Palmer amaranth is an annual weed of great economic significance in the southeastern United States. Infestation has been reported to cause severe yield loss in many cropping systems (Bensch et al. Reference Bensch, Horak and Peterson2003; Burke et al. Reference Burke, Schroeder, Thomas and Wilcut2007; Rowland et al. Reference Rowland, Murray and Verhalen1999). Furthermore, the ability of this species to prolifically produce seed favors its propagation and dispersal of germplasm that is the result of genetic recombination (Keeley et al. Reference Keeley, Carter and Thullen1987). The selection pressure associated with overreliance on glyphosate to control Palmer amaranth coupled with the aforementioned abundant seed production have resulted in rapid spread of glyphosate-resistant biotypes of this weed (Culpepper et al. Reference Culpepper, Grey, Vencill, Kichler, Webster, Brown, York, Davis and Hanna2006; Norsworthy et al. Reference Norsworthy, Griffith, Scott, Smith and Oliver2008; Scott et al. Reference Scott, Steckel, Smith, Mueller, Oliver and Norsworthy2007; York et al. Reference York, Whitaker, Culpepper and Main2007). There are no costs of fitness associated with seed production for the glyphosate-resistant biotype (Webster and Grey Reference Webster and Grey2015). Increasing resistance to protoporphyrinogen oxidase–inhibiting (WSSA Group 14) herbicides has been further complicating options for potential chemical control of Amaranthus for agronomists, particularly in the mid-South in Palmer amaranth (Giacomini et al. Reference Giacomini, Umphres, Nie, Mueller, Steckel, Young, Scott and Tranel2017; Shoup et al. Reference Shoup, Al-Khatib and Peterson2003). Solely adopting the use of WSSA Group 14 herbicides for Palmer amaranth control in response to glyphosate resistance could theoretically increase the occurrence of resistance to protoporphyrinogen oxidase–inhibiting herbicides in this species. Therefore, combining the use of herbicides with several different mechanisms of action for Palmer amaranth control by including chemistries such as auxinic herbicides (WSSA Group 4) is a valuable resistance management strategy.
Auxinic herbicides were the first class of selective chemical weed control agents in agriculture, used for broadleaf weed control in cereal crops (Grossmann Reference Grossmann2000; Norman et al. Reference Norman, Minarik and Weintraub1950). The first commercially available herbicide with this mechanism of action, 2,4-D, was introduced to the market about 70 yr ago. Since then, many structural analogues of herbicides of this class have been discovered and introduced into various agronomic applications. Along with such diversification of this mechanism of action has come a wider weed control spectrum, increasing the utility and importance of this group of herbicides across many different agronomic systems (Cobb and Reade Reference Cobb and Reade2010). The most current improvements to these chemistries include the introduction of new salts and products labeled for use on WSSA Group 4 herbicide–resistant crops. The new choline salt of 2,4-D, contained in the combination product Enlist Duo™, is now labeled for application on Enlist™ corn (Zea mays L.), soybean [Glycine max (L.) Merr.], and cotton (Gossypium hirsutum L.) (Anonymous 2017). In addition, the new diglycolamine salt of dicamba in XtendiMax™ with VaporGrip™ Technology is now labeled for use on Roundup Ready 2 Xtend™ crops (Anonymous 2015b). Another new salt of dicamba is the N,N-Bis-(3-aminopropyl)methylamine salt formulated for the Engenia™ herbicide (Anonymous 2016). New salts of WSSA Group 4 herbicides such as these are formulated to result in reduced volatility and drift potential, which are problematic with these chemistries. These improvements will likely lead to increased use of these herbicides, particularly with the advent of resistance to other mechanisms of action.
In many large-scale systems involving WSSA Group 4 herbicides, herbicide applications must be made at various times of the day. Agronomists may apply herbicides very early or late in the day when temperatures and sunlight intensity are reduced in order to increase the time of spray droplet retention on leaves prior to evaporation, or because farm size may require that applications be made at these times of day due to general time constraints (Prasad et al. Reference Prasad, Foy and Crafts1967; Sellers et al. Reference Sellers, Smeda and Johnson2003). However, weed control resulting from auxinic herbicide use has been shown to vary depending upon time of application; this has been reported to occur with members of the phenoxyalkanoic, benzoic, and pyridinecarboxylic acid chemical classes that comprise the majority of WSSA Group 4 herbicides (Bovey et al. Reference Bovey, Haas and Meyer1972; Skuterud et al. Reference Skuterud, Bjugstad, Tyldum and Semb Tørresen1998; Stewart et al. Reference Stewart, Nurse and Sikkema2009; Weaver and Nylund Reference Weaver and Nylund1963). The causes of variation in weed control related to application time of day are not well understood, partially due to the complexity of this particular mechanism of action (Song Reference Song2014). Any reduced efficacy of WSSA Group 4 herbicides caused by this phenomenon is of concern due to potential for yield loss, selection for resistance, or other adverse consequences resulting from compromised weed control. Investigation into the mechanisms conferring time-of-day effects are thus warranted to better understand the potential for this phenomenon and ways to counter it in agronomic systems.
