Introduction
Downy brome and Japanese brome are two nonnative winter annual grasses that have invaded the western United States, with downy brome present in the cold deserts, western Great Plains, and western forests; and Japanese brome found mainly in the western Great Plains (Germino et al. Reference Germino, Belnap, Stark, Allen and Rau2016). These annual grasses can have substantial impacts in cropped (Blackshaw Reference Blackshaw1993; Rydrych and Muzik Reference Rydrych and Muzik1968) and rangeland areas (Haferkamp and Heitschmidt Reference Haferkamp and Heitschmidt1999; Ogle et al. Reference Ogle, Reiners and Gerow2003). Downy brome has been found to reduce winter wheat (Triticum aestivum L.) biomass by up to 59% and grain yield by up to 68% (Blackshaw Reference Blackshaw1993). Japanese brome has been shown to impact grass yield in rangelands; its removal from a western wheatgrass [Pascopyrum smithii (Rydb.) Á. Löve] rangeland increased standing crop yield by 220 kg ha−1 and tillers by 153 m−2 (Haferkamp and Heitschmidt Reference Haferkamp and Heitschmidt1999). Downy brome also impacts ecosystem processes by competing with native grasses (Francis and Pyke Reference Francis and Pyke1996; Nasri and Doescher Reference Nasri and Doescher1995; Vasquez et al. Reference Vasquez, Sheley and Svejcar2009), changing fire regimes (Brooks et al. Reference Brooks, D’Antonio, Richardson, Grace, Keeley, DiTomaso, Hobbs, Pellant and Pyke2004; Whisenant Reference Whisenant, McArthur, Romney, Smith and Tueller1990), altering available nitrogen (Rimer and Evans Reference Rimer and Evans2006; Sperry et al. Reference Sperry, Belnap and Evans2006), increasing soil organic carbon storage (Norton et al. Reference Norton, Monaco, Norton, Johnson and Jones2004; Ogle et al. Reference Ogle, Ojima and Reiners2004), and modifying nutrient cycling (Belnap and Phillips Reference Belnap and Phillips2001; Norton et al. Reference Norton, Monaco, Norton, Johnson and Jones2004). There are currently no studies assessing these ecological impacts for Japanese brome.
Although there have been attempts to manage downy brome and Japanese brome with prescribed fire, grazing, tillage, and biological control in range- and wildlands (Brooks et al. Reference Brooks, Brown, Chambers, D’Antonio, Keeley and Belnap2016; Cox and Anderson Reference Cox and Anderson2004; DiTomaso et al. Reference DiTomaso, Brooks, Allen, Minnich, Rice and Kyser2006; Ehlert et al. Reference Ehlert, Mangold and Engel2014; Germino et al. Reference Germino, Belnap, Stark, Allen and Rau2016; Harmoney Reference Harmoney2007; Lehnhoff et al. Reference Lehnhoff, Rew, Mangold, Seipel and Ragen2019; Masters and Sheley Reference Masters and Sheley2001; Metier et al. Reference Metier, Rew and Rinella2018; Monsen et al. Reference Monsen, Stevens and Shaw2004; Vermeire et al. Reference Vermeire, Heitschmidt and Haferkamp2008; Whitson and Koch Reference Whitson and Koch1998), herbicides are still the most widespread management tool, though they are often used in combination with grazing and seeding in rangelands (Kelley et al. Reference Kelley, Fernandez-Gimenez and Brown2013; Monaco et al. Reference Monaco, Mangold, Mealor, Mealor and Brown2017). Herbicides are also the most widely used tool in cropping systems (Radosevich et al. Reference Radosevich, Holt and Ghersa2007). Glyphosate is commonly used to control weedy species during the fallow phase in cropping systems and during restoration of range- and wildlands. Rangeland field studies reported high (Morris et al. Reference Morris, Morris and Surface2017) to very high levels of downy brome control after one (>97%) (Cox and Anderson Reference Cox and Anderson2004; Whitson and Koch Reference Whitson and Koch1998) and three (>92%) consecutive applications (Whitson and Koch Reference Whitson and Koch1998) of glyphosate. In the greenhouse, Park and Mallory‐Smith (Reference Park and Mallory‐Smith2004) found an average of 85% reduction of downy brome biomass when treated with glyphosate compared with an untreated control. Less is known about the efficacy of glyphosate on Japanese brome, though Waller and Schmidt (Reference Waller and Schmidt1983) reported glyphosate provided good control of Japanese brome. However, because glyphosate is a broad-spectrum herbicide, it is not suitable for all situations (Baker et al. Reference Baker, Garner and Lyon2009; Morris et al. Reference Morris, Monaco and Rigby2009; Owen et al. Reference Owen, Sieg and Gehring2011).
