Introduction
Among warm-season grasses used for lawns and sport fields, zoysiagrass (Zoysia spp.) stands out for its large genetic variation for traits such as shade, heat, and drought tolerance. Zoysiagrass is native to China, Japan, and other parts of Southeast Asia, but it has been in the United States since the late 1800s. Currently, there are two primary species of zoysiagrass commonly used for turf: Zoysia japonica Steud., which exhibits coarse-textured, wide rigid leaves, and Zoysia matrella (L.) Merr., which has very narrow flexible leaves that provide a fine texture. Besides these two, there is also Zoysia matrella (L.) var. pacifica Goudsw., the finest-textured and densest zoysiagrass, which usually can be seen in Asian-themed gardens as a puffy turf surface (Patton et al. Reference Patton, Schwartz and Kenworthy2017; Unruh et al. Reference Unruh, Trenholm and Cisar2005). In addition to texture, these two species differ in cold tolerance and aggressiveness; Z. japonica has a faster growth rate and exhibits excellent cold tolerance. Both Z. matrella and Z. matrella var. pacifica have good wear tolerance and adapt to conditions from the Southeast region up to the northern transitional zone of the United States (Patton et al. Reference Patton, Schwartz and Kenworthy2017; Unruh et al. Reference Unruh, Trenholm and Cisar2005).
Broadleaf weeds are considered easy to control in zoysiagrass, as there are many herbicides registered for selective control and suppression. Moreover, mechanical mowing and manual weeding can also efficiently subject broadleaf weeds to a competitive disadvantage against the turfgrass. Conversely, warm-season grassy weeds such as bermudagrass (Cynodon spp.) and crabgrass (Digitaria spp.) are considerably more difficult to manage, because there are only a few selective herbicides providing effective control without causing significant injury to zoysiagrass. Furthermore, most of those selective herbicides are either PRE or POST and only control small plants. Thus, selective systemic herbicides that can effectively control larger annual or creeping perennial grasses are needed to ensure zoysiagrass purity over time.
Acetyl-coenzyme A carboxylase (ACCase) is the target site for cyclohexanedione and aryloxyphenoxypropionate (AOPP) herbicides (Burton et al. Reference Burton, Gronwald, Somers, Gengenbach and Wyse1989; Rendina et al. Reference Rendina, Felts, Beaudoin, Craig-Kennard, Look, Paraskos and Hagenah1988). ACCase catalyzes the ATP-dependent carboxylation of acetyl-CoA to form the precursor for fatty-acid biosynthesis. In susceptible plants, such as monocotyledonous grasses, fluazifop-P-butyl inhibits ACCase. This inhibition leads to the depletion of cellular malonyl CoA, blocking fatty-acid and lipid formation (Burton et al. Reference Burton, Gronwald, Somers, Gengenbach and Wyse1989; Carr et al. Reference Carr, Davies, Cobb and Pallet1985).
Variation in response to ACCase herbicides in both weed and turfgrass species is of great interest. Studies have been conducted to characterize the tolerance to ACCase herbicides in other turfgrass species, including Cynodon spp., seashore paspalum (Paspalum vaginatum Sw.), tall fescue [Lolium arundinaceum (Schreb.) Darbysh.], and centipedegrass [Eremochloa ophiuroides (Munro) Hack] (Heckart et al. Reference Heckart, Parrott and Raymer2010; Johnson Reference Johnson1987; Johnson and Duncan Reference Johnson and Duncan1997; McCalla et al. Reference McCalla, Richardson, Karcher and Boyd2004; Unruh et al. Reference Unruh, Stephenson, Brecke and Trenholm2006). However, those studies were intended to identify a safe label rate for each species (in most cases unsuccessful) and not to identify lines that could be used to enhance the tolerance to the herbicide through breeding. If tolerance to fluazifop-P-butyl can be introduced into a turfgrass species, systemic selective control of a wide range of grassy weeds may be achievable without injuring the turfgrass. The inheritance of the variation in response to herbicides has been studied in several species other than turfgrass. Sources of variation in tolerance to herbicides have been identified as either resulting from a single major dominant (Grogan et al. Reference Grogan, Eastin and Palmer1963) or recessive (Hayes et al. Reference Hayes, Pfeiffer and Rana1965) allele. Also, multiple genes, each partially contributing to the overall phenotype, have been documented conferring tolerance. For example, siduron tolerance in foxtail barley (Hordeum jubatum L.) was found to be controlled by at least three dominant complementary major genes (Schooler et al. Reference Schooler, Bell and Nalewaja1972). Quantitative patterns of variation in response to herbicides were also observed in common flax (Linum usitatissimum L.), when exposed to atrazine and MCPA (Comstock and Andersen Reference Comstock and Andersen1968; Stafford et al. Reference Stafford, Comstock and Ford1968), although this variation was concluded to be mainly due to environmental factors. Quantitative variation in herbicide tolerance has also been reported in perennial ryegrass (Lolium perenne L.) when treated with several herbicides (Wright Reference Wright1968).