Translocation has been correlated with phytotoxicity in previous research with WSSA Group 4 herbicides and members of other herbicide mechanisms of action (Beriault et al. Reference Beriault, Horsman and Devine1999; Geiger and Bestman Reference Geiger and Bestman1990; Goggin et al. Reference Goggin, Cawthray and Powles2016). It is therefore of interest whether or not herbicide translocation and phytotoxicity are processes that are affected by time of application, and if so, by what mechanisms. Manipulating transport of auxins has been reported to also influence the translocation and activity of herbicides in the WSSA Group 4 mechanism of action, particularly 2,4-D (Goggin et al. Reference Goggin, Cawthray and Powles2016). The auxin translocation inhibitors 2,3,5-triiodobenzoic acid (TIBA) and 5-[N-(3,4-dimethoxyphenylethyl)methylamino]-2-(3,4-dimethoxyphenyl)-2-isopropylvaleronitrile hydrochloride (verapamil) are known to inhibit the action of auxin transporters of the PIN family and ATP-binding cassette subfamily B family (ABCB), respectively (Shukla et al. Reference Shukla, Ohnuma and Ambudkar2011; Zhu and Geisler Reference Zhu and Geisler2015). Diflufenzopyr (DFFP), a synthetic semicarbazone, is combined with dicamba in the product Distinct® for use in corn, pastures, and rangeland and blocks movement of both natural plant auxins and dicamba (Anonymous 2015a). It has been suggested that DFFP may improve herbicide efficacy by limiting translocation to growing points and meristematic tissue, thereby reducing regrowth following herbicide application (Bowe et al. Reference Bowe, Landes, Best, Schmitz and Graben1999; Lym and Deibert Reference Lym and Deibert2005). This takes place presumably through inhibition of PIN proteins via binding to proteins known to interact with the phytotropin naphthylphthalamic acid, itself an auxin transport inhibitor (Brunn et al. Reference Brunn, Subramanian, Walters, Patel and Reagan1994; Grossmann et al. Reference Grossmann, Caspar, Kwiatkowski and Bowe2002; Hess et al. Reference Hess, Subramanian, Brunn and Jain1998; Subramanian et al. Reference Subramanian, Bernasconi, Patel and Reagan1997). Treating plants with translocation inhibitors such as TIBA, verapamil, and DFFP and then applying Group 4 herbicides at different times of day may grant insights into the role of translocation behind the time-of-day effect, particularly by observing any resulting alterations in efficacy. The purpose of this research was to investigate the effect of time of application on both dicamba and 2,4-D translocation, and how different application timings and translocation inhibitors influence dicamba efficacy on Palmer amaranth.
Materials and Methods
Absorption and Translocation Experiments
Experiments using radiolabeled herbicides were conducted in Tifton, GA, from August to October 2013. Palmer amaranth seed collected in Macon County, GA, was sown in the greenhouse in 12.7-cm-diameter cups with a 17.8-cm depth filled with a 3:1 potting mix (Scotts Miracle-Gro Company, 14111 Scottslawn Road, Marysville, OH 43040) to sand mixture. Plants were thinned within 1 wk of emergence, and individual plants were allowed to grow to the 3- to 5-leaf stage (12- to 14-cm tall) prior to herbicide treatments. Supplemental light was provided in the greenhouse at 400 μmol m−2 s−1 to ensure plants received 14 h of light per day. Daytime greenhouse temperatures ranged from 29 to 32 C, and nighttime temperatures ranged from 18 to 21 C. Relative humidity in the greenhouse ranged from 75% to 95%.
Applications of dicamba and 2,4-D were made at 7 AM (sunrise), 2 PM (6 h after sunrise), and 12 AM (6 h before sunrise). At each application time, corresponding plants had the most acropetal fully expanded leaf covered with a plastic sheath prior to nonlabeled herbicide application. The 2,4-D (Weedar® 64, 0.455 kg ae L−1, Nufarm Americas, 11901 South Austin Avenue, Alsip, IL 60803) was applied broadcast at 0.28 kg ae ha−1 and dicamba (Clarity®, 0.479 kg ae L−1, BASF Corporation, 26 Davis Drive, Research Triangle Park, NC 27709) at 0.14 kg ae ha−1. Directly following the drying of the spray droplet, plants were moved to the laboratory. Plastic sheaths were removed, and the most acropetal fully expanded leaf of each plant was treated with radiolabeled herbicide. The 2,4-D treatment had a total of 5.17 kBq of [14C]2,4-D (ring-labeled, specific activity 7.449 MBq mg−1, 99.7% radiochemical purity) applied, and the dicamba treatment had a total of 3.30 kBq of [14C]dicamba (ring-labeled, specific activity 1.658 MBq mg−1, 99.5% radiochemical purity) applied. No surfactants were added to the radiolabeled working mix. Radiolabeled herbicide applications were made using a micro-applicator (Burkard Manufacturing, Rickmansworth, Hertfordshire, UK) with ten 1 μl drops applied to each plant. Applications made at 7:00 AM and 12:00 AM were done under low-intensity green light to reduce interference with diurnal plant rhythms. Once the droplet containing the [14C]herbicide dried, plants were moved to a growth chamber set to a 32 C/21 C day/night temperature with a 14-hlight (450 μmol m−2 s−1) and 10-h dark photoperiod, with light from 7 AM to 9 PM and 50% relative humidity.