Graminicides are grass-specific POST herbicides that inhibit acetyl-CoA carboxylase (ACCase) enzyme, specifically the production of phospholipids required for cell membrane production (Délye et al. Reference Délye, Wang and Darmency2002). Graminicides are used in annual cropping systems (Foy and Witt Reference Foy and Witt1992; Marquardt and Johnson Reference Marquardt and Johnson2013), and usage will likely increase with the release of wheat varieties with ACCase herbicide tolerance traits where quizalofop-P-ethyl is recommended as the herbicide (e.g., CoAXium® wheat, CoAXium Wheat Production System, CO). They are also widely used in forestry (Clay et al. Reference Clay, Dixon and Willoughby2006), but they are used less in rangeland and restoration scenarios (James et al. Reference James, Sheley, Erickson, Rollins, Taylor and Dixon2013). These herbicides, including clethodim, sethoxydim, fluazifop-P-butyl, and quizalofop-P-ethyl (hereafter fluazifop and quizalofop), are phytotoxic to grasses, but unlike glyphosate they do not affect forbs or shrubs (Kukorelli et al. Reference Kukorelli, Reisinger and Pinke2013). For this reason, they may be particularly useful at sites dominated by annual grasses, where few perennial grasses and some desired forbs and shrubs exist.
Research on the effect of these graminicides on downy brome and Japanese brome is limited, though what exists is encouraging. Dense downy brome cover was reduced over a 5-yr period with sethoxydim (∼70%), fluazifop (95%), and quizalofop (99%) applied at label rates in a field study at Oregon State University (Brewster and Spinney Reference Brewster and Spinney1989). Similarly, high rates of biomass reduction were observed in a greenhouse study for sethoxydim (85%), clethodim, fluazifop, and quizalofop (all >98%) when applied at the recommended herbicide label rates (Ball et al. Reference Ball, Frost and Bennett2007).
The goal of this study was to build on previous work and examine the efficacy of glyphosate and graminicides to control downy brome and Japanese brome. Specifically, we evaluated the effect of herbicide type, application rate, and plant size (target plant height and leaf number at time of application) on different downy brome and Japanese brome accessions in a controlled setting. Our first objective was to evaluate the efficacy of glyphosate and four graminicides (clethodim, sethoxydim, fluazifop, and quizalofop) on downy brome biomass at high and low label-recommended application rates of each herbicide when applied across five different plant heights using one downy brome accession. Our second objective was to compare the efficacy of glyphosate and the same four graminicides at high and low label-recommended application rates across three accessions of both downy brome and Japanese brome, applied at one plant height.
Materials and Methods
Herbicide Type and Rate Applied to Downy Brome of Different Heights (Experiment 1)
The efficacy of downy brome control was evaluated for four graminicides (clethodim, sethoxydim, fluazifop, and quizalofop) and glyphosate. All herbicides were applied at two rates (low and high label-recommended rates for downy brome [and Japanese brome where stated]; Table 1) to plants that had reached five predefined aboveground heights (5, 8.5, 11, 15.5, and 17 cm).
Table 1. Herbicide common and trade names and the recommended low and high rates used for our downy brome and Japanese brome experiments.

a BASF Agricultural Products, 26 Davis Drive, PO Box 13528, Research Triangle Park, NC 27709, USA, https://agriculture.basf.com/us/en/Crop-Protection.html.
b Valent USA LLC Agricultural Products, 1333 N California Blvd, Suite 600, Walnut Creek, CA 94596, USA, http://www.valent.com/.
c Syngenta, PO Box 18300, Greensboro, NC 27419, USA, http://www.syngenta-us.com/.
d Corteva Agriscience (DuPont), 9330 Zionsville Road, Indianapolis, IN 46268, USA, http://www.corteva.us.
e Bayer CropScience, 2 T.W. Alexander Drive, Research Triangle Park, NC 237709, USA, http://www.bayercropscienceus.com.
