Introduction
Acetyl-coenzyme A carboxylase (EC 6.4.1.2; ACCase) is an essential enzyme involved in fatty-acid synthesis and formation of cell membranes within plants. In Poaceae species, ACCase-inhibiting herbicides competitively bind to the homomeric form of this enzyme, blocking the carboxylation of acetyl-CoA and production of malonyl-CoA, a precursor for lipids. Herbicides with this site of action, such as fluazifop-P-butyl, an aryloxyphenoxypropionate herbicide, are commonly used to selectively target grass weeds in broadleaf crops. This selectivity is due to the multimeric form of ACCase that is insensitive to these herbicides and is present within the chloroplasts in non-Poaceae species, while in susceptible Poaceae species only the homomeric form is found in both cytosol and chloroplast (Yu et al. Reference Yu, Collavo, Zheng, Owen, Sattin and Powles2007).
The efficiency of fluazifop-P-butyl as a systemic POST herbicide to control target weeds is largely determined by retention and absorption rate on the leaf surface, de-esterification to the active acid form once absorbed, translocation through the phloem, and finally, transport to meristematic cells, where its activity is more injurious within plants (Carr et al. Reference Carr, Davies, Cobb and Pallet1985; Hendley et al. Reference Hendley, Dicks, Monaco, Slyfield, Tummon and Barrett1985). Fluazifop-P-butyl is registered for grass weed control in zoysiagrass (Zoysia spp.) due to the moderate level of tolerance observed in this species. However, the degree of this tolerance is highly dependent on the cultivar and application frequency and timing (Anonymous 2009; Johnson Reference Johnson1992; Leon et al. Reference Leon, Unruh, Brecke and Kenworthy2014). For example, Leon et al. (Reference Leon, Unruh, Brecke and Kenworthy2014) reported that cultivars such as ‘Zeon’ and ‘PristineFlora’ exhibited up to 30% more injury and needed 2 to 4 more weeks to recover to acceptable injury levels (i.e., <20%) than ‘Empire’ and ‘Emerald’ after treatment with 175 or 263 g ai ha−1 of fluazifop-P-butyl.
Resistance to ACCase-inhibiting herbicides has been documented for 48 Poaceae species worldwide (Heap Reference Heap2018). The main mechanisms of tolerance/resistance reported in grass species are metabolic detoxification and reduced binding affinity at the site of action. Despite previous reports and research on several species, including rigid ryegrass (Lolium rigidum Gaudin), wheat (Triticum aestivum L.), and barley (Hordeum vulgare L.) (Preston et al. Reference Preston, Tardif, Christopher and Powles1996; Vila-Aiub et al. Reference Vila-Aiub, Neve and Powles2005), a clear understanding of the mechanism for metabolic tolerance of fluazifop-P-butyl remains elusive. The other type of tolerance (also called evolved resistance in cases that resulted from selection from an originally susceptible population) is found in the target site, usually caused by a single base-pair mutation in the carboxyl transferase (CT) domain of the ACCase enzyme inhibiting binding of ACCase-targeting herbicides. The seven mutations previously reported as conferring resistance to ACCase-inhibiting herbicides can be grouped into two regions within the ACCase gene; one that contains the Ile-1781-Leu mutation and the 1999 to 2096 region that contains five possible mutations, which are Trp-1999-Cys, Trp-2027-Cys, Ile-2041-Asn (Val), Asp-2078-Gly, Cys-2088-Arg, and Gly-2096-Ala substitutions (Délye et al. Reference Délye, Zhang, Michel, Matejicek and Powles2005; Devine Reference Devine1997; Liu et al. Reference Liu, Harrison, Chalupska, Gornicki, O’Donnell, Adkins, Haselkorn and Williams2007). The nature of variation in tolerance to fluazifop-P-butyl for zoysiagrass is not known and could possibly be due to either mutations at any of the seven amino acid positions previously reported as conferring ACCase-inhibitor tolerance, or differences in uptake, translocation, and metabolism. The most common mutation that confers fluazifop-P-butyl tolerance/resistance is the Ile-1781-Leu substitution, of which the codon number is in reference to that of blackgrass (Alopecurus myosuroides Huds.) (Powles and Yu Reference Powles and Yu2010).
The objective of the present study was to characterize the physiological factors (e.g., absorption/translocation, target-site sensitivity, metabolism) associated with zoysiagrass tolerance to fluazifop-P-butyl using germplasm with different levels of sensitivity to this herbicide. Better understanding of the physiological and genetic mechanisms responsible for this tolerance would benefit selection and breeding efforts to develop elite zoysiagrass lines with both desirable characteristics for turfgrass use and better tolerance to ACCase-inhibiting herbicides for increased selective control of grassy weeds.