Four separate harvest timings were used for each herbicide treatment to analyze absorption and translocation trends over time. Harvests were made 6, 12, 24, and 48 h after radiolabeled herbicide application. Plants were sectioned into four fractions: treated leaf, shoot tissue above the treated leaf, shoot tissue below the treated leaf, and roots. Plant tissues were oxidized using a biological oxidizer (OX-500, R. J. Harvey Instrument, 123 Patterson Street, Hillsdale, NJ 07642), and radioactivity was measured using liquid scintillation spectrometry (LS 6000 TA, Beckman Instruments, 2500 Harbor Boulevard, Fullerton, CA 92634). Treated leaves were washed twice with 10 ml of a 1:1 water:ethanol solution to remove nonabsorbed radiolabeled herbicide; [14C]herbicide removed from washing was added to radioactivity from plant fractions to determine percent recovery. Percent absorption was calculated as the total [14C]herbicide recovered from plant tissue fractions divided by the total recovered [14C]herbicide. Specific radioactivity was calculated as total [14C]herbicide in roots or shoots divided by dry weight of the corresponding plant fraction.
A completely randomized design was used with four replications. ANOVA was applied to absorption, translocation, specific radioactivity, and final [14C]herbicide distribution data using the GLM procedure in SAS (SAS Studio, SAS Institute, 100 SAS Campus Drive, Cary, NC 27513). Means were separated using Fisher’s protected LSD test at α=0.05. Nonlinear regression analysis and model selection were performed using the ‘drc’ (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015) and ‘qpcR’ (Ritz and Spiess Reference Ritz and Spiess2008) packages in R (R v. 3.3.3, R Foundation for Statistical Computing, Vienna, Austria) to determine maximum [14C]herbicide absorption (Amax), maximum 14C translocation (Tmax) out of the treated leaf, and the time required for 90% of absorption or translocation (t 90) to occur. Nonlinear regression analysis was performed according to the method outlined by Kniss et al. (Reference Kniss, Vassios, Nissen and Ritz2011). Models used a rectangular hyperbolic model,
where y is absorption or translocation, βmax is the Amax or Tmax, and t is time after application. Rectangular hyperbolic models were selected over asymptotic regression and linear models based on values calculated using Akaike’s information criterion corrected for small sample sizes. Parameters for Amax or Tmax and t 90 were compared across treatments using likelihood ratio tests. Plotting of rectangular hyperbolic models was carried out using Sigmaplot (Sigmaplot 11, Systat Software, 2107 N First Street, Suite 360, San Jose, CA 95131).
Phytotoxicity Experiments
Growth chamber experiments were conducted in Athens, GA, from July to September 2016 to evaluate the effect of translocation inhibitors and time of application on dicamba phytotoxicity to Palmer amaranth. Palmer amaranth seed was obtained from the University of Georgia Iron Horse Plant Sciences Farm in Watkinsville, GA, and sowed in the greenhouse in 354-ml plastic cups in a potting mix (Fafard® 2, Sun Gro Horticulture, 770 Silver Street, Agawam, MA 01001). Plants were allowed to grow to the 3- to 5-leaf stage and were transplanted into opaque 125-ml bottles (Thermo Fisher Scientific, 168 Third Avenue, Waltham, MA 02451) containing deionized water and a 20-20-20 liquid-soluble fertilizer at a rate containing the nitrogen content of a 17% strength Hoagland solution (liquid fertilizer rate was determined from a preliminary rate titration experiment to determine the highest fertilizer rate that did not cause burning of meristematic tissue; unpublished data). Plants were then placed in a growth chamber set to a 16-h light/8-h dark photoperiod, with light from 8:00 AM to 12:00 AM (600 µmol m−2 s−1), at 25C and with ambient relative humidity of 50%. Plants were allowed to acclimate to growth chamber conditions for 72 h prior to treatment.