The experiment was established as a randomized complete block design with 11 treatments (10 herbicides and an untreated control) by 5 heights by 7 replicates (385 experimental units). The experimental unit was 1 downy brome seedling per pot. The experiment was performed twice (Trial 1: November 2014 through May 2015; and Trial 2: November 2015 through May 2016) in a greenhouse with a 16-h photoperiod at 22 ± 4°C daytime temperatures and 17 ± 6°C nighttime temperatures. At 30 d after seeding, the plants assigned to the three tallest height groups were transferred to a cold chamber (4°C, 12-h photoperiod) for 6 wk to vernalize and were then returned to the greenhouse. Plants in the two shorter height groups did not receive the vernalization treatment, because at 30 d they were already close to their desired height for herbicide application. Plant height was determined using the average height of three randomly selected extended leaves. Pots were watered equally and as needed. Plants were sprayed when the average replicate height reached its predefined target (5, 8.5, 11, 15.5, and 17 cm). The number of leaves per plant was recorded at the time of spray application. For all herbicide treatments, a nonionic surfactant (X-77 Spreader, Loveland Products, 3005 Rocky Mountain Ave, Loveland, CO 80538) was added at a rate of 0.25% v/v. Herbicides were applied using a moving nozzle sprayer (DeVries Manufacturing, 86956 State Highway 251, Hollandale, MN 56045) calibrated to deliver 94 L ha−1 of spray solution (i.e., water plus herbicide plus surfactant) at 276 kPa. Plants were harvested at the root crown at 45 d after herbicide application; all remaining plant tissue was dried at 40°C for 72 h and weighed.
Herbicide Type and Rate Applied to Three Downy Brome and Japanese Brome Accessions (Experiment 2)
The efficacy of the same four graminicides and glyphosate, applied at two application rates, was assessed on three downy brome and Japanese brome accessions. Seed accessions of both species were collected from three grassland locations to determine whether there were site-specific differences in response to herbicides. Downy brome and Japanese brome seeds were collected from “disturbed” restoration sites on Decker (45.056780°N, 106.840467°W) and Spring Creek (45.139351°N, 106.921612°W) coal mines, north of Decker, MT, in the Powder River Basin. Nomenclature is based on Lesica (Reference Lesica2012). The remaining downy brome site was in rangeland at the Montana State University Red Bluff Agricultural Research Ranch in Norris, MT (45°52′N, 111°68′W; also used in Experiment 1), and the Japanese brome site was Burke Park in Bozeman, MT (45°67′N, 111°03′W). These two sites are hereafter referred to as “undisturbed.” This experiment was conducted over a 7-mo period (November 2015 through May 2016) in a greenhouse with the same temperature and light and watering conditions as Experiment 1.
The experiment was designed as a randomized complete block design: 11 treatments (10 herbicides and an untreated control) by 2 species by 3 accessions by 7 replicates (462 experimental units). For this experiment, we used one plant height (11 cm) as our target plant size. After 30 d, seedlings were placed in the cold chamber (4°C, 12-h photoperiod) to vernalize for 6 wk and were then returned to the greenhouse. When the average height of the plants within a replicate reached 11 cm, the same herbicide treatments were applied using the same procedures as described for Experiment 1. Similarly, the number of leaves at time of herbicide application was recorded. Again, aboveground biomass was harvested at 45 d after herbicide application, and the resulting plant biomass was harvested and dried.
Data Analysis
Data were analyzed with linear mixed-effects models using the lmerTest and lme4 (Bates et al. Reference Bates, Maechler, Bolker and Walker2015) packages. Least-squares means and Tukey pairwise comparisons were evaluated using the lsmeans (Lenth Reference Lenth2016) package. Data analysis was performed using R v. 3.3.2 (R Core Team 2016). The most parsimonious model was selected using Akaike information criterion (AIC) with a decrease in AIC score of 2 being considered a better fit. In all models, the biomass response variable was natural log (ln) transformed to satisfy model assumptions.
For Experiment 1, a linear mixed-effects model was created in which the response variable was plant biomass (ln) at time of harvest for each replicate. Initially, a full model was run with fixed effects for treatment (all herbicide and rate combinations), height at time of application (5, 8.5, 11, 15.5, or 17 cm), and trial (1 and 2), along with the interactions among treatment and height, trial and height, and treatment, trial, and height, as well as a random effect for replicate. Individual models were then created for each plant height group to better elucidate the efficacy of herbicide treatments. For 5-cm, 8.5-cm, and 17-cm plant heights, fixed effects were herbicide, trial, and the interaction between herbicide and trial. (Data from the 5-cm plant height treated with the clethodim low rate during Trial 2 in Experiment 1 were excluded due to a problem with the spray chamber during application.) For the 11-cm and 15.5-cm plants, herbicide and trial were included as fixed effects, and no interaction term was necessary. In all models, a random effect was included for replicate.