Materials and methods
Fluazifop-P-butyl tolerance screening
A greenhouse study was conducted in 2014 and 2015 at the West Florida Research and Education Center in Jay, FL, to characterize fluazifop-P-butyl tolerance of 27 zoysiagrass lines (Table 1). These lines included Zoysia japonica Steud. (coarse-textured) and Zoysia matrella (L.) Merr. (fine-textured) species, which both exhibited different levels of tolerance to fluazifop-P-butyl and desirable traits for turf use (Leon et al. Reference Leon, Unruh, Brecke and Kenworthy2014). Sprigs of zoysiagrass lines were harvested from planting trays kept under greenhouse conditions, and immediately afterward soil was washed off the roots. All plant materials were transplanted from trays into PVC containers (3.8-cm diameter by 21-cm depth), and a total of 30 plants were propagated for each zoysiagrass line. The potting mix used for propagation contained 50% peat moss, 20% processed pine bark, 20% perlite, and 10% vermiculite. After planting, zoysiagrass lines were maintained in the greenhouse (28 ± 2 C) for 13 wk for establishment and acclimation.
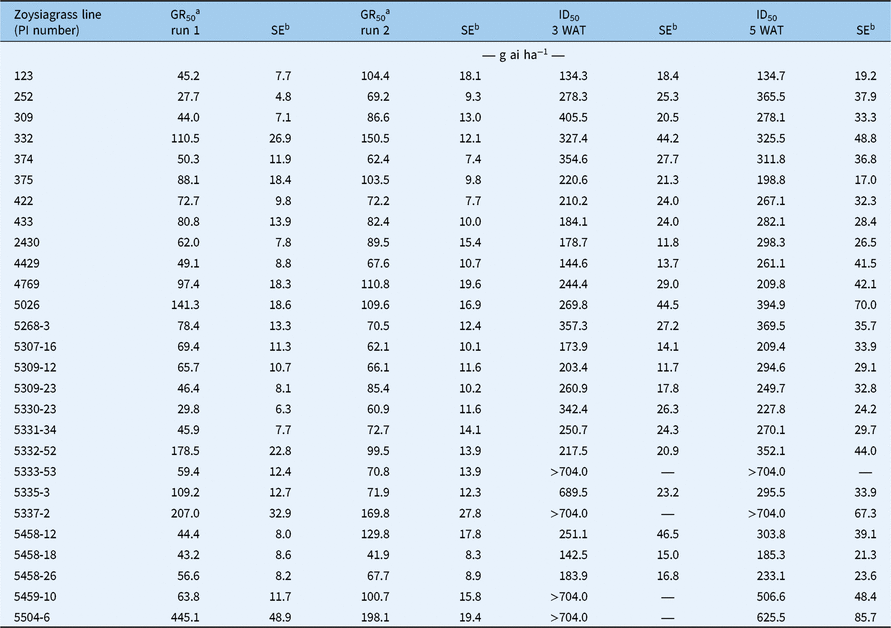
Table 1. Fluazifop-P-butyl rates required for 50% reduction of aboveground biomass (GR50) at 5 wk after treatment (WAT) and 50% injury (ID50) at 3 and 5 WAT.

a Due to treatment by experimental run interactions (P > 0.05), the two runs are presented separately for GR50.
b SE: standard error with N = 3 for GR50 at 5 WAT and N = 6 for ID50 at 3 WAT and 5 WAT.
All cultivars were watered daily throughout the duration of the experiment and mowed weekly at 3.5 cm before herbicide treatments were applied. Mowing was stopped at 1 wk before herbicide application to allow enough biomass to accumulate. Fluazifop-P-butyl (Fusilade® II, 240 g ai L−1, Syngenta Crop Protection, Greensboro, NC) was then applied at 88, 176, 352, and 704 g ai ha−1, equivalent to 1, 2, 4, and 8 times the labeled rate for zoysiagrass (Anonymous 2009) in a spray chamber calibrated to deliver 187 L ha−1. A nontreated control was included for each line. After herbicide application, zoysiagrass lines were immediately placed in four growth chambers (Conviron PGR15, Controlled Environments, Pembina, ND) set to maintain 28 ± 2 C, 70% relative humidity, and a 14-h photoperiod with 415 μmol m−2s−1 photosynthetically active radiation. Experimental units (i.e., individual containers with a given zoysiagrass line) were shuffled weekly within and among growth chambers to minimize site effects.
Percent injury was visually evaluated at 1, 3, and 5 wk after treatment (WAT) with 0% and 100% indicating no injury symptoms and death of all turf tissue, respectively. Clippings were collected from each container by mowing at 1.7 mm at 5 WAT and dried at 60 C for 5 d to determine dry biomass. The experiment was conducted and analyzed as a completely randomized design with three replications. This experiment was conducted twice.