Herbicide treatments included dicamba at 0.56 kg ae ha−1, dicamba at 0.56 kg ae ha−1 plus a root treatment of verapamil dissolved in ethanol, dicamba at 0.56 kg ae ha−1 plus a root treatment of TIBA dissolved in ethanol, and dicamba plus DFFP (Distinct®, 0.200 kg ae kg−1 diflufenzopyr and 0.500 kg ae kg−1 dicamba, BASF Corporation, 26 Davis Drive, Research Triangle Park, NC 27709) at 0.78 kg ae ha−1. An untreated control was included, as well as a control root treatment with ethanol but no translocation inhibitor. All herbicide treatments were made either 8 h after exposure to darkness (HAED) or 8 h after exposure to light (HAEL) to simulate morning and midday applications, respectively. All dicamba treatments included a nonionic surfactant at 0.25% v/v. Root treatments were made by dissolving technical-grade verapamil (verapamil hydrochloride 98%, Sigma-Aldrich, 3050 Spruce Street, St Louis, MO 63103) or TIBA (2,3,5-triiodobenzoic acid 98+%, Alfa Aesar, 2 Radcliff Road, Tewksbury, MA 01876) into 99.5% reagent-grade ethanol to obtain a 12.5 mM stock solution of each. At 8 h prior to herbicide treatments, bottles to be administered verapamil or TIBA treatments were spiked with 100 μl of the corresponding stock solution to obtain a 10 μM concentration of each translocation inhibitor in the growing solution. Water and fertilizer growing solution was changed every 3 d to prevent algae formation and reduction in dissolved oxygen, with the first change of growing solution occurring the day after herbicide treatment. Plants administered with translocation-inhibitor root treatments prior to herbicide application were only exposed to inhibitors for 24 h total, as successive changes of growing solution did not contain inhibitors.
Phytotoxicity was measured on a visual percent scale where 0% indicated no injury and 100% indicated complete desiccation. Phytotoxicity measurements were made at 3, 7, 10, and 14 d after application (DAA). At 14 DAA, fresh shoot weight was harvested, and root length was measured. Roots and shoots were then placed in a drying oven at 50C for 96 h, and dry mass was obtained. Data were analyzed using the GLM procedure in SAS, and means were separated using Fisher’s protected LSD test at α=0.05. The experiment was repeated.
Results and Discussion
Absorption
Experiment by treatment interactions were detected prior to nonlinear regression of absorption for both herbicides (P<0.0001); results are therefore presented separately across experimental repetitions. Experiment by treatment interactions were not detected (unpublished data) for final absorption and specific radioactivity; results are therefore combined over experimental repetitions. Absorption of [14C]2,4-D and [14C]dicamba initially increased over time with all application timings, with the rate of increase in absorption decreasing after approximately 12 h after application (HAA) (Figure 1). Significant differences in t 90 were detected across application times for Experiment 1 with 2,4-D, where t 90 was significantly lower with 7:00 AM applications at 18 h compared with 2:00 PM applications (Table 1). Despite significant differences in Amax in nonlinear regression analysis, final absorption at 48 HAA was not statistically different across application timings for dicamba and 2,4-D (Table 2). Final absorption was 48% to 57% for [14C]2,4-D and 45% to 51% for [14C]dicamba. Specific radioactivity was also not statistically different across application times for both herbicides. Specific radioactivity of [14C]2,4-D in roots was 454 to 837 Bq g−1, and specific radioactivity in shoots was 2,124 to 2,840 Bq g−1. In [14C]dicamba experiments, specific radioactivity in roots was 615 to 768 Bq g−1, and specific radioactivity in shoots was 1,194 to 1,474 Bq g−1.
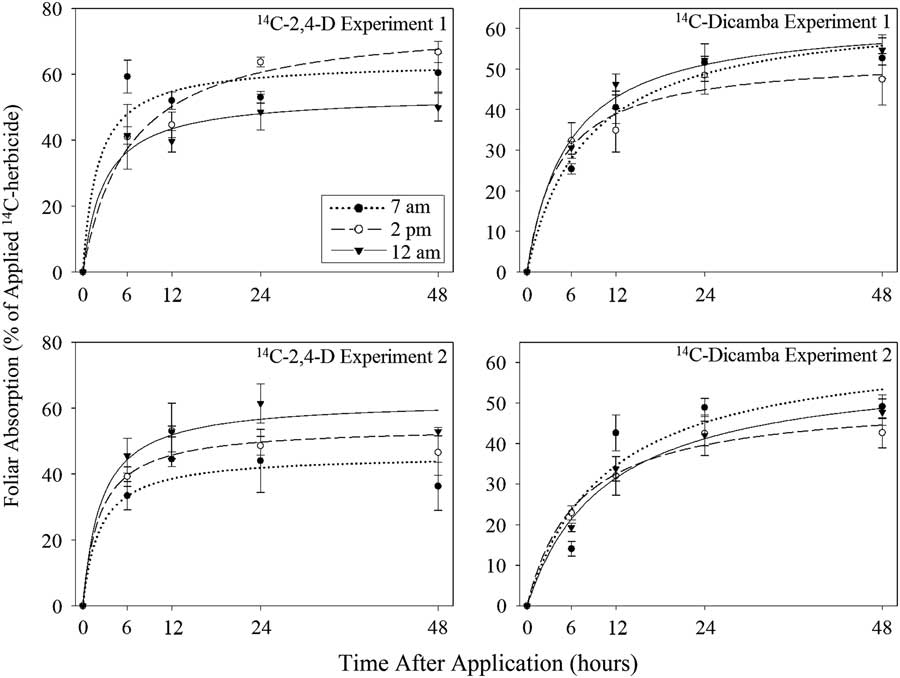