As herbicide application timing is also often based on number of leaves, we developed a second model in which number of leaves, rather than height, was used as an explanatory variable. The most parsimonious linear mixed-effects model had plant biomass (ln) at time of harvest as the response variable with fixed effects for treatment (all herbicide and rate combinations), trial (1 and 2), and number of leaves (ln) at time of application, along with the interactions between treatment and number of leaves, trial and number of leaves, and a random effect for replicate.
Similar models were created for plant biomass (ln) at time of harvest for Experiment 2. Fixed effects included herbicide treatment (all herbicide and rate combinations), accession (Decker, Spring Creek, or undisturbed), and species (Japanese brome or downy brome), as well as the interaction between herbicide and species. There was no difference between the Spring Creek and Decker mine accessions (P = 0.3393), so they were combined in the final analysis and are hereafter referred to as “disturbed.” A random effect was included for replicate.
Results and Discussion
Our results demonstrate that fluazifop, quizalofop, clethodim, sethoxydim, and glyphosate can all reduce downy brome and Japanese brome biomass, especially when applied shortly after germination—with a tendency for fluazifop and quizalofop to be most effective. Our study demonstrates that targeting smaller plants, specifically plants 11 cm or smaller with less than 12 leaves, provides more reliable results. In Experiment 1, plants that were shorter (≤11 cm) with fewer leaves (≤12 leaves) at time of herbicide application were most affected, with biomass reduced by more than 50% of the control for all but the low glyphosate treatment at 11 cm. However, little or no reduction in biomass was observed when herbicides were applied at the 17-cm height. A similar pattern was observed across herbicides for Experiment 2, where treatments were only applied to 11-cm plants: quizalofop and fluazifop were again the most effective, and the low rate of glyphosate was the least effective at reducing biomass at 45 d after treatment.
The Importance of Plant Size
Efficacy of different herbicides applied at two rates was assessed across growth stages (height and number of leaves). The main effect of trial was significant for all downy brome height groups. For the shortest height groups (5 cm and 8.5 cm), there was greater biomass reduction in the first than the second trial (Supplementary Tables S1 and S2), with the opposite pattern for the taller groups (Figure 1; Supplementary Tables S4 and S5). All herbicide treatments reduced downy brome biomass when applied to the two shortest groups of plants (5 cm and 8.5 cm) compared with the control (Supplementary Tables S1 and S2, respectively). This was also true for 11-cm plants, with the exception of the low rate of glyphosate (Supplementary Table S3), and for 15.5-cm plants with the low rate of glyphosate and sethoxydim (Supplementary Table S4). The tallest plants (17 cm) showed less response, with neither rate of glyphosate nor a low rate of sethoxydim reducing plant biomass compared with the control in the first trial and only the low rate of glyphosate reducing biomass in the second trial (Supplementary Table S5).
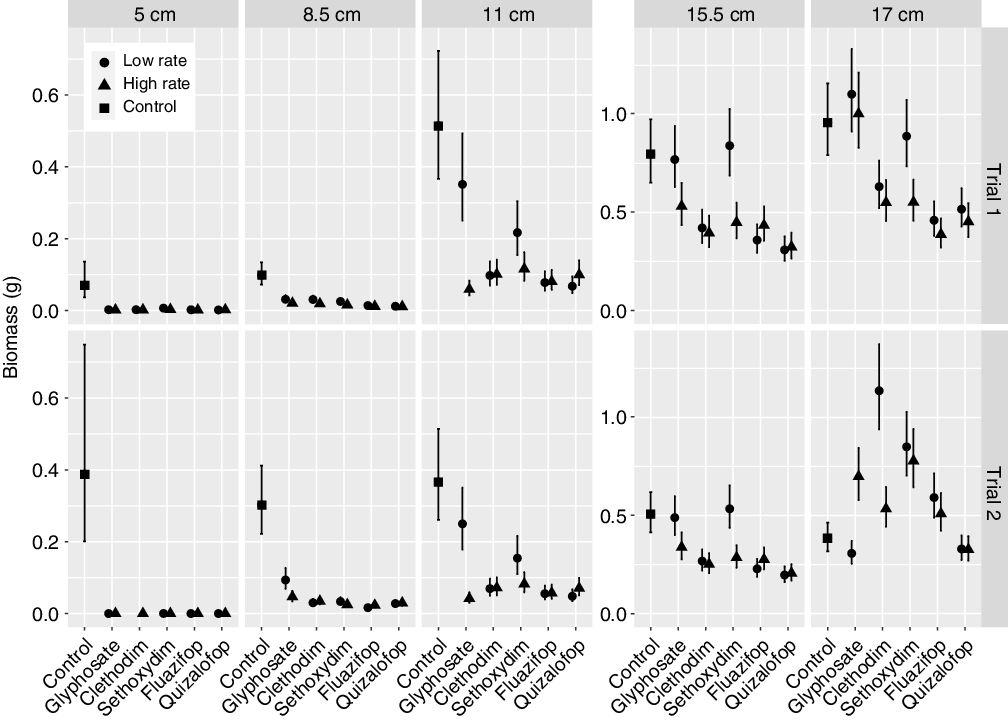
Figure 1. Effect of different herbicides and application rates on individual downy brome biomass (g) for plants treated at different growth stages (height) in the two trials of Experiment 1. Mean plant biomass (symbols) and SE (vertical line) of the individual plants within a replicate are presented, using least-squares means (back-transformed natural log values) from the mixed-effects model. See Supplementary Tables S1–S5 for further statistical comparison and text for pairwise comparisons.