Development of genotypes with increased tolerance to ACCase-inhibiting herbicides would allow selective POST control of annual and perennial grass weeds in zoysiagrass turf. However, this requires identifying ACCase tolerance variation in germplasm that can be used for breeding. A previous study screening 27 experimental lines of zoysiagrass (Liu et al. Reference Liu, MacDonald, Unruh, Kenworthy, Trenholm and Leon2019a) identified zoysiagrass genotypes exhibiting up to 10-fold difference in biomass reduction and greater than 4-fold difference in percent injury levels when treated with fluazifop-P-butyl. This wide variation of this desirable trait may facilitate the development of new AOPP-tolerant varieties. Identifying and crossing lines with high tolerance would permit segregation of lines that tolerate label or higher than label rates that could then be generated and selected. Liu et al. (Reference Liu, MacDonald, Unruh, Kenworthy, Trenholm and Leon2019a) suggested that multiple non–target site mechanisms may be responsible for the tolerance observed in zoysiagrass, but their research focused on physiological and biochemical factors, and the inheritance of the tolerance was not studied.
The genetics of quantitative resistance are difficult to study, as the effect of each gene is small and often influenced by the environment or by epistatic interactions with other genes (Carlborg and Haley Reference Carlborg and Haley2004; Huang et al. Reference Huang, Richards, Carbone, Zhu, Anholt, Ayroles, Duncan, Jordan, Lawrence, Magwire, Warner, Blankenburg, Han, Javaid, Jayaseelan, Jhangiani, Muzny, Ongeri, Perales, Wu, Zhang, Zou, Stone, Gibbs and Mackay2012). By crossing genotypes with contrasting levels of herbicide tolerance, it is possible to generate a progeny population segregating for the tolerance trait. The pattern of segregation can be used to evaluate genetic models involved in the control of the trait. This approach for genetic evaluation assumes that genotypic variation can be distinguished from environmental variation and that the quantitative effects of each gene are similar and additive (Lindhout Reference Lindhout2002). The main hypothesis of the present study was that fluazifop-P-butyl tolerance is a quantitative trait controlled by multiple genetic factors. It was also hypothesized that segregating populations will exhibit a wide range of tolerance levels within the phenotypes of the parental lines. The present research was conducted under the premise that a better understanding of the genetic mechanisms involved in tolerance to ACCase will help inform the design of breeding strategies to incorporate this tolerance into advanced lines of zoysiagrass.
Materials and Methods
Plant Material
Three Zoysia spp. lines from the University of Florida zoysiagrass germplasm collection and previously characterized by Liu et al. (Reference Liu, MacDonald, Unruh, Kenworthy, Trenholm and Leon2019a) as fluazifop-P-butyl tolerant (5337-2, 5459-10, and 5504-6) and three more-sensitive lines (123, 252, and 5330-23), here arbitrarily referred to as susceptible, were propagated from field plots and transferred to controlled conditions (28 ± 5 C, 75% to 85% relative humidity, and natural illumination providing a 12- to 14-h photoperiod) in 2017 and 2018. These parental lines were planted in 28 cm by 54 cm by 6 cm trays and maintained under greenhouse conditions with adequate fertilization, daily irrigation, and weekly mowing at 3.5 cm. Each tolerant line was crossed with each susceptible line in a full pair-wise approach including reciprocal crosses to explore any potential maternal inheritance. Viable seeds were harvested from all crosses; however, there were only six crosses that yielded enough progeny to properly evaluate segregation patterns: 5337-2 × 123 and reciprocal, 5337-2 × 252 and reciprocal, and 5337-2 × 5330-23 and reciprocal. Seeds were germinated in growth chambers, and seedlings were transplanted to trays and maintained in the greenhouse as previously described. F1 lines were then propagated into PVC containers (3.8-cm diameter by 21-cm depth) and maintained under the same greenhouse environment as the parents until their canopy completely covered the soil surface of each container.
Fluazifop-P-Butyl Susceptibility Evaluation
Mowing was ceased 1 wk before herbicide application to allow enough biomass to accumulate for injury and growth assessments. F1 lines and respective parental lines were treated with fluazifop-P-butyl (Fusilade® II, Syngenta Crop Protection, Greensboro, NC) at 176 g ai ha−1 in a spray chamber calibrated to deliver 187 L ha−1, which is 2X the labeled rate for weed control in zoysiagrass (Anonymous 2009). This rate was selected because it allows visual estimation of the differences in fluazifop-P-butyl injury without causing plant death. Also, it allows the quantification of growth reduction (Liu et al. Reference Liu, MacDonald, Unruh, Kenworthy, Trenholm and Leon2019a). For each line, a nontreated control was maintained for comparison. Percent injury was estimated visually at 3 and 6 wk after treatment (WAT). Injury symptoms included leaf chlorosis, purpling, and necrosis, as well as plant stunting. At 6 WAT, clippings were collected 1.7 mm above the soil surface and dried at 60 C for 5 d to determine dry biomass. Percent biomass growth reduction (GR) was calculated by dividing the collected biomass of treated plants by nontreated plants, and then deducting the result from 1. This experiment had three replications and was repeated to ensure reproducibility of results.