Figure 1 Absorption of [14C]2,4-D and [14C]dicamba in Palmer amaranth applied at three different application timings in two separate experiments in Tifton, GA, in 2013. Error bars represent standard error of the mean at each application timing and harvest. [14C]2,4-D Experiment 1: 7 AM timing: r 2 =0.86, n=20, y=(63.17×t)/(0.11×17.94+t), SE=8.96; 2 PM timing: r2=0.87, n=20, y=(76.26×t)/(0.11×56.41+t), SE=9.50; 12 AM timing: r2=0.88, n=20, y=(53.48×t)/(0.11×25.64+t), SE=7.02. [14C]2,4-D Experiment 2: 7 AM timing: r 2 =0.68, n=20, y=(45.46×t)/(0.11×20.32+t), SE=11.25; 2 PM timing: r2=0.78, n=20, y=(53.99×t)/(0.11×20.33+t), SE=10.16; 12 AM timing: r2=0.90, n=20, y=(62.08×t)/(0.11×21.88+t), SE=7.54. [14C]dicamba Experiment 1: 7 AM timing: r 2 =0.91, n=19, y=(65.03×t)/(0.11×71.82+t), SE=6.28; 2 PM timing: r2=0.81, n=20, y=(53.04×t)/(0.11×39.14+t), SE=8.74; 12 AM timing: r2=0.94, n=19, y=(62.77×t)/(0.11×49.50+t), SE=5.31. [14C]dicamba Experiment 2: 7 AM timing: r 2 =0.86, n=20, y=(65.16×t)/(0.11×95.63+t), SE=7.88; 2 PM timing: r2=0.91, n=19, y=(50.88×t)/(0.11×61.26+t), SE=5.28; 12 AM timing: r2=0.92, n=19, y=(59.54×t)/(0.11×96.19+t), SE=5.43.
Table 1 Nonlinear regression results for maximum absorption (Amax) and time required for 90% of maximum absorption (t 90) of [14C]2,4-D and [14C]dicamba in Palmer amaranth at three separate application timings.

a Values followed by different letters within columns, herbicides, and experimental runs are significantly (P≤0.05) different. Uppercase and lowercase letters are used to denote that likelihood ratio tests were performed separately for each experimental run within each herbicide.
b Determined by rectangular hyperbolic model (Equation 1): y=(Amax×t)/(0.11×t 90+t), where y is observed absorption, Amax is maximum absorption, t 90 is time required to reach 90% of maximum absorption, and t is time after application.
Table 2 Final absorption and specific radioactivity from [14C]2,4-D and [14C]dicamba applications in Palmer amaranth at three separate application timings.

a Absorption and root and shoot specific radioactivity are values measured at last harvest at 48 h after application.
b Significance of application timing factor on absorption and root- and shoot-specific radioactivity.
c Abbreviation: NS, not significant.
d LSD (0.05), least significant difference of application timing effects on absorption and specific radioactivity. Means were separated using Fisher’s LSD test at α=0.05.
Foliar absorption of 2,4-D has been reported to be under control of several environmental factors that directly relate to time of application. Previous research has correlated increasing temperature with increased foliar absorption of the dimethylamine and triethanolamine salts of 2,4-D (Pallas Reference Pallas1960; Sharma and Vanden Born Reference Sharma and Vanden Born1970). Potential reasoning behind this phenomenon has suggested it is due to alteration of the viscosity of lipids in the cuticle and general effects on cellular metabolism (Norris and Bukovac Reference Norris and Bukovac1969; Richardson Reference Richardson1977). Interestingly, light has been shown to have a positive correlation with 2,4-D penetration in cotton, sunflower (Helianthus annuus L.), and common bean (Phaseolus vulgaris L.) (Sargent and Blackman Reference Sargent and Blackman1972). This may be due to changes in energy transfer, as improved uptake of 2,4-D under higher light intensities in common bean has been reported to be inhibited by the halting of ATP production (Sargent and Blackman Reference Sargent and Blackman1969). Midday applications coincided with higher temperatures; however, given that experiments were initiated in the greenhouse and treated in a laboratory, it is assumed relative humidity was consistent. Significant effects of time of application on any absorption parameters were not detected for dicamba (Tables 1 and 2). There are likely mechanisms conferring differential efficacy for dicamba, as a member of the same mechanism of action as 2,4-D; however, these may be of less magnitude due to differences in activity across these herbicides. Very little research is available on factors affecting dicamba uptake, and further research is necessary.