When the data from Experiment 1 were analyzed using number of leaves at time of spraying (continuous variable) instead of height at time of spraying, the results yielded similar patterns (Figure 2). As the number of leaves at time of spraying increased, the efficacy of all herbicide treatments decreased (Figure 2; Supplementary Table S6; P = 0.0018), and generally the herbicides worked best on plants with fewer than 12 leaves (ln 2.48). There was little difference among herbicide treatments applied at the high rate, but fluazifop and quizalofop were more effective at reducing plant biomass at low rates (Figure 2; pairwise comparisons not shown).
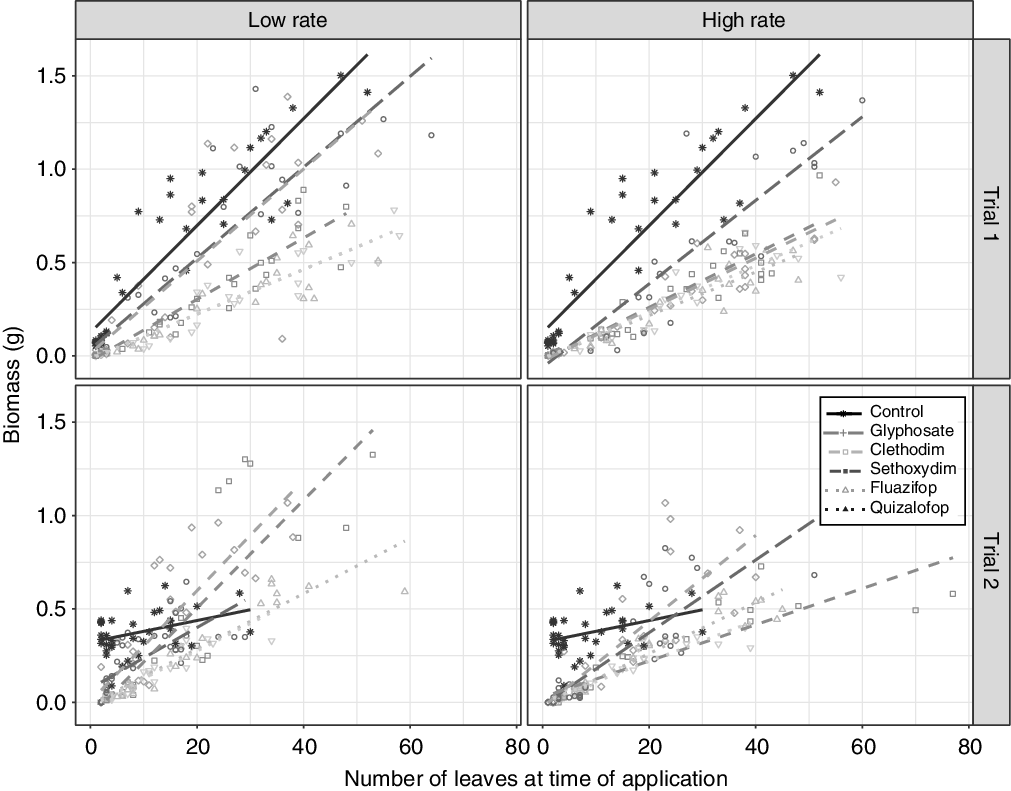
Figure 2. Effect of different herbicides and application rates on individual downy brome biomass (g) for plants treated at different growth stages (number of leaves) in the two trials of Experiment 1. Points represent individual plants. See Supplementary Table S6 for further statistical comparison.