Data were subjected to a polynomial regression analysis in SigmaPlot (Systat Software, San Jose, CA) to determine the fluazifop-P-butyl rate required to cause 50% visual injury (ID50) and 50% biomass reduction (GR50) for each zoysiagrass line. An exponential raise to a maximum model was used for this purpose:
where y represents GR50 or ID50, a is the intercept, b is the rate of response, and x is the herbicide rate (g ai ha−1). ANOVA and mean separation (Tukey’s honestly significance difference [HSD] at 5% significance level) were conducted using R statistical software (v. 3.5.0, R Foundation for Statistical Computing, Vienna, Austria) to compare ID50 and GR50 among zoysiagrass lines. If treatment by experimental run interactions were significant, analyses were conducted separately per run.
Characterization of tolerance mechanisms
Previously reported mutation sites within the ACCase coding sequence that confer tolerance, cytochrome P450–mediated metabolism, differences among the rates of absorption, translocation, and metabolism were all examined to identify mechanisms responsible for the diverse levels of fluazifop-P-butyl tolerance observed in the tested lines. For this purpose, the five most-susceptible and three most-tolerant zoysiagrass lines were identified and selected from the 27 lines for further analysis. The lines that exhibited the highest values in both GR50 and ID50 were considered the most tolerant, and those that exhibited the lowest values were considered the most susceptible. Line selection also took into account plant material availability, so when two lines had similar GR50 and ID50 values, the line that had more plant material for propagation was chosen for the experiments.
ACCase gene sequencing
Two regions of the ACCase gene of these six lines were sequenced to determine whether previously reported mutations conferring resistance to ACCase-targeting herbicides could be involved in their differential sensitivity to fluazifop-P-butyl. Approximately 0.5 g of fresh leaf tissue was sampled from each line, placed in 1.5-ml centrifuge tubes, dipped in liquid nitrogen, and then ground to fine powder with a TissueLyser II (Qiagen, Hilden, Germany). Genomic DNA extraction was performed with the DNeasy® Plant Mini Kit (Qiagen, Hilden, Germany).
For the Ile-1781-Leu target region, primers ACCF5/ACCR5 (Délye et al. Reference Délye, Matejicek and Gasquez2002) were used for PCR amplification, and primers POA3F/POA3R (Tate Reference Tate2012) were used for the region containing the 1999–2096 mutation sites. PCR was performed with denaturation for 30 min at 94 C, followed by 20 cycles of 30 s at 94 C, 45 s at 53 C, and 45 s at 72 C. DNA electrophoresis was then performed with 1% agarose gels to verify that PCR-amplified fragment size matched targeting regions. Verified PCR products were stored at −60 C until being sent to the University of Florida Interdisciplinary Center for Biotechnology Research for Sanger sequencing. Sequences were aligned and compared with a goosegrass [Eleusine indica (L.) Gaertn.] ACCase nucleotide sequence (gb|KF700369.1|) using the Basic Local Alignment Search Tool (BLAST) to evaluate the seven known mutation sites.
Cytochrome P450 inhibition
Previous studies have reported that application of organophosphate insecticides such as phorate reduced cytochrome P450 monooxygenase activity as well as its mediated metabolism of certain herbicides within crops such as cotton (Gossypium hirsutum L.) and maize (Zea mays L.), resulting in increased herbicide injury (Baerg et al. Reference Baerg, Barrett and Polge1996; Ferhatoglu et al. Reference Ferhatoglu, Avdiushko and Barrett2005). This is because organophosphates, upon oxidation by cytochrome P450, can release the sulfur that covalently binds to the apoprotein, rendering it inactive (Werck-Reichhart et al. Reference Werck-Reichhart, Hehn and Didierjean2000). The insecticide phorate suppresses P450 activity, lowering the ability of plants to metabolize the herbicide, thus reducing the tolerance to the herbicide. To test whether cytochrome P450–mediated metabolism is involved in zoysiagrass fluazifop-P-butyl tolerance, greenhouse experiments were carried out during April 2015 and August 2015 in Gainesville, FL.
The three most-tolerant (5337-2, 5459-10, and 5504-6) and three most-susceptible zoysiagrass lines (123, 5458-18, and 375) were propagated in conical containers (3.8-cm diameter by 21-cm depth) as described for the tolerance screening study. Once the plants reached full canopy closure and density, insecticide and herbicide treatments were applied.
Phorate (Thimet®, American Cyanamid, Wayne, NJ) was evenly applied to the soil surface below the canopy within each pot at a rate of 90 kg ha−1. Fluazifop-P-butyl was applied at 352 g ha−1 2 d later to allow enough time for zoysiagrass plants to absorb the phorate. The treatments were: phorate plus fluazifop-P-butyl, fluazifop-P-butyl alone, phorate alone, and a nontreated control. Visual injury was estimated weekly after treatment, and at 5 WAT, clippings were collected, dried, and weighed as described earlier. Percent biomass reduction was calculated by dividing the biomass of clippings from pots that received treatments by that of the nontreated control.
The experiment was conducted as a completely randomized design with three replications and was repeated. ANOVA of percent injury and biomass reduction was performed using a mixed linear model in which fluazifop-P-butyl rates, phorate rates, zoysiagrass line, and their interaction were considered as fixed effects, while replication and experimental run were considered as random effects. ANOVA and mean separation (Tukey’s HSD at 5% level) were performed using R statistical software.