Data Analysis
Data were analyzed as a completely randomized design with six replications. Because there were no crossover interactions between experimental run and treatments, the analyses were conducted by pooling data from both experimental runs. Both GR and percent injury data from each zoysiagrass F1 and parental line were analyzed with PROC GLIMMIX in SAS (SAS Institute, Cary, NC), considering cross as a fixed effect (Carmer et al. Reference Carmer, Nyquist and Walker1989). In this way, all crosses were compared, allowing identification of any reciprocal or maternal effects. Also, F1 trait segregation patterns were visualized using histograms, and multiple genetic inheritance models exploring different expected segregation patterns were evaluated and tested with the chi-square test (α = 0.05). Inheritance models included single, two, three, and four loci, considering two alleles per locus and testing for fluazifop-P-butyl tolerance as a dominant, codominant, or recessive allele.
Results and Discussion
Generation of F1 Lines
Reciprocal crosses were made between susceptible and tolerant parents; however, pollination between certain lines was not successful due to infertility or low pollen production. In total, 250 F1 lines were generated: 30 from 5337-2 × 123, 29 from 123 × 5337-2, 29 from 5337-2 × 252, 26 from 252 × 5337-2, 38 from 5337-2 × 5330-23, 92 from 5330-23 × 5337-2, 3 from 5504-6 × 5330-23, and 3 from 5504-6 × 252. F1 lines from all these crosses were later evaluated for fluazifop-P-butyl sensitivity, with the exception of the latter two crosses because of limited progeny.
Transgressive Segregation
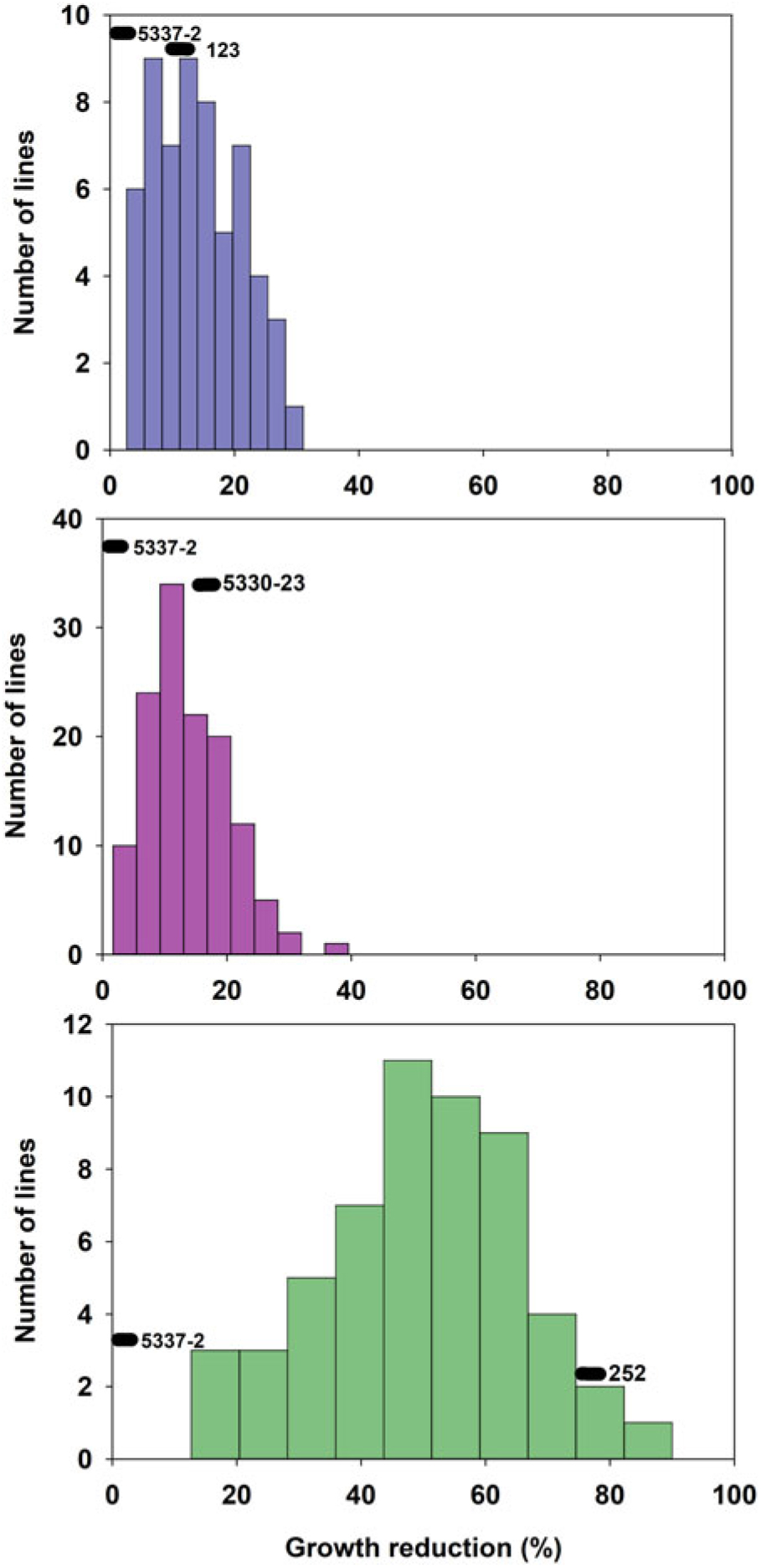
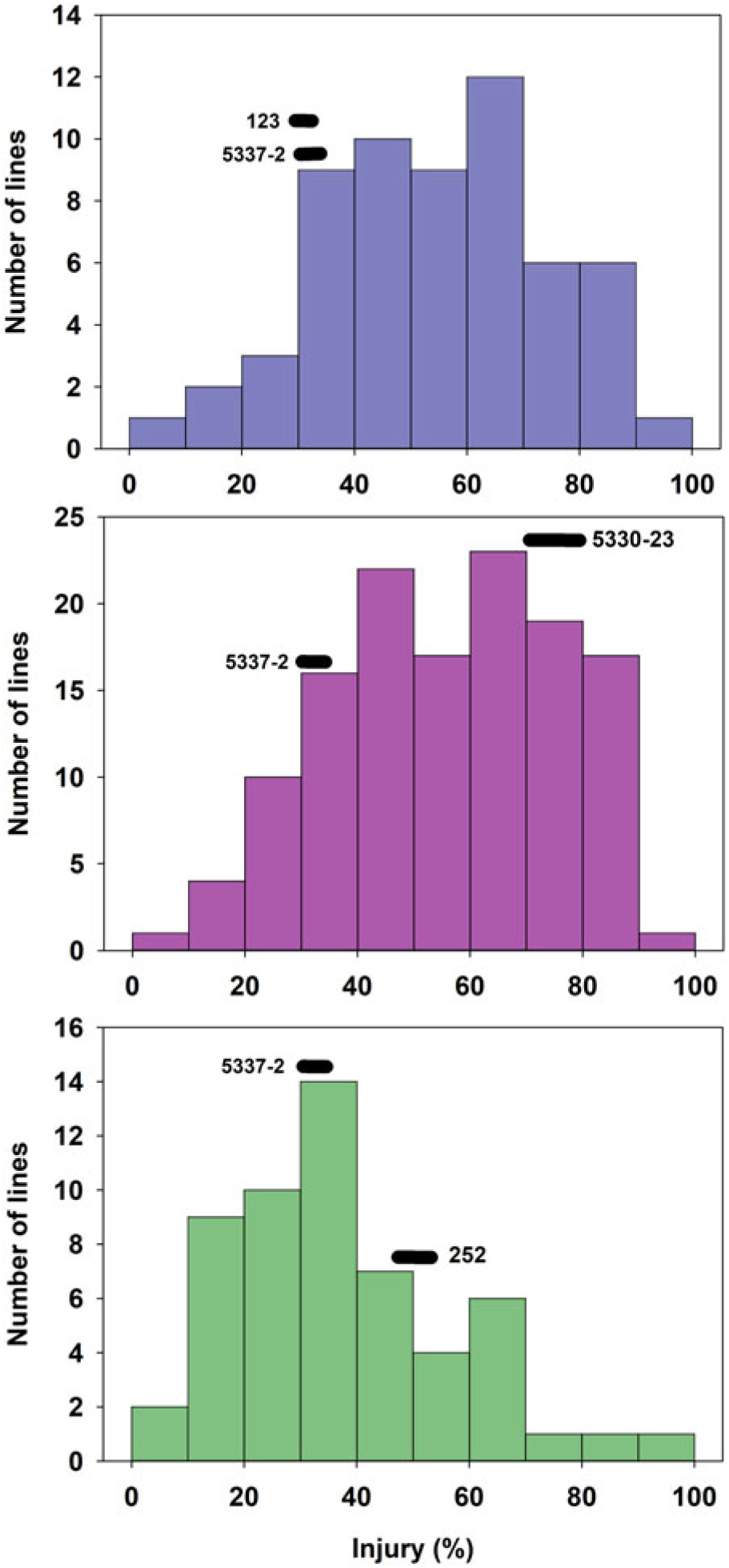
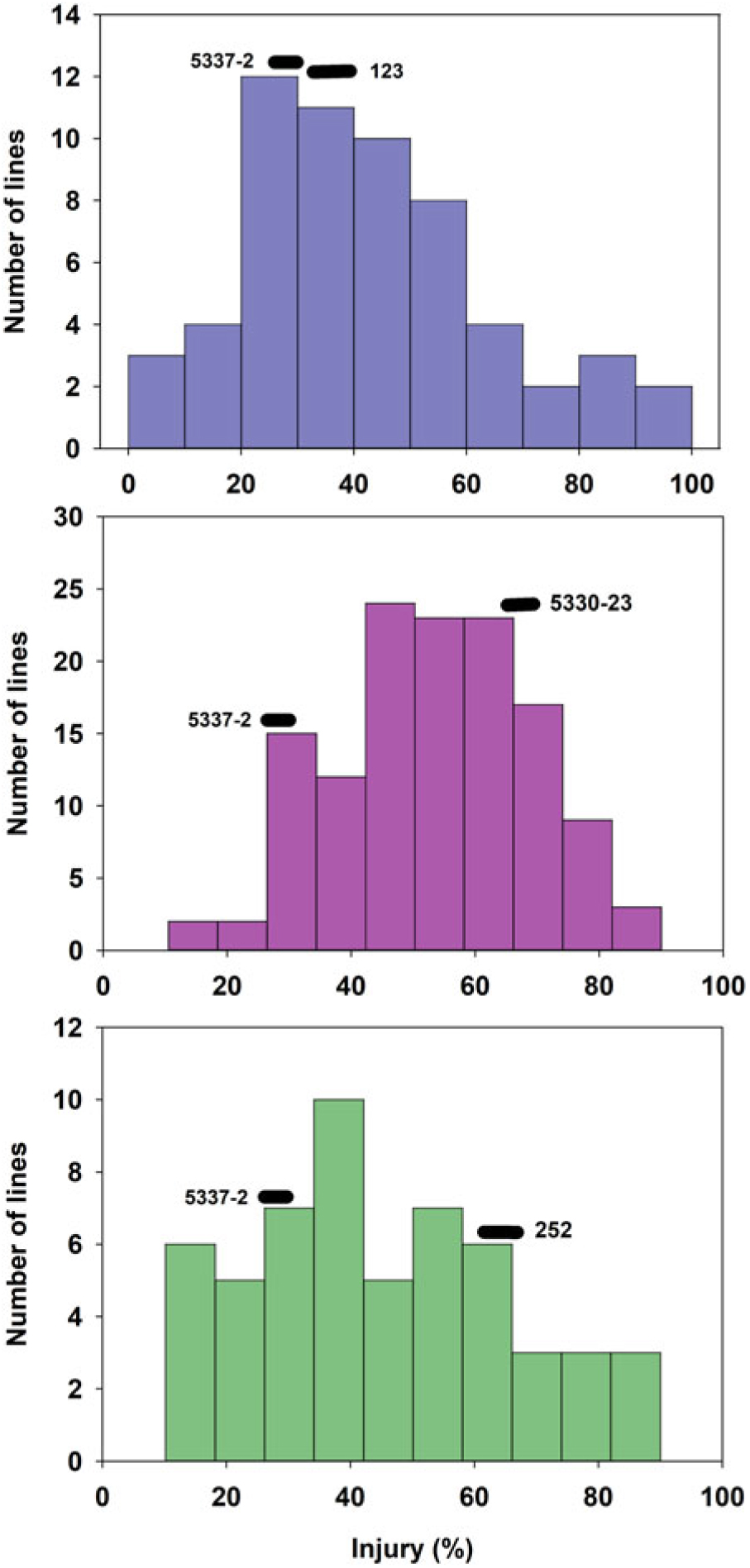
The tolerant parental line 5337-2 exhibited less than 5% GR, while the susceptible parental lines 123, 5330-23, and 252 suffered 10%, 18%, and 75% GR, respectively (Figure 1). It is worth noting that Liu et al. (Reference Liu, MacDonald, Unruh, Kenworthy, Trenholm and Leon2019a) determined that the tolerance to fluazifop-P-butyl in 5337-2 was not the result of any known resistance mutations in the ACCase gene and was likely due to non–target site mechanisms, although those researchers did not find any major differences in fluazifop-P-butyl metabolism between this line and susceptible lines. Although differences between the tolerant and susceptible lines were also detected for injury, those differences did not follow the same trend as for GR. For example, the tolerant parent 5337-2 suffered up to 35% injury at 3 WAT (Figure 2), but injury was decreasing (28%) by 6 WAT (Figure 3). The parental line 123 had similar injury to 5337-2 at 3 WAT (35%), but the former had slightly increased injury (40%) at 6 WAT. The susceptible line 252, which had the highest GR among parental lines (Figure 1), only suffered intermediate injury, while 5330-23, which suffered intermediate GR, exhibited the highest injury among parental lines, reaching 78% and 67% at 3 and 6 WAT, respectively (Figures 2 and 3). The fact that the ranking in sensitivity to fluazifop-P-butyl among parental lines differed for injury and GR suggested that the genetic mechanisms controlling these two responses might be different.