Not all studies provide information on plant height or number of leaves at the time of application, making comparisons between our work and that of others difficult. However, studies on a frequently used herbicide in rangeland found no difference in downy brome control when imazapic (acetohydroxyacid synthase branched-chain amino acid inhibitor) was applied to plants with 2 to 4 leaves compared with plants with 5 to 10 leaves, in agreement with our results. In contrast, Mangold et al. (Reference Mangold, Parkinson, Duncan, Rice, Davis and Menalled2013) found that downy brome control increased when imazapic was applied to plants at the 1- to 2-leaf stage compared with the 3- to 4-leaf stage, a finer differentiation than we observed. However, all these studies demonstrate that brome control varies on a finer scale (i.e., 2.5 cm height intervals) than is often recommended on herbicide labels. While logistical constraints of large-scale herbicide applications (timing of precipitation, weather patterns, plant growth patterns, access, etc.) often hamper timely application, both plant height and number of leaves are simple to assess in the field, and this practice should be adhered to more carefully.
Efficacy of Herbicides and Rates
Overall, glyphosate was not as effective at reducing biomass as the graminicides, with the low rate of glyphosate often performing worst. That said, for the shortest plants (5 cm), there were no biomass differences between low and high rates of any herbicide treatments, in either trial (Figure 1). However, for the middle height groups, the low rate of glyphosate performed less well. For the 8.5-cm height, in both trials the glyphosate applied at low rate resulted in higher biomass than both fluazifop (Trial 1 low, P = 0.0496; Trial 2 low, P < 0.0001; Trial 1 high, P = 0.0028; Trial 2 high, P < 0.0001) and quizalofop (Trial 1 low, P = 0.0044; Trial 2 low, P < 0.0001; Trial 2 high, P = 0.0008; Trial 2 high, P = 0.0002) treatment rates; and in the second trial only, the glyphosate low rate resulted in higher biomass than both rates of sethoxydim (low, P = 0.0016; high, P < 0.0001) and clethodim (low, P = 0.0002; high, P = 0.0029). For the 11-cm height, the low rate of glyphosate had higher biomass than both rates of fluazifop, quizalofop, and clethodim (P < 0.0001 for all), as well as the high rates of sethoxydim (P = 0.0006) and glyphosate (P < 0.0001). There was a similar trend for the 15.5-cm height: the low rate of glyphosate had higher biomass than both rates of fluazifop (low, P < 0.0001; high, P = 0.0102), quizalofop (P < 0.0001 for both) and clethodim (low, P = 0.0044; high, P = 0.0007), and the sethoxydim high rate (P = 0.0235). For the 17-cm height, trial was again significant, and there was an interaction with herbicide. In the first trial, the glyphosate low rate had higher biomass than both rates of fluazifop and quizalofop (P < 0.0001 for all) and clethodim (low, P = 0.0121; high, P = 0.0002) and the sethoxydim high rate (P = 0.0003). However, in the second trial, the glyphosate low rate had lower biomass than both rates of fluazifop (low, P = 0.0008; high, P = 0.0414), clethodim (low, P < 0.0001; high, P = 0.0131), and sethoxydim (P < 0.0001 for both) and the glyphosate high rate (P < 0.0001).
All graminicides performed well, with fluazifop and quizalofop outperforming clethodim and sethoxydim in all but the shortest group (Figure 1). Response to fluazifop and quizalofop was similar, with low rates generally performing as well or better than the high rates. In the first trial for the 8.5-cm height, the low rate of quizalofop (P = 0.0062) and fluazifop (P = 0.004) resulted in less biomass than the clethodim low rate, and the high rate of quizalofop had less biomass than the low rates of clethodim (P = 0.0012) and sethoxydim (P = 0.0309): there were no differences among graminicides in Trial 2. For the 11-cm height in both trials, the low rate of quizalofop (P = 0.0002) and fluazifop (P = 0.0026) and high rate of fluazifop (P = 0.0048) had less biomass than the sethoxydim low rate. For the 15.5-cm height in both trials, both rates of fluazifop (low, P < 0.0001; high, P = 0.0009) and quizalofop (P < 0.0001 for both) had less biomass than the sethoxydim low treatment. Similarly, for the 17-cm height in Trial 1, both rates of fluazifop (low, P = 0.0007; high, P < 0.0001) and quizalofop (low, P = 0.0177; high, P = 0.0004) had less biomass than the sethoxydim low rate. In the second trial, the fluazifop high rate (P = 0.0365) had less biomass than the sethoxydim low rate, and both quizalofop rates had less biomass than both sethoxydim rates (P < 0.0001 for all). The only difference between fluazifop and quizalofop was in the 17-cm height group in Trial 2, where both quizalofop rates (low, P = 0.0058; high, P = 0.0046) outperformed the fluazifop low rate.