Translocation and 14C Distribution
Experiment by treatment interactions were detected for nonlinear regression of translocation out of the treated leaf for both herbicides (P=0.0081 and P=0.0103 for 2,4-D and dicamba, respectively); results are therefore presented separately across experimental repetitions. Translocation out of the treated leaf initially increased over time with [14C]2,4-D and [14C]dicamba applications at all timings, with the rate of increase in translocation out of the treated leaf decreasing at approximately 12 h after application (Figure 2). Significant differences in Tmax were observed for [14C]herbicide translocation out of the treated leaf due to time of application in one of two experiments for both herbicides (Table 3). Results from Experiment 2 showed significant differences in Tmax of [14C]2,4-D out of the treated leaf, with the highest value for Tmax resulting from 7:00 AM applications and the lowest Tmax resulting from 12:00 AM applications. The range in Tmax out of the treated leaf across application times was similar for both experiments, ranging from 51% to 100% in Experiment 1 and 48% to 100% in Experiment 2. Trends for t 90 corresponding to translocation out of the treated leaf varied similarly to Tmax in both experiments. The Tmax for [14C]dicamba translocation out of the treated leaf was significantly higher with 7:00 AM application in Experiment 1. The range in Tmax out of the treated leaf ranged from 42% to 76% in Experiment 1 and 76% to 87% in Experiment 2. The variation in t 90 followed a trend similar to that of Tmax in both [14C]dicamba experiments.
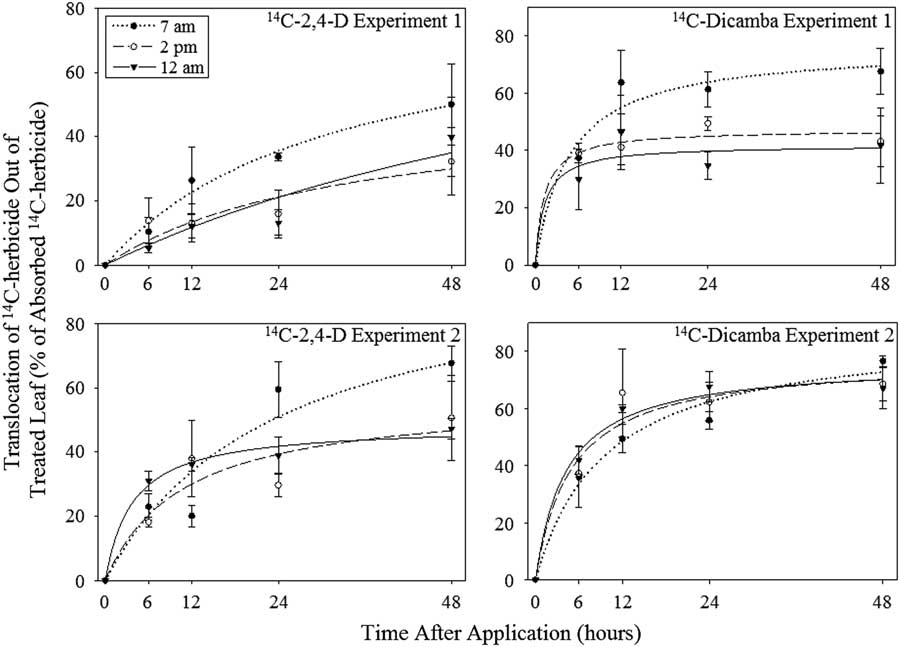
Figure 2 Translocation of [14C]2,4-D and [14C]dicamba out of the treated leaf in Palmer amaranth at three different application timings in two separate experiments in Tifton, GA, in 2013. Error bars represent standard error of the mean at each application timing and harvest. [14C]2,4-D Experiment 1: 7 AM timing: r 2 =0.63, n=20, y=(82.46×t)/(0.11×285.30+t), SE=13.68; 2 PM timing: r2=0.36, n=20, y=(50.84×t)/(0.11×301.40+t), SE=13.18; 12 AM timing: r2=0.57, n=20, y=(100.00×t)/(0.11×826.90+t), SE=11.61. [14C]2,4-D Experiment 2: 7 AM timing: r 2 =0.82, n=20, y=(100.00×t)/(0.11×214.89+t), SE=11.88; 2 PM timing: r2=0.53, n=20, y=(57.03×t)/(0.11×97.09+t), SE=15.71; 12 AM timing: r2=0.87, n=20, y=(48.30×t)/(0.11×33.76+t), SE=6.32. [14C]dicamba Experiment 1: 7 AM timing: r 2 =0.81, n=19, y=(76.03×t)/(0.11×41.73+t), SE=12.42; 2 PM timing: r2=0.78, n=20, y=(47.24×t)/(0.11×11.11+t), SE=9.50; 12 AM timing: r2=0.54, n=17, y=(41.81×t)/(0.11×11.33+t), SE=15.67. [14C]dicamba Experiment 2: 7 AM timing: r 2 =0.86, n=20, y=(87.25×t)/(0.11×86.30+t), SE=10.32; 2 PM timing: r2=0.82, n=19, y=(77.26×t)/(0.11×44.28+t), SE=12.57; 12 AM timing: r2=0.90, n=19, y=(76.33×t)/(0.11×38.25+t), SE=8.89.