Absorption and translocation of [14C]fluazifop-P-butyl
Greenhouse studies were conducted in May 2017 and August 2017 in Gainesville, FL. The six previously selected zoysiagrass lines were propagated and grown in pots (6.35 cm by 9.5 cm) filled with sand to simplify root washing at the end of the experiment. After propagation, plants were acclimated in a greenhouse, with daily irrigation and weekly fertilization. Once full canopy closure and density were reached for all lines, six fully expanded leaves of uniform size were selected from each pot, ensuring that they were evenly distributed within the pots. A 1-μL droplet of [14C]fluazifop-P-butyl (specific activity 87.9 μCi mg−1; labeled at the phenyl ring) containing a total of 1.4 kBq of radioactivity was applied to a fully expanded leaf from each grass line between the midrib and leaf margin on the adaxial surface. A broadcast application of fluazifop-P-butyl at 88 g ha−1 was immediately performed afterward on these spotted plants inside a spray chamber calibrated to deliver 187 L ha−1 to better simulate herbicide activity at the whole-plant level. Treated zoysiagrass plants were then maintained under greenhouse conditions. Plants were harvested at 1, 3, 7, and 10 d after herbicide application and split into three parts: treated leaf, shoots (except the treated leaf), and roots. Roots were washed carefully with water to remove the sand while seeking to maximize root recovery. Half of the plant was used for combustion and subsequent determination of fluazifop-P-butyl translocation, while the other half was stored at −20 C for metabolism analysis via thin-layer chromatography (TLC).
Herbicide extraction was performed using the methodology reported by Carr et al. (Reference Carr, Davies, Cobb and Pallet1985). Treated leaves were washed by submerging them three times in 5 ml of 50% methanol for 20 s, and all leaf wash was placed in glass scintillation vials. The combined leaf wash was then dried on a hot plate under a hood and resuspended in 15 ml of ScintiVerse™ BD Cocktail (Fisher Scientific, Hampton, NH) before scintillation counting. All plant tissue was oven-dried at 60 C, ground with a mill to pass a 2-mm screen, and then combusted in a biological oxidizer (Model OX-500, R.J. Harvey Instrument, Hillsdale, NJ). Radioactivity of unabsorbed herbicide in the leaf wash and absorbed herbicide within the combustion products of shoots/treated leaves/roots were quantified with liquid scintillation spectroscopy. Percent 14C distribution was calculated by dividing the radioactivity recovered in that specific plant segment (treated leaf, nontreated shoot, and root) by total radioactivity recovered in each plant. Percent absorption of fluazifop-P-butyl was determined by dividing total radioactivity recovered in the plant by the total radioactivity recovered from plant tissue and leaf washes. The experiment was conducted as a completely randomized design with three replications and repeated.
Foliar absorption data were subjected to a quadratic regression analysis, and coefficients of correlation and standard error values were determined in SigmaPlot. The time required to reach 50% herbicide absorption was calculated using regression analysis (Equation 1) for each zoysiagrass line. ANOVA of percent 14C distribution was performed using a mixed linear model with harvest timing, zoysiagrass line, and their interaction as fixed factors, and replications as a random factor. Harvest timing effect was not significant (P = 0.74), and as a result data were pooled over the four evaluation timings. Mean separation (Tukey’s HSD at 5% significance level) was then performed on pooled data using R statistical software.
Metabolism of [14C]fluazifop-P-butyl in six zoysiagrass lines
Also following the methodology reported by Carr et al. (Reference Carr, Davies, Cobb and Pallet1985), study of metabolites was carried out at each harvest timing. Leaves directly spotted with [14C]fluazifop-P-butyl as described earlier were placed in 1.5-ml microcentrifuge tubes and ground with liquid nitrogen. Tubes were then filled with 300 µl of acetone solution for each 100 mg biomass, vortexed for 30 s, and placed in a sonication bath for 1 h. Tubes were then centrifuged for 5 min, and the extract solution was transferred to new tubes. The extraction procedure was conducted three times using fresh acetone solution, and aliquots were combined.
Pooled extracts were spotted on aluminum-backed TLC plates (Whatman, Clifton, NJ) with radiolabeled fluazifop-P-butyl standards. Samples were then developed to 15 cm in benzene: glacial acetic acid (50:8 v/v) at room temperature. Plates were air-dried, and metabolites were determined with an AR-2000 radio-TLC Imaging Scanner (Eckert & Ziegler Group, Berlin, Germany).
The experiment was a completely randomized design with three replications and was repeated. ANOVA was performed considering harvest timings, zoysiagrass lines, and their interactions as fixed effects, while replication and experimental repetition were regarded as random effects. No experiment by treatment interactions were detected. Treatment means were separated within each harvest timing using Tukey’s HSD at the 5% significance level.
