifically, synthetic auxins were found to improve GR waterhemp and Palmer amaranth control over treatments not containing synthetic-auxin herbicides in soybean (Meyer et al. Reference Meyer, Norsworthy, Young, Steckel, Bradley, Johnson, Loux, Davis, Kruger, Bararpour and Ikley2015). In combination with future technologies, the use of companion crops, cover crops, narrow row spacing, high seeding rates, and crops with different herbicide-resistant traits are all potential tools to reduce the evolution of herbicide resistance. The continuing evolution of singly and multiply resistant weed populations is a threat to the economic viability of field crop production in Ontario.