Table 3 Nonlinear regression results for maximum translocation (Tmax) and time required for 90% of maximum translocation (t 90) out of treated leaf of [14C]2,4-D and [14C]dicamba in Palmer amaranth at three separate application timings.

a Values followed by different letters within columns, herbicides, and experimental runs are significantly (P ≤ 0.05) different. Uppercase and lowercase letters are used to denote that likelihood ratio tests were performed separately for each experimental run within each herbicide.
b Determined by rectangular hyperbolic model (Equation 1): y=(Tmax×t)/(0.11×t 90+t), where y is observed translocation, Tmax is maximum translocation, t 90 is time required to reach 90% of maximum translocation, and t is time after application.
Experiment by treatment interactions were not detected (unpublished data) for [14C]herbicide distribution to any plant fraction for both herbicides; results are therefore combined across experimental repetitions. Fate of recovered [14C]herbicide (48 HAA) for [14C]2,4-D experiments was only significantly different across application times with distribution to the root (Figure 3). With 7:00 AM applications, approximately 10% of recovered [14C]2,4-D was found in the root, while 4% to 5% was detected with the 2:00 PM and 12:00 AM applications. Fate of [14C]dicamba was only significantly different across application times with distribution above the treated leaf, as 9% more [14C]dicamba was recovered in this fraction when applied at 7:00 AM compared with 2:00 PM or 12:00 AM.

Figure 3 Distribution of [14C]2,4-D and [14C]dicamba in Palmer amaranth applied at three different application timings in Tifton, GA, in 2013. Results were pooled over two experiments. Vertical bars represent the standard error of the mean. Different letters across application timings within each plant fraction indicate a significant difference in percent recovered 14C. Data are from distribution of [14C]herbicide at 48 HAA.
Data for final [14C]herbicide distribution suggest that the ability for Palmer amaranth to translocate both [14C]2,4-D and [14C]dicamba is potentially increased with early morning applications. Nonlinear regression data (Tmax) also suggest that ability to translocate [14C]dicamba and [14C]2,4-D may be enhanced with 7:00 AM applications. Pallas (Reference Pallas1960) reported increased translocation of 2,4-D when applied at 7:00 AM. Coincidentally, likely as a function of early-morning application, the same research by Pallas (Reference Pallas1960) reported increased translocation with increased humidity. Again, humidity can likely be dismissed as a contributing factor in our research, as it was kept relatively constant in the growth chamber. Little research has been done on the effect of light on the translocation of these herbicides, however the effect of light on the translocation of the now-banned auxinic herbicide 2,4,5-T was noted to be species specific in a study by Brady (Reference Brady1969).
Phytotoxicity
Application time was a significant factor in phytotoxicity from herbicide treatments at 7 DAA (P=0.0270); however, it was not significant at all other rating dates (Table 4). Treatment was not significant as a factor at all rating dates. The greatest variation in dicamba phytotoxicity across time of application was observed in combination with verapamil root treatments, with phytotoxicity being 34% lower with applications at 8 HAEL compared with those at 8 HAED at 14 DAA. Contrasts between application times of dicamba treatments containing verapamil root treatments showed statistical significance 7 DAA onward. TIBA root treatments resulted in the most consistent phytotoxicity from dicamba across application times, varying only 2% to 5% across all rating times. Plants receiving the 100-μl ethanol control root treatment displayed <9% phytotoxicity throughout the experiment.
Table 4 Phytotoxicity of dicamba in combination with three auxin transport inhibitors on Palmer amaranth from two separate application timings.Footnote a

a Abbreviations: DAA, days after application; DFFP, diflufenzopyr; HAED, hours after exposure to darkness; HAEL, hours after exposure to light; TIBA, 2,3,5-triiodobenzoic acid; NS, not significant.
b *, significant at 0.05 probability level.
c LSD (0.05) treatment: least significant difference of herbicide+inhibitor treatment effects on phytotoxicity; LSD (0.05) timing: least significant difference of application timing effects on phytotoxicity. Means were separated using Fisher’s LSD test at α=0.05.
d Timing: significance of application timing factor on phytotoxicity; treatment: significance of herbicide+inhibitor treatment effects on phytotoxicity; timing*treatment: significance of the interaction of application timing and herbicide+inhibitor treatment effect on phytotoxicity.
e Results from orthogonal contrasts comparing the effect of time of application across individual herbicide+inhibitor treatments.
Time and treatment were not significant as factors for fresh and dry shoot weights (Table 5). The contrast indicated that plants treated with dicamba containing the verapamil root treatment had greater reductions in dry shoot weight with applications at 8 HAEL than 8 HAED (P=0.0144). This is consistent with contrasts on phytotoxicity from the verapamil root treatment at 10 and 14 DAA.