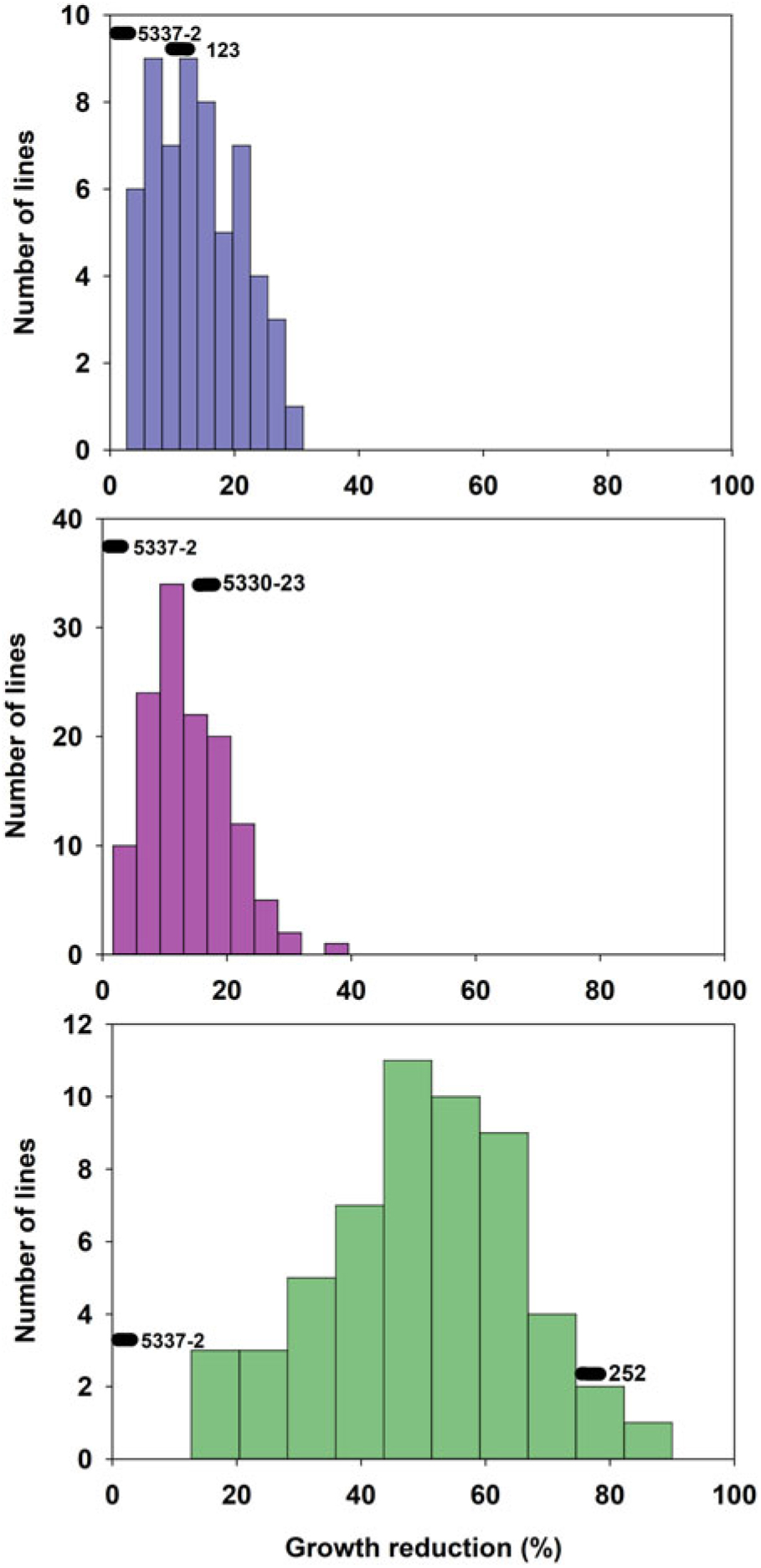
Figure 1. Frequency distribution of 244 zoysiagrass F1 lines from crosses of a tolerant line (5337-2) and three lines (123, 252, and 5330-23) varying in susceptibility to fluazifop-P-butyl based on growth reduction determined by clippings collected at 6 wk after treatment in response to fluazifop-P-butyl (176 g ai ha−1) under greenhouse conditions in Gainesville, FL, averaged across three replications and two experimental runs.
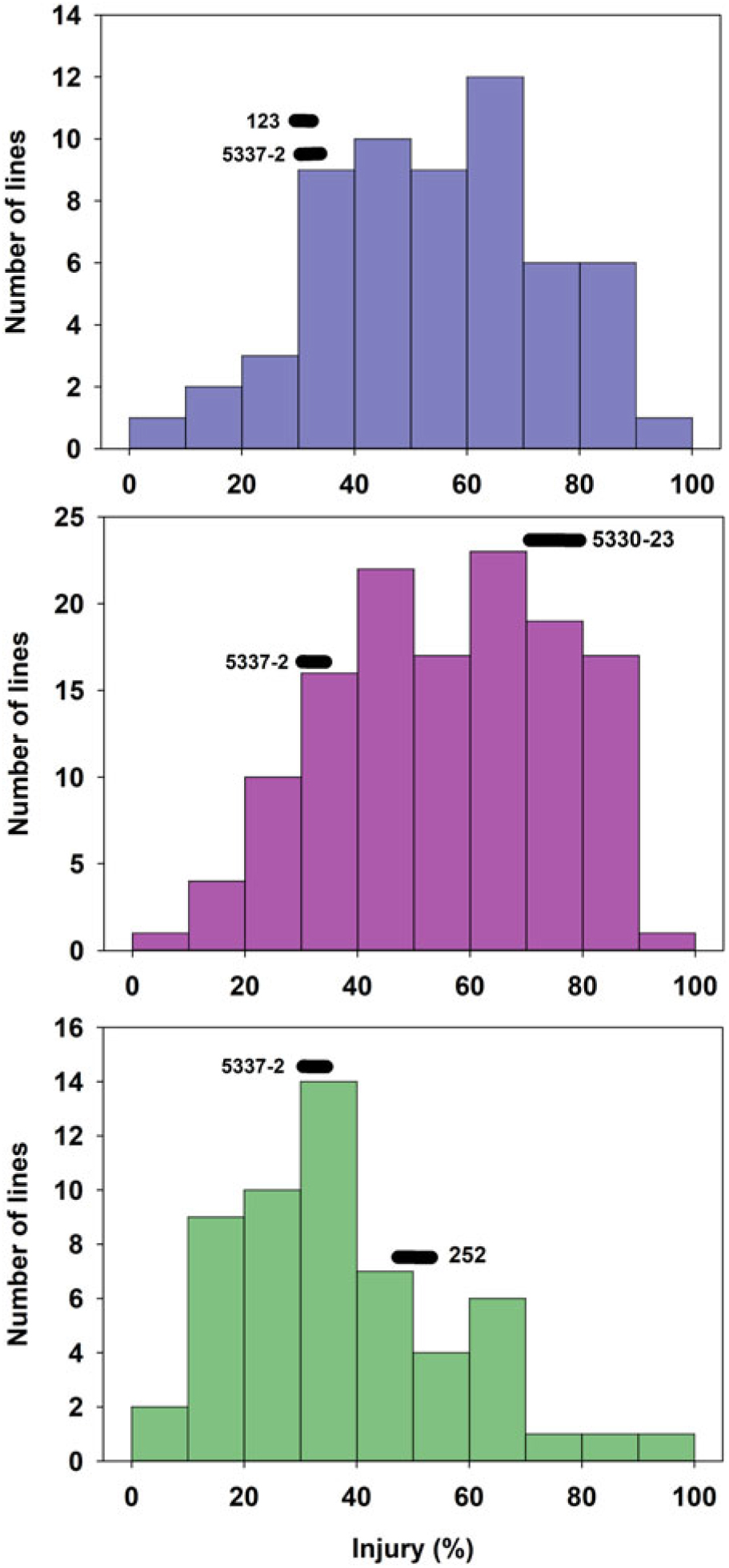
Figure 2. Frequency distribution of 244 zoysiagrass F1 lines from crosses of a tolerant line (5337-2) and three lines (123, 252, and 5330-23) varying in susceptibility to fluazifop-P-butyl based on injury at 3 wk after treatment with fluazifop-P-butyl (176 g ai ha−1) under greenhouse conditions in Gainesville, FL, averaged across three replications and two experimental runs.

Figure 3. Frequency distribution of 244 zoysiagrass F1 lines from crosses of a tolerant line (5337-2) and three lines (123, 252, and 5330-23) varying in susceptibility to fluazifop-P-butyl based on injury at 6 wk after treatment with fluazifop-P-butyl (176 g ai ha−1) under greenhouse conditions in Gainesville, FL, averaged across three replications and two experimental runs.
GR and injury in the progeny ranged from 1% to 95% and 0% to 100%, respectively (Figures 1–3). GR of F1 lines from 5337-2 × 123 and 5337-2 × 5330-23 was consistently below 40%, while F1 lines from 5337-2 × 252 had GR values ranging from 0 to 95 (Figure 1; Table 1). A large proportion of F1 lines from 5337-2 × 123 were transgressive segregants for GR and injury, with almost two-thirds of the F1 lines suffering more GR and injury at 6 WAT than the susceptible parental line 123. For 5337-2 × 5330-23 and 5337-2 × 252, approximately 25% of the F1 lines were transgressive segregants. For all families, the majority of transgressive segregation exhibited higher susceptibility than parental lines, but at 3 WAT a small number of lines had lower injury than the tolerant parental line 5337-2. However, at 6 WAT the low level of GR and injury observed in 5337-2 reduced the level of transgressive segregation of F1 lines.
Table 1. Differences in response to fluazifop-P-butyl (176 g ai ha−1) between zoysiagrass progeny from different crosses (reciprocal crosses were pooled) and between progeny from each reciprocal cross. a