Results and discussion
Fluazifop-P-butyl tolerance screening
Experiment by genotype interactions were detected for percent biomass reduction (P < 0.05) for GR50, therefore data from the two experimental runs are presented separately for this variable. Zoysiagrass lines differed in both GR50 and ID50 with differences of at least 4-fold between the most tolerant and most susceptible lines depending on the experimental run (Table 1). For example, lines 252 and 5330-23 had the lowest GR50 in Experiment 1 at 28 and 30 g ha−1, respectively, while line 5504-6 had the highest value at 445 g ha−1, which represented more than a 10-fold difference in fluazifop-P-butyl tolerance in Experiment 1. In Experiment 2, the most susceptible line was 5458-18, with GR50 of 42 g ha−1, which was almost 5-fold lower compared with the tolerant line 5504-6 at 198 g ha−1 (Table 1). It is not clear why there were differences between experimental runs, but it is possible that minor variations during the propagation of the plants (e.g., stolon and rhizome numbers and size or rooting vigor) could have influenced the response to the herbicide.
Line 123 had the lowest ID50 at 3 WAT at 134 g ha−1, and the highest ID50 was observed for lines 5337-2 and 5504-6 at >704 g ha−1 (>4-fold difference). At 5 WAT, line 123 remained as the zoysiagrass line with the lowest ID50, while lines 5337-2 and 5504-6 maintained the highest ID50 at 901 g ha−1 and 625.5 g ha−1 respectively, representing a 6- to 8-fold difference in injury. It is noteworthy that line 252, while having the lowest GR50 considering both experimental runs, exhibited intermediate ID50 levels at 5 WAT. This was because fluazifop-P-butyl decreased new tissue growth even when applied at low rates, but foliar tissue quality was not dramatically reduced. Stunted growth has been associated with lower translocation of systemic herbicides within the phloem, and consequentially less injury might be observed as less herbicide reaches the target sites (Devine and Hall Reference Devine and Hall1990; Hunter Reference Hunter1995). The fact that the sensitivity to fluazifop-P-butyl was expressed independently as either chlorosis and necrosis (i.e., visual injury) or stunted growth suggests that those responses are controlled by different mechanisms.
Based on these screenings, the three most-tolerant lines (5459-10, 5337-2, and 5504-6) and the five most-susceptible lines (123, 252, 375, 5330-23, and 5458-18) were selected for further characterization of fluazifop-P-butyl tolerance. Due to limited plant material, three susceptible lines were randomly allocated to the following experiments, so lines 123, 375, and 5458-18 were used for the cytochrome P450 experiment, and lines 123, 252, and 5330-23 were used for the rest of the experiments.
Cytochrome P450–mediated detoxification and enhanced metabolism
Contrary to our hypothesis that tolerant lines would exhibit similar injury to susceptible lines when phorate was applied before fluazifop-P-butyl treatment, the combined effect of these two chemicals did not increase fluazifop-P-butyl injury or growth reduction compared with the herbicide alone (Table 2), suggesting that cytochrome P450 monoxygenase metabolism is not responsible for the differences in fluazifop-P-butyl tolerance among zoysiagrass lines.
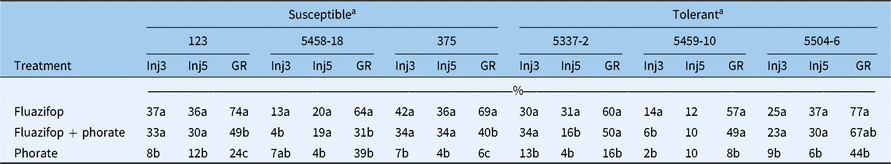
Table 2. Effect of phorate and fluazifop-P-butyl applications on injury evaluated 3 and 5 wk after treatment (WAT) (Inj3 and Inj5, respectively), and growth reduction based on nontreated controls (GR) at 5 WAT on three tolerant and three susceptible zoysiagrass lines.

a Values within columns followed by the same letter are not statistically different based on Tukey’s honestly significant difference (HSD; α = 0.05).
There were minor variations in fluazifop-P-butyl activity in some lines as a result of phorate applications. For example, phorate minimized growth reduction for the susceptible lines 123, 375, and 5458-18, while it reduced injury of line 5458-18 at 3 WAT (Table 2). These responses were not observed for any of the tolerant lines. Hidayat and Preston (Reference Hidayat and Preston2001) reported a similar antagonizing effect from the organophosphate insecticide malathion, which when applied with fluazifop-P-butyl, reduced injury on large crabgrass [Digitaria sanguinalis (L.) Scop]. This enhanced tolerance triggered by phorate application in susceptible zoysiagrass lines could be the result of increased metabolic responses to this chemical. However, the exact mechanism for fluazifop-P-butyl and phorate interaction remains unclear and open for future research.
TLC analysis detected no clear differences in the metabolite profile between the six lines tested (data not shown). Therefore, tolerant lines did not degrade the bioactive acid form of fluazifop through a unique metabolic process that would explain differences in sensitivity compared with susceptible lines.
