Introduction
Weeds greatly reduce crop yield by competing with crops for nutrients, light, water, and space, which are the main restraining factors for food production in agriculture (Vigueira et al. Reference Vigueira, Olsen and Caicedo2013). Catchweed bedstraw (Galium aparine L.) is an annual dicot weed that is widely distributed in major wheat (Triticum aestivum L.) production regions in China (Sun et al. Reference Sun, Wang, Zhang, Liu and Bian2011). Wheat is the second most important crop in China. Acetyl-CoA carboxylase- and acetolactate synthase (ALS)-inhibiting herbicides are the two major classes of POST herbicides that have been used to control grass and broadleaf weeds, respectively, in wheat for the past two decades. Galium aparine has become one of the most notorious weeds in the wheat fields of China due to its competitive advantage and single-herbicide use pattern. In recent years, many farmers have found it difficult to control G. aparine using tribenuron-methyl in some Chinese winter wheat fields.
ALS, also known as acetohydroxyacid synthase, is a key enzyme that catalyzes the first step in the biosynthesis of branched-chain amino acids (BCAAs; valine, leucine, and isoleucine) (Duggleby et al. Reference Duggleby, McCourt and Guddat2008). ALS-inhibiting herbicides, including sulfonylurea (SU), triazolopyrimidine (TP), pyrimidinyl thiobenzoate (PTB), imidazolinone (IMI), and sulfonylamino-carbonyl-triazolinone (SCT), bind the enzyme at the substrate access channel, which results in BCAA starvation in plant cells and, ultimately, plant death (Corbett and Tardif Reference Corbett and Tardif2006; Singh and Shaner Reference Singh and Shaner1995). ALS-inhibiting herbicides are widely used throughout the world because they provide broad-spectrum weed control and high herbicidal activity combined with low mammalian toxicity (Mazur and Falco Reference Mazur and Falco1989). Following the persistent and extensive use of ALS inhibitors in the past few decades, 160 weeds species have been documented with resistance to these herbicides (Heap Reference Heap2018). In most cases, target-site resistance caused by mutations in the target enzyme is mainly responsible for resistance to ALS-inhibiting herbicides. To date, 28 resistance-conferring mutations have been identified at eight sites (Ala-122, Pro-197, Ala-205, Asp-376, Arg-377, Trp-574, Ser-653, Gly-654) in more than 50 weed species (Tranel et al. Reference Tranel, Wright and Heap2018). In contrast, non–target site resistance has been less studied and has been reported in only a few ALS-resistant weeds, including turnipweed [Rapistrum rugosum (L.) All.] (Hatami et al. Reference Hatami, Gherekhloo, Rojano-Delgado, Osuna, Alcántara, Fernández, Sadeghipour and Prado2016), corn poppy (Papaver rhoeas L.) (Rey-Caballero et al. Reference Rey-Caballero, Menéndez, Osuna, Salas and Torra2017), threeleaf arrowhead (Sagittaria trifolia L.) (Zhao et al. Reference Zhao, Fu, Yu, Huang, Yan, Li, Shafi, Zhu, Wei and Ji2017), and flixweed [Descurainia sophia (L.) Webb ex Prantl] (Yang et al. Reference Yang, Li, Shen, Xu, Liu, Deng, Li and Zheng2018b).