Table 5 Fresh and dry shoot mass, root mass, and root length of Palmer amaranth plants treated with dicamba in combination with three auxin transport inhibitors at two separate application timings.Footnote a

a Abbreviations: DFFP, diflufenzopyr; HAED, hours after exposure to darkness; HAEL, hours after exposure to light; TIBA=2,3,5-triiodobenzoic acid; NS, not significant.
b *, significant at 0.05 probability level; ***, significant at 0.001 probability level.
c LSD (0.05) treatment: least significant difference of herbicide+inhibitor treatment effects on fresh and dry shoot biomass, root biomass, and root length; LSD (0.05) time: least significant difference of application timing effects on fresh and dry shoot biomass, root biomass, and root length. Means were separated using Fisher’s LSD test at α=0.05.
d Timing: significance of application timing factor on phytotoxicity; treatment: significance of herbicide+inhibitor treatment effects on phytotoxicity; timing*treatment: significance of the interaction of application timing and herbicide+inhibitor treatment effect on phytotoxicity.
e Results from orthogonal contrasts comparing the effect of time of application across individual herbicide+inhibitor treatments.
The effect of treatment was significant on dry root weight (P=0.0116). TIBA root treatments resulted in greater dry root weight reductions than any other translocation inhibitor and was the only translocation inhibitor that resulted in significantly reduced root length from dicamba alone at both application timings. Contrast of dry root weight between application times of dicamba alone resulted in a highly significant difference (P<0.0001), with greater reductions in dry root weight at 8 HAED than 8 HAEL. Interestingly, contrasts of dry root weight between the two application times of dicamba when combined with the verapamil root treatment show a highly significant difference as well (P<0.0001). A highly significant effect of treatment on root length was detected (P<0.0001). The greatest reduction in root length from dicamba application was observed with the TIBA treatment, reducing root length 3 and 2 cm at 8 HAED and 8 HAEL, respectively. DFFP, verapamil, and TIBA treatments resulted in considerable stability of reduced root length across application times. DFFP actually resulted in significantly increased root length compared with dicamba alone at 8 HAEL, potentially due to concentration of dicamba in the meristem preventing root translocation.
TIBA appeared to stabilize many responses of Palmer amaranth to dicamba across application times. This suggests that PIN proteins may play a part in differential phytotoxicity observed across application timings. Diurnal regulation of PIN proteins has been reported in previous research, and the inhibition of PIN proteins through TIBA treatment could theoretically eliminate any difference in regulation across different times of day (Friml et al. Reference Friml, Wisniewska, Benkova, Mendgen and Palme2002). The exacerbation of the difference in phytotoxicity and dry shoot biomass seen across application times when verapamil, an inhibitor of ABCB transporters, was combined with dicamba treatment may have a major implication for mechanisms relating dicamba translocation and phytotoxicity in Palmer amaranth. A critical factor (e.g., an ABCB protein–membrane interaction) involved in conferring phytotoxicity from dicamba treatment that is sensitive to verapamil may have been highly limiting at 8 HAED, but responsibility of this factor for dicamba-induced phytotoxicity may have been relieved by 8 HAEL due to diurnal fluctuation in activity. This could conceivably be a result of ABCB-mediated translocation having a more negative association with phytotoxicity at dawn, but a more positive relationship with phytotoxicity at midday. Based on the different trends observed across root treatments, the effect of translocation on phytotoxicity appears to be transport protein–specific; how these different proteins contribute to phytotoxicity demands further investigation.
It can be noted that t 90 appeared to be somewhat related to Tmax for both herbicides, suggesting that faster rates of translocation in general may be associated with a reduction in the maximum amount of herbicide that is able to be translocated to certain plant fractions. This phenomenon may be linked to the fact that increased phytotoxicity, if indeed a result of quicker movement (i.e., lower t 90) of herbicide to the target site, effectively limits in some way a plant’s machinery that is necessary for sustained movement (i.e., increased Tmax) of phloem-mobile herbicides (Geiger and Bestman Reference Geiger and Bestman1990). This reasoning supports a hypothesis in which early-application timing results in slower saturation of the available target sites throughout the plant as a whole, and resulting decreases in phytotoxicity allow for such sustained movement of herbicide. Further investigation of the relationship of speed of translocation to maximum translocation is necessary.
These studies illustrate that much remains to be investigated concerning herbicide activity depending on time of application. The network of interactions between environmental parameters associated with time of day, herbicide translocation, and phytotoxicity are complex and involve physiological as well as molecular and genetic responses in plants. These interactions have real consequences for agronomists, as compromised weed control can directly affect yield of many crops and select for resistance development in weeds such as Palmer amaranth. The identification of mechanisms responsible for these variations in herbicide phytotoxicity requires continued research to maintain the security of viable weed control options, particularly in species for which resistance has become extremely problematic.