Screening for ACCase mutations
ACCase gene sequencing demonstrated that neither tolerant nor susceptible zoysiagrass lines contained any of the mutations at the 1781, 1999, 2027, 2041, 2078, 2088, and 2096 sites previously associated with resistance to ACCase inhibitors (Table 3). These results confirm previous findings by Tate (Reference Tate2012) in which no mutation was reported for the 1781 site in Z. japonica or at the other four sites in Z. matrella. However, in their study, Z. matrella was not screened for the 1781 site mutation.
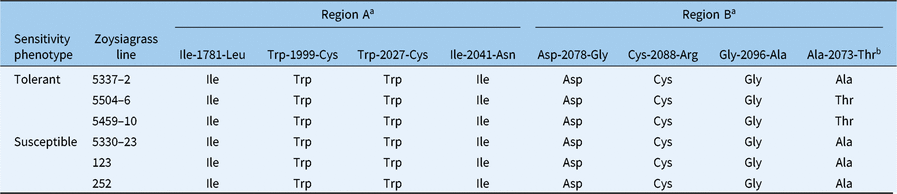
Table 3. Amino acids at eight target-site locations of the ACCase CT domain in tolerant (5337-2, 5504-6, and 5459-10) and susceptible (5330-23, 123, and 252) zoysiagrass lines.

a Region A contains the Ile-1781-Leu substitution, and region B contains the 1999–2096 region within the amino acid sequence of ACCase.
b Codon with mutation not previously reported in the literature.
It was concluded that, for the six genotypes tested, the source of fluazifop-P-butyl tolerance is not due to target-site mutations at any of the seven known sites within the ACCase coding sequence. This was expected, as tolerance observed in our study was considerably lower than the level of resistance conferred by those mutations in other species. Tang et al. (Reference Tang, Zhou, Chen and Zhou2014) reported that in Asia Minor bluegrass (Polypogon fugax Nees ex Steud.), an Ile-2041-Asn substitution led to a fluazifop-P-butyl–resistant genotype with a R/S ratio of 364 in GR50 (R = 182 g ha−1, S = 0.5 g ha−1). A mutant of L. rigidum with a less sensitive form of ACCase has also been reported to have a GR50 for fluazifop-P-butyl >6,000 g ha−1 (Tardif et al. Reference Tardif, Hokum and Powles1993). Eleusine indica with an Asn-2097-Asp substitution was found to increase GR50 up to 150-fold compared with the wild-type biotype (San Cha et al. Reference San Cha, Najihah, Sahid and Chuah2014). Those differences in sensitivity are considerably larger than the observations made from our collection of zoysiagrass lines.
Interestingly, an alanine to threonine mutation was observed at location 2073 in two of the three tolerant lines, 5504-6 and 5459-10, which resulted from a GCT to ACT base pair substitution (Table 3). This mutation was absent in all susceptible lines. To further study whether this mutation is indeed responsible for fluazifop-P-butyl tolerance in zoysiagrass, crosses and heritability studies must be conducted to confirm cosegregation of the mutation and the tolerant phenotype (Haughn and Somerville Reference Haughn and Somerville1986; Souza Machado et al. Reference Souza Machado, Bandeen, Stephenson and Lavigne1978). Because lines 5337-2 and 5504-6 had similar ID50 and GR50, and the former did not have the Ala-2073-Thr substitution, while the latter did, it is possible that this is a null mutation or that both lines have different tolerance mechanisms. Even if this mutation is involved in fluazifop-P-butyl tolerance, the level of tolerance it confers is considerably lower compared with other mutations previously reported in other species (Délye et al. Reference Délye, Zhang, Michel, Matejicek and Powles2005; Devine Reference Devine1997; Liu et al. Reference Liu, Harrison, Chalupska, Gornicki, O’Donnell, Adkins, Haselkorn and Williams2007), which might limit its value for breeding purposes.
Absorption, translocation, and metabolism
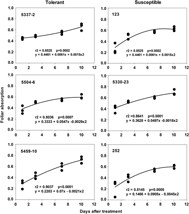
Foliar absorption of radiolabeled fluazifop-P-butyl for all six zoysiagrasses increased during the 10 d after application (Figure 1), but susceptible and tolerant lines exhibited both slow and high uptake rates. Therefore, reduced uptake may not be the sole mechanism for tolerance. However, susceptible line 5330-23 exhibited the fastest absorption rate, reaching 50% absorption 3.1 d after application, while tolerant line 5459-10 took almost twice as long (Figure 1). Thus, this observation suggests that in the case of line 5330-23, high susceptibility could be partially attributed to faster absorption.