The SU herbicide tribenuron-methyl has been used in winter wheat fields in China since 1988. The continuous use of tribenuron-methyl has led to the evolution of resistant G. aparine populations in the Henan, Shandong, Anhui, and Jiangsu provinces of China (Gao et al. Reference Gao, Zhang, Wang, Guo, Wang, Ma, Gao, Liu and Li2016; Peng et al. Reference Peng, Wang, Duan and Yang2008; Wang et al. Reference Wang, Xiao, Lou, Sun and Xu2017). The Pro-197-Leu, Pro-197-Ala, Pro-197-Ser, Pro-197-Thr, Trp-574-Leu, and Trp-574-Gly mutations in ALS have been reported to be the target-site resistance mechanisms to tribenuron-methyl in G. aparine (Cui et al. Reference Cui, Wang, Xu and Li2016; Sun et al. Reference Sun, Wang, Zhang, Liu and Bian2011; Zhang et al. Reference Zhang, Li, Guo, Li and Wang2016). In this study, 20 G. aparine populations were collected from the Henan and Shandong provinces of China, where tribenuron-methyl can no longer effectively control G. aparine. The objectives of this study were: (1) to investigate the level of resistance to tribenuron-methyl in the G. aparine populations; (2) to identify the molecular basis of resistance; and (3) to evaluate the cross-resistance to ALS-inhibiting herbicides for G. aparine populations with specific ALS mutations.
Materials and Methods
Plant Material
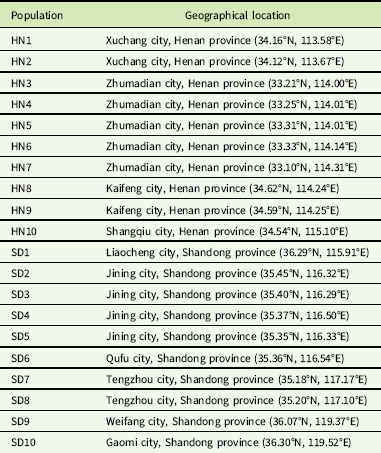
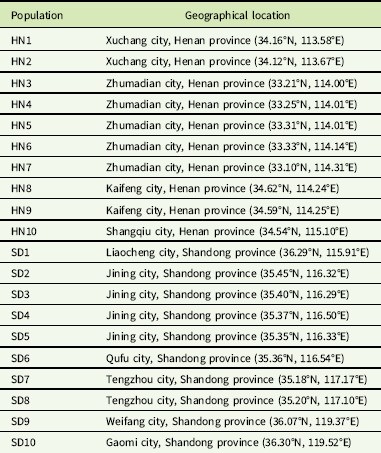
A total of 20 G. aparine populations (HN1-10 and SD1-10) collected from Shandong and Henan provinces in June 2016 and 2017 were used in this study (Table 1). Seeds of the 19 putative resistant populations were collected randomly from wheat fields for which the tribenuron-methyl application history was unknown. Seeds of the susceptible population (HN10) were harvested from roadside areas in Kaifeng, Henan province, that had never been treated with herbicides. The dry seeds were placed in paper bags and stored at 4 C for 2 wk to break dormancy and then kept at room temperature until the experiment.
Table 1 Geographical location of 20 G. aparine populations collected in Henan and Shandong provinces in China.
The seeds were first soaked with sodium hypochlorite for 10 min and then rinsed thoroughly with water. The treated seeds were germinated in petri dishes in an artificial climate chamber (18/15 C, 12/12 h day/night). After 15 d, seedlings at the 2-leaf stage were transplanted to 7-cm-diameter plastic pots (9 plants pot−1) containing loamy soil. The pots were placed in the greenhouse (about 30/20 C, natural light) and watered as necessary.
Dose Response to Tribenuron-Methyl
Approximately 10 d after transplanting, when the plants had grown to the 4-leaf stage, they were sprayed with tribenuron-methyl using a laboratory sprayer delivering 450 L ha−1 at 0.2 MPa. The tribenuron-methyl doses were 0, 0.072, 0.36, 1.8, 9, 45, 225, and 450 g ai ha−1 for the susceptible population (HN10), and 0, 0.072, 0.36, 1.8, 9, 45, 225, 450, 900, and 1,800 g ha−1 for the putative resistant population. The recommended rate for tribenuron-methyl was 15 g ha−1. At 21 d after treatment (DAT), the aboveground plant biomass was sampled, and the fresh weight was recorded. Each herbicide treatment had three replications, and the experiment was conducted two times.