In our second experiment that evaluated the efficacy of herbicide type and rate on different downy and Japanese brome accessions, we observed the same patterns at the same growth stage(s) as in Experiment 1. However, herbicides caused notably greater biomass reduction to Japanese brome than downy brome (Figure 3; Supplementary Table S7; P < 0.0001). Overall, the graminicides performed better than the low rate of glyphosate, which was the least effective at controlling both downy and Japanese brome (Figure 3). The low rate of glyphosate resulted in greater downy brome biomass than both rates of fluazifop (P < 0.0001 for both), quizalofop (low, P < 0.0001; high, P < 0.0025) and clethodim (P < 0.0001 for both), as well as the glyphosate high rate (P < 0.0001). The most effective herbicides were fluazifop and quizalofop, with low rates performing well, again similar to Experiment 1. Both fluazifop rates and the quizalofop low rate (P < 0.0001 for all) had less biomass than the sethoxydim low rate; and the fluazifop low treatment had less biomass than the sethoxydim high treatment (P = 0.0168). Low-rate application of sethoxydim was generally the least effective of the graminicides and produced greater biomass than both rates of clethodim (low, P < 0.0001; high, P = 0.0008).

Figure 3. Effect of different herbicides and application rates on individual plant biomass (g) for undisturbed and disturbed downy brome (left) and Japanese brome (right) accessions treated at one growth stage (11-cm mean plant height) for Experiment 2. Mean plant biomass (symbols) and SE (vertical line) of the individual plants within a replicate are presented, using least-squares means (back-transformed natural log values) from the mixed-effects model. See Supplementary Table S7 for statistical comparison and text for pairwise comparisons.
In summary, application rate did not affect the efficacy of graminicides when applied to smaller plants (≤11 cm, ≤12 leaves), with low rates often performing better, but both application rates reduced plant biomass by at least 50% compared with the control. However, for glyphosate, application rate did matter. Glyphosate applied at the high rate was more effective than the low rate when applied to <11-cm plants in Experiments 1 and 2. Thus, for glyphosate, the high application rate was necessary to ensure adequate control; this will likely be especially important in a field setting, where target plant heights could vary. Park and Mallory‐Smith (Reference Park and Mallory‐Smith2004) applied glyphosate at a rate of 0.420 kg ai ha−1 to downy brome plants in the 3- to 4-leaf stage and found an average of 85% control. In our study, this same treatment (8.5-cm-height group treated with our low glyphosate rate) only provided an average of 68% control of downy brome. In a Wyoming field experiment, Whitson and Koch (Reference Whitson and Koch1998) applied glyphosate to downy brome plants at the 2- to 8-leaf stage at 0.42, 0.55, 0.69, and 0.83 kg ai ha−1 and achieved >99% decrease in live canopy cover in all treatments. This is far greater control than we achieved with our glyphosate treatments in our comparable (11-cm) group. It has been shown that higher rates of imazapic can increase the effectiveness of downy brome control (Morris et al. Reference Morris, Monaco and Rigby2009), but broad-spectrum herbicides like glyphosate and imazapic can also damage desired species (Kyser et al. Reference Kyser, Wilson, Zhang and DiTomaso2013). Because graminicides are grass specific, using a higher rate to control bromes should not increase the damage to non-target shrub and broadleaf species (Kukorelli et al. Reference Kukorelli, Reisinger and Pinke2013), but our results suggest that the low label rate of fluazifop or quizalofop should provide good control, as well as provide a good alternative to broad-spectrum herbicides in restoration scenarios.