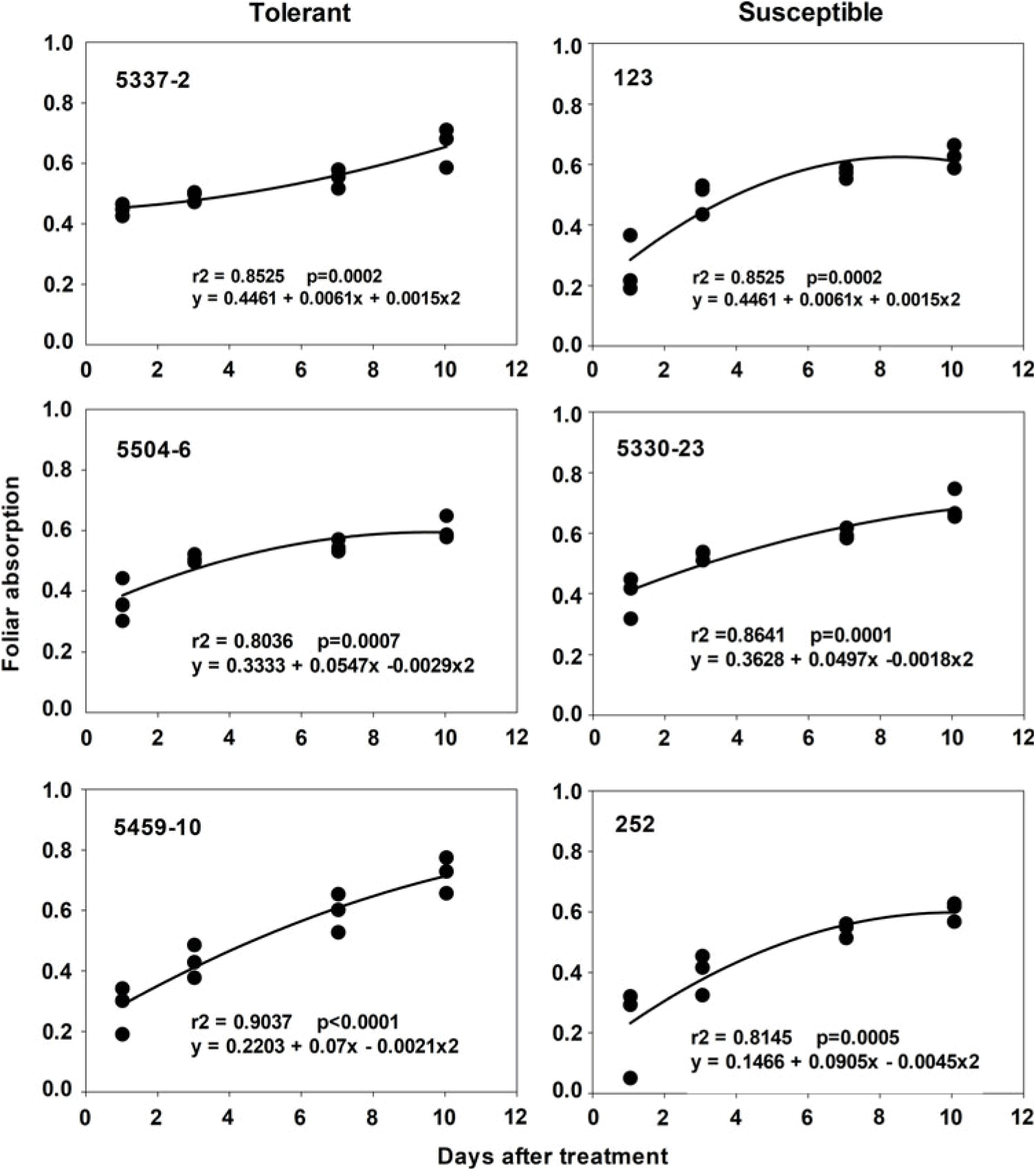
Figure 1. Foliar absorption of [14C]fluazifop-P-butyl in three tolerant and three susceptible zoysiagrass lines. For each plant at each harvest timing, three observations were made. Foliar absorption ranges from 0 to 1, with 0 representing no absorption and 1 representing total absorption based on total radioactivity recovered from plant tissue and leaf washes.