The herbicide dose resulting in 50% plant growth reduction (GR50) was determined by the following log-logistic equation (Seefeldt et al. Reference Seefeldt, Jensen and Fuerst1995):
where C is the lower limit, D is the upper limit, b is the slope, x is the herbicide dose, and y represents plant fresh weight as percentage of the control. The resistance level was indicated by the resistance index (RI), which was calculated as the ratio of the GR50 of resistant populations to that of the susceptible population.
ALS Gene Sequencing
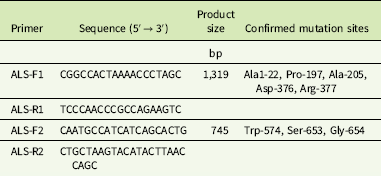
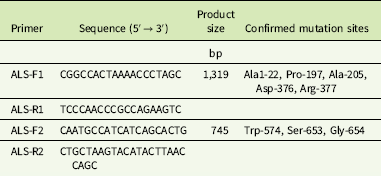
Total DNA was extracted from tender leaves using the DNAquick Plant Kit (Tiangen, Beijing, China). Two pair of primers (ALS-F1/ALS-R1, ALS-F2/ALS-R2) (Table 2) were designed based on the G. aparine ALS sequences (GenBank accession GU377313) to amplify partial fragments of the ALS gene containing the known eight resistance-conferring mutation sites. The 50-μl polymerase chain reaction (PCR) reactions contained 100 ng DNA, 25 μl of 2 × Taq PCR Master Mix, 21 μl of ddH2O, and 2 μl (10 μM) of each primer (Tiangen). PCR was run according to the following program: 3 min denaturing at 95 C, 35 cycles of 30 s denaturation at 95 C, 30 s annealing at 56 C, 90 s elongation at 72 C, and a final extension for 10 min at 72 C.
Table 2 Primer pairs designed for ALS gene amplification.
PCR products were visualized on 1% agarose gels and purified with TIANgel Midi Purification Kit (Tiangen). The purified product was sequenced in the forward and reverse directions with the Sanger method by a commercial corporation (Sangon Biotech, Shanghai, China). Sequence data were assembled, aligned, and analyzed using DNAMAN v. 5.22 software (Lynnon Biosoft, QC, Canada).
Generation of Purified Resistant Subpopulations
Seedlings of the susceptible (HN10) and resistant (HN4, HN7, HN8, HN5, and HN3) populations were cultured to the 4-leaf stage. Plants of resistant populations were sprayed with tribenuron-methyl at rates of 45 g ha−1, but plants of the susceptible population were not treated with herbicide. After 30 d, the ALS mutation types of surviving individuals from resistant populations were determined according to the method described earlier. Single plants homozygous for specific ALS mutations were isolated and transplanted into 20-cm-diameter plastic pots to produce seeds individually in the greenhouse. In this way, six purified subpopulations, homozygous for wild type, Pro-197-Ser, Pro-197-His, Pro-197-Leu, Asp-376-Glu, and Trp-574-Leu mutations, were obtained and used for subsequent cross-resistance determination experiments. At least 10 progeny plants of each purified subpopulation were randomly selected for ALS sequencing to confirm the ALS mutation.
Cross-Resistance Patterns to ALS-inhibiting Herbicides
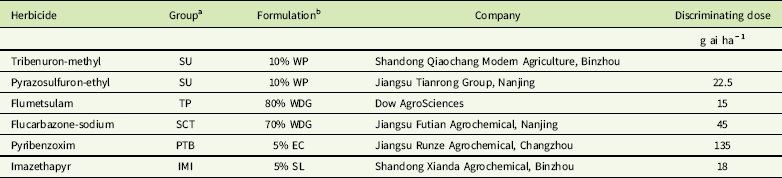
Seed germination and plant growth of purified subpopulations were the same as those described earlier. When resistant and susceptible plants grew to the 2-leaf stage, herbicide screening was conducted using 1-, 2-, and 4-fold the discriminating doses known to control the susceptible population. The herbicides and discriminating doses are listed in Table 3. Plant survival was determined at 21 DAT. The experiment was conducted with four replicates (at least 30 plants per dose). Resistance was confirmed when ≥50% individuals survived the herbicide treatment at the discriminating dose and susceptibility as ≤10% survival (Deng et al. Reference Deng, Yang, Zhang, Jiao, Mei, Li and Zheng2017; Yu et al. Reference Yu, Han, Li, Purba, Walsh and Powles2012).