All herbicides were more effective at reducing Japanese brome biomass compared with downy brome. Biomass of untreated (control) Japanese brome plants was greater than biomass of untreated downy brome plants, but in contrast, Japanese brome plants treated with herbicides all had lower biomass than their downy brome counterparts that received the same application (Figure 3; Supplementary Table S7). Furthermore, all of the graminicides reduced Japanese brome accessions in comparison with the low rate of glyphosate (P < 0.0001 for all treatments). Other studies have found that glyphosate is effective at reducing downy brome biomass (Cox and Anderson Reference Cox and Anderson2004; Morris et al. Reference Morris, Morris and Surface2017; Park and Mallory‐Smith Reference Park and Mallory‐Smith2004; Whitson and Koch Reference Whitson and Koch1998), and in the only study to test effectiveness on Japanese brome, Waller and Schmidt (Reference Waller and Schmidt1983) stated that it provided excellent control of Japanese brome in a Nebraska tallgrass prairie, though no data were reported. This also agrees with our findings, but for both Japanese and downy brome, the low glyphosate rate performed significantly worse than the high rate, where the biomass was 457% and 395% greater, respectively. There is limited information addressing graminicides’ ability to control downy brome (Ball et al. Reference Ball, Frost and Bennett2007; Brewster and Spinney Reference Brewster and Spinney1989), but in the few studies that do, graminicides provided good control. Additionally, our study agrees with Ball et al. (Reference Ball, Frost and Bennett2007), who found that quizalofop and fluazifop are generally the most effective, and sethoxydim the least effective of these herbicides. Our study is the first we know of that tests the efficacy of these graminicides on Japanese brome.
Differences in Populations
Biomass of downy and Japanese brome accessions from disturbed sites was greater than for the undisturbed downy brome across all herbicide treatments (Figure 3; Supplementary Table S7; P < 0.0001); however, pairwise comparisons showed there was no difference between the disturbed and undisturbed Japanese brome accessions. There is evidence to suggest that plant characteristics such as cold tolerance (Bykova and Sage Reference Bykova and Sage2012), germination success (Hardegree et al. Reference Hardegree, Moffet, Flerchinger, Cho, Roundy, Jones, James, Clark and Pierson2013), and vernalization requirements (Lawrence et al. Reference Lawrence, Hauvermale and Burke2018) can vary across downy brome accession. Additionally, some downy brome accessions have developed resistance to both acetolactate synthase (ALS) inhibitors (Mueller-Warrant et al. Reference Mueller-Warrant, Mallory-Smith and Hendrickson1999; Park and Mallory‐Smith Reference Park and Mallory‐Smith2004) and graminicides (Ball et al. Reference Ball, Frost and Bennett2007; Park and Mallory‐Smith Reference Park and Mallory‐Smith2004). While we found differences in herbicide control among accessions, geography as well as disturbance history may be a factor. The disturbed sites (Spring Creek and Decker mine) are located within 25 km of one another, so they are more likely to be genetically similar to each other, and this could be why there was no difference between them. The undisturbed sites have received little if any herbicide management and low disturbance (e.g., grazing) pressure, but they are also geographically distant from the disturbed sites.
Conclusion
Herbicide control of annual bromes is important, as these two species have invaded large areas of the western United States (Chambers et al. Reference Chambers, Roundy, Blank, Meyer and Whittaker2007; Duncan et al. Reference Duncan, Jachetta, Brown, Carrithers, Clark, DiTomaso, Lym, McDaniel, Renz and Rice2004; Haferkamp et al. Reference Haferkamp, Young, Grings, Karl, Heitschmidt and MacNeil1992; Knapp Reference Knapp1996; Whisenant Reference Whisenant, McArthur, Romney, Smith and Tueller1990), their ranges are expanding (Bradley Reference Bradley2009; Bradley et al. Reference Bradley, Curtis and Chambers2016), and they are negatively impacting many different ecosystems (Blackshaw Reference Blackshaw1993; Haferkamp and Heitschmidt Reference Haferkamp and Heitschmidt1999; Ogle et al. Reference Ogle, Reiners and Gerow2003; Rydrych and Muzik Reference Rydrych and Muzik1968). Our results demonstrate that graminicides, specifically fluazifop and quizalofop, can be used to successfully control annual bromes. In wheat-dominated agroecosystems of the northwestern United States there are more frequent reports of downy brome populations resistant to ALS herbicides (Barroso and Gourlie Reference Barroso and Gourlie2019), and the introduction of ACCase-resistant wheat and the associated application of quizalofop will help to reduce these populations. In highly disturbed rangeland restoration ecosystems, these graminicides could provide a useful tool and improve control efficacy, but evaluation under field conditions where desired species are present is required before recommendations can be made.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wet.2019.112
Acknowledgments
Thanks to Kaylee Schmitz who helped with field sampling. EAL, JM, and LJR are supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture Hatch: NMLehnhoff-17H, MONB00359, and MONB00363, respectively. No conflicts of interest have been declared.