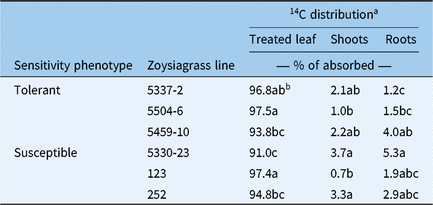
All six evaluated zoysiagrasses retained the majority of 14C in the treated leaf with limited translocation during the 10-d period after application (Table 4). Similar results have been found in quackgrass [Elymus repens (L.) Gould] and green foxtail [Setaria viridis (L.) P. Beauv.], for which more than 90% of absorbed fluazifop-P-butyl remained in the treated leaf (Boydston Reference Boydston1992; Hendley et al. Reference Hendley, Dicks, Monaco, Slyfield, Tummon and Barrett1985). Interestingly, susceptible line 5330-23, which exhibited the highest absorption rate (Figure 1), was also the line that had the highest percentage of absorbed radioactivity translocated out of the treated leaf, with 3.7% and 5.3% of applied radioactivity being recovered in aboveground and belowground tissue, respectively. However, line 123, another susceptible line, had the same level of radioactivity retained in the treated leaf as the tolerant lines (Table 4). This lack of a clear difference between tolerant and susceptible lines could potentially be attributed to the presence of different mechanisms of fluazifop-P-butyl tolerance among zoysiagrass lines, which is likely a consequence of the high genetic diversity present in zoysiagrass germplasm (Goggin et al. Reference Goggin, Kaur, Owen and Powles2018; Kimball et al. Reference Kimball, Zuleta, Kenworthy, Lehman and Milla-Lewis2012; Moore et al. Reference Moore, Zuleta, Patton, Schwartz, Aranaz and Milla-Lewis2017; Patton et al. Reference Patton, Schwartz and Kenworthy2017a; Petit et al. Reference Petit, Duhieu, Boucansaud and Délye2010). Furthermore, it has been reported that multiple non–target site herbicide resistance mechanisms can be simultaneously present in a single individual, explaining the overall resistant phenotype (Petit et al. Reference Petit, Duhieu, Boucansaud and Délye2010).
Table 4. Distribution in plant tissue of absorbed radiolabeled [14C]fluazifop-P-butyl following foliar applications to three tolerant and three susceptible zoysiagrass lines.

a Data were pooled over four harvest timings. Means followed by the same letter are not significantly different at the 5% level using Tukey’s honestly significant difference (HSD) test.
Practical implications
Fluazifop-P-butyl can be used for grass weed control in zoysiagrass establishment. However, the rate necessary to ensure weed control and zoysiagrass safety is largely dependent on the cultivar (Leon et al. Reference Leon, Unruh, Brecke and Kenworthy2014; McElroy and Breeden Reference McElroy and Breeden2006; Patton et al. Reference Patton, Trappe, Doroh and McElroy2017b). Based on our results, fluazifop-P-butyl tolerance could be composed of multiple non–target site (NTS) mechanisms likely controlled by multiple genes that vary among different Zoysia genotypes, and as a result of this, conventional breeding approaches for creating more fluazifop-P-butyl–tolerant zoysiagrass lines will be challenging. If those NTS mechanisms are controlled by multiple genes with small additive effects, even with the use of marker-assisted selection, ensuring high levels of inheritance might be difficult, and a large number of crosses and progeny screening will be needed. Also, it is possible that those NTS genes might exhibit linkage disequilibrium with undesirable traits, increasing the difficulty of generating cultivars with both herbicide tolerance and desirable agronomic and quality traits.
Another challenge is the stability of the tolerance trait. Liu et al. (Reference Liu, MacDonald, Unruh, Kenworthy, Trenholm and Leon2017) reported that fluazifop-P-butyl injury varied depending on the time of the year when the application is made and that the magnitude of this seasonal effect on herbicide susceptibility was also influenced by the cultivar. Even if a line with a single tolerance mechanism were to be generated, the complex nature of fluazifop-P-butyl tolerance may lead to unstable phenotypes due to large genotype by environment interactions, and thereby may limit their commercial value. Therefore, the best strategy for breeding zoysiagrass with improved herbicide tolerance might be through target-site mutations, for example, by introducing in the ACCase gene a point mutation conferring tolerance using either transgenic, gene-editing, or traditional mutagenesis methods (Jander et al. Reference Jander, Baerson, Hudak, Gonzalez, Gruys and Last2003; Shah et al. Reference Shah, Horsch, Klee, Kishore, Winter, Tumer, Hironaka, Sander, Gasser, Aykent, Siegel, Roger and Fraley1986; Sun et al. Reference Sun, Zhang, Wu, He, Ma, Hou, Guo, Du, Zhao and Xia2016). This approach is more convenient than using conventional selection and breeding from germplasm, as was studied here, because of easy identification and fast introduction of desirable traits, and as previously pointed out, tolerance caused by mutations at the target site is usually much higher and more stable than NTS mechanisms such as reduced absorption/translocation and enhanced metabolism.
Finally, the present research illustrates how a species can exhibit different minor additive NTS mechanisms such as translocation rate and compartmentation after absorption that might combine in different ways to provide enough tolerance/resistance to label rates. The fact that multiple NTS tolerance/resistance mechanisms can be present within a population, and that individuals within that population might have different combinations of those mechanisms, has important implications for the study of how natural and human selection operate on NTS resistance evolution in weeds.
Author ORCID
Ramon G. Leon https://orcid.org/0000-0002-1924-3331
Acknowledgments
No conflicts of interests have been declared. This research received no specific grant from any funding agency or the commercial or not-for-profit sectors. The authors thank Syngenta Crop Protection Inc. for providing 14C-radiolabeled fluazifop-P-butyl and Nicole Benda, Mike Dozier, Mike Durham, and Rocio van der Laat for their technical support.