Table 3 ALS-inhibiting herbicides used in this study and discriminating doses applied to Galium aparine in cross-resistance assays.
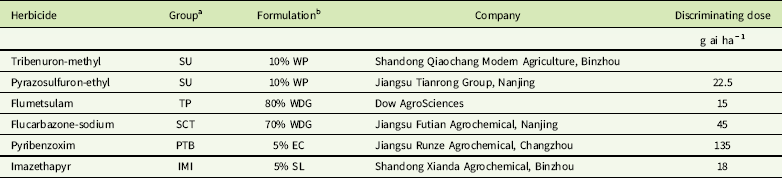
a IMI, imidazolinone; PTB, pyrimidinyl thiobenzoate; SCT, sulfonylamino-carbonyl-triazolinone; SU, sulfonylurea; TP, triazolopyrimidine.
b EC, emulsifiable concentrate; SL, soluble concentrate; WDG, water-dispersible granule; WP, wettable powder.
Results and Discussion
Dose Response to Tribenuron-methyl
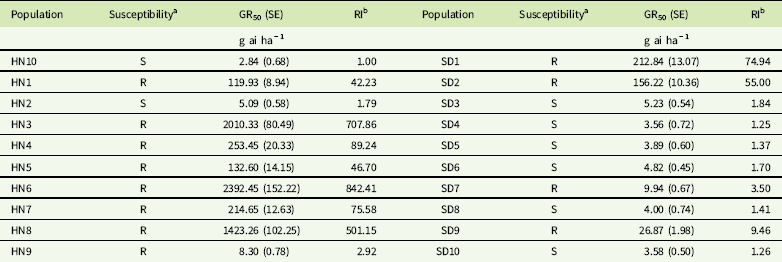
The dose–response experiments indicated that 12 out of 20 populations showed different resistance levels to tribenuron-methyl. The HN3, HN6, and HN8 populations were highly resistant to tribenuron-methyl with 707.8, 842.4, and 501.2-fold RIs compared with the susceptible population. Two populations (HN9 and SD7) had low resistance levels (2 ≤ RI <5), one population (SD9) had moderate resistance levels (5 ≤ RI <10), and nine populations (HN1, HN3, HN4, HN5, HN6, HN7, HN8, SD1, and SD2) had high resistance levels (RI ≥ 10) to tribenuron-methyl (Table 4).
Table 4 Herbicide dose causing 50% fresh weight reduction (GR50) and resistance index (RI) values for tribenuron-methyl in Galium aparine populations.
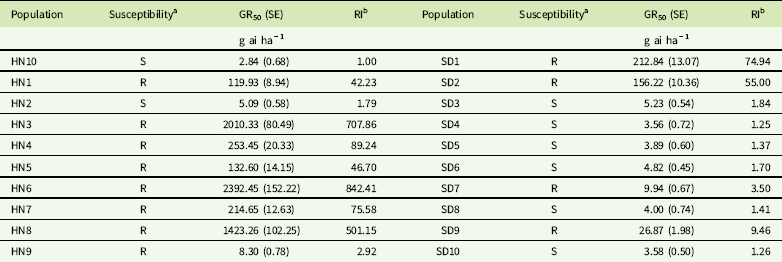
a R, resistant; S, susceptible.
b RI = GR50 value of resistant populations/GR50 value of susceptible population (HN10).