a Experiments were performed under greenhouse conditions in Gainesville, FL.
b Growth reduction (in percent based on a nontreated control) was determined by clippings collected at 6 wk after treatment (WAT); percent injury was visually rated at 3 and 6 WAT.
c Tolerant line 5337-2 and three lines (123, 252, and 5330-23) varying in susceptibility.
Dominance
Although Zoysia spp. are allotetraploids (Patton et al. Reference Patton, Schwartz and Kenworthy2017), simplified genetic inheritance models can help describe the dominance nature of a trait of interest. In this case, we assumed that fluazifop-P-butyl tolerance is determined by two alleles per locus, but multiple loci might be involved in the control of this trait. As Figures 1–3 clearly show, the frequency of phenotypes for F1 lines generated by the different crosses suggests that fluazifop-P-butyl tolerance is expressed as a quantitative trait in Zoysia spp. as originally hypothesized. However, there were different levels of dominance between the tolerant and susceptible phenotypes depending on the parental lines (Table 2). For example, assuming the simplest model of a single gene or a single major quantitative trait locus (QTL) with both parental lines being heterozygotes, the phenotypic distribution of the F1 lines of the crosses 5337-2 × 5330-23 and 5337-2 × 252 matched the expected ratios of a dominant allele for tolerance for injury at 3 and 6 WAT (Table 2). Conversely, for 5337-2 × 123 the model that best matched the phenotypic frequencies was the one in which the tolerance allele is recessive in relation to the susceptible allele, and this was true for both injury and for GR. Other more complex models also fit the F1 lines’ phenotypic distribution of this latter cross, including: (1) two loci with codominant alleles with a heterozygote parent for tolerance and a homozygote recessive susceptible parent and (2) each parent carrying a single tolerance allele in one of the loci reciprocally. No other models, including three and four loci, were significant (unpublished data), and this was due to the continuous nature of the phenotypic differences among F1 lines. The fact that crosses differed not only for genetic inheritance patterns but also for dominant/recessive relationship between tolerance and susceptibility to fluazifop-P-butyl is a strong indication that the parental lines studied have different genetic mechanisms controlling this trait.
Table 2. Chi-square tests for genetic inheritance models for crosses of a tolerant line (5337-2) and three lines (123, 252, and 5330-23) varying in susceptibility to fluazifop-P-butyl based on growth reduction determined by clippings collected at 6 wk after treatment (WAT), injury at 3 WAT, and injury at 6 WAT in response to fluazifop-P-butyl (176 g ai ha−1) under greenhouse conditions in Gainesville, FL, averaged across three replications and two experimental runs. a

a Frequency of phenotypes was evaluated considering transgressive F1 lines (i.e., tails of histograms outside the parental phenotypic range) the most tolerant or most susceptible phenotypes. In most cases, these transgressive lines were grouped with the parental lines.
Maternal Inheritance
The results also indicated that genetic differences in fluazifop-P-butyl sensitivity are not exclusively dependent on the nuclear genome, and extranuclear regulatory mechanisms (i.e., maternal/cytoplasmic inheritance) might also influence the inheritance of this trait (Hatfield et al. Reference Hatfield, Shoemaker and Palmer1985; Reboud and Zeyl Reference Reboud and Zeyl1994; Scott and Putwain Reference Scott and Putwain1981). Thus, reciprocal effects were found for GR in the cross 5337-2 × 5330-23, and for injury at both 3 WAT and 6 WAT in the cross 5337-2 × 252. When the susceptible lines 5330-23 and 252 were the pollen source, this led to greater GR and injury, respectively, than when they were the maternal parent (Table 1). This might suggest that organelles, likely chloroplasts, where the plastidic ACCase form plays a major role in fatty-acid biosynthesis, can at least partially influence the tolerance to fluazifop-P-butyl. Thus, nuclear and extranuclear genetic factors in 5337-2 might have complementary roles in determining the level of tolerance, but the former might play a more important role than the latter. Organellar and nuclear genomes in plants interact to modulate gene expression. These interactions occur as part of normal growth and metabolic processes and also in response to stresses, including those that generate reactive oxygen species (Woodson and Chory Reference Woodson and Chory2008), and can increase the injury caused by fluazifop-P-butyl (Liu et al. Reference Liu, Li, Sun, Zhou, Yang, Li, Matsumoto and Luo2017).
Practical Implications
Based on the extensive segregation observed in F1 progeny and the differences between injury and GR phenotypes (Figures 1–3), it was concluded that fluazifop-P-butyl tolerance in zoysiagrass is a quantitative trait likely affected by multiple genes controlling different physiological processes (Tables 1 and 2).
The results suggested that the large genetic diversity present in zoysiagrass can be used in breeding programs to develop cultivars with enhanced tolerance to fluazifop-P-butyl. Under this scenario, it might be possible to develop a new zoysiagrass variety that not only maintains biomass but also exhibits low injury when exposed to fluazifop-P-butyl. Because the tolerance is a polygenic trait, research should be conducted to identify the responsible QTLs to enable the use of marker-assisted selection and avoid the need to regularly conduct herbicide-sensitivity screenings (Délye et al. Reference Délye, Gardin, Boucansaud, Chauvel and Petit2011; Gupta et al. Reference Gupta, Langridge and Mir2010; Schneider et al. Reference Schneider, Brothers and Kelly1997). However, as with most traits controlled by multiple mechanisms, environmental conditions also inevitably play a role in the outcome of herbicide applications, and fluazifop-P-butyl sensitivity in zoysiagrass has been documented to vary depending on the environment and time of the year (Liu et al. Reference Liu, Unruh, Kenworthy, MacDonald, Trenholm and Leon2019b). This significantly increases the difficulty of breeding a commercial line that performs predictably under variable environmental conditions or during different times of the year. Therefore, screening for naturally occurring, single base mutations in the ACCase target site that conferred high levels of tolerance may be a more cost-effective strategy for developing tolerant lines for commercial use, as single mutations at the target site have been reported to have the potential of conferring resistance to multiple herbicides targeting the same enzyme (Bernasconi et al. Reference Bernasconi, Woodworth, Rosen, Subramanian and Siehl1995; Gressel and Valverde Reference Gressel and Valverde2009; Heckart et al. Reference Heckart, Parrott and Raymer2010; Whaley et al. Reference Whaley, Wilson and Westwood2007). Breeding that leads to the release of commercial lines with this tolerance trait would allow for the use of higher rates of fluazifop-P-butyl (if the benefit balances out the cost of label changes), ensuring effective control of grassy weeds, including both common and hybrid Cynodon spp., all accomplished without sacrificing the aesthetic value or growth rate of zoysiagrass.
Author ORCIDs
Ramon G. Leon https://orcid.org/0000-0002-1924-3331
Acknowledgments
No conflicts of interests have been declared. This research received no specific grant from any funding agency or the commercial or not-for-profit sectors. The authors thank Nicole Benda, Mike Dozier, and Rocio van der Laat for their technical support.