These results indicated that 63% of all populations had evolved resistance to tribenuron-methyl. This suggested that tribenuron-methyl resistance in G. aparine has become widespread in winter wheat fields of the Shandong and Henan provinces. Wang et al. (Reference Wang, Xiao, Lou, Sun and Xu2017) reported that 12 of 20 G. aparine populations collected from wheat fields in Jiangsu Province have evolved resistance to tribenuron-methyl. Cui et al. (Reference Cui, Wang, Xu and Li2016) reported that 19 of 25 G. aparine populations in five provinces of wheat-growing areas were resistant to tribenuron-methyl. The occurrence frequency of resistant G. aparine in wheat production areas in China was similar and high (>50%), which indicated the resistance to tribenuron-methyl in G. aparine is very serious. The persistent and intensive use of the same herbicide or herbicides with the same mode of action has resulted in the rapid evolution of herbicide-resistant weeds. Tribenuron-methyl was used to control broadleaf weeds for nearly 30 yr in winter wheat fields in China. Several weeds, including G. aparine (Cui et al. Reference Cui, Wang, Xu and Li2016; Zhang et al. Reference Zhang, Li, Guo, Li and Wang2016), D. sophia (Cui et al. Reference Cui, Zhang, Wei, Zhang, Li, Zhang and Wang2011; Deng et al. Reference Deng, Liu, Yang, Mei, Li and Zheng2015), shepherd’s-purse [Capsella bursa-pastoris (L.) Medik.] (Cui et al. Reference Cui, Li, Wang, Wang, Wei and Cao2012; Zhang et al. Reference Zhang, Guo, Li, Wu, Zhao, Liu and Wang2017), and water starwort [Myosoton aquaticum (L.) Moench] (Liu et al. Reference Liu, Bi, Li, Yuan and Wang2013, Reference Liu, Wu, Guo, Du, Yuan and Wang2015), have evolved resistance to tribenuron-methyl. Thus, an appropriate herbicide rotation or mixture, such as MCPA-sodium or fluroxypyr, and other management strategies are important for controlling the tribenuron-methyl–resistant weeds or minimizing the prevalence of these resistant populations.
Identification of Target-Site Mutations
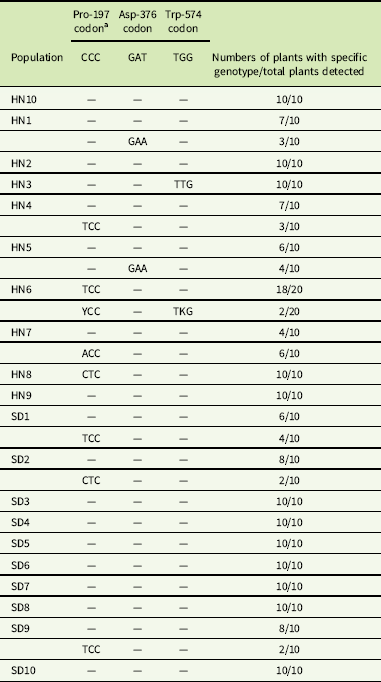
Two fragments of the ALS gene of 1,319 and 745 bp were assembled into one 1,959-bp sequence and aligned with a known ALS gene of G. aparine (GenBank accession GU377313). The results indicated that the 1,959-bp sequence of the gene fragments from all populations exhibited 99% homology with the documented ALS gene. An amino acid mutation was identified at Pro-197, Asp-376, and Trp-574 in 10 resistant G. aparine populations (Table 5). Three different nucleotide mutations, namely, CCC to CTC (HN8 and SD2), TCC (HN4, HN6, SD1 and SD10), and ACC (HN7), resulting in leucine (Leu), serine (Ser), and histidine (His) amino acid substitutions, were detected at the Pro-197 codon of the ALS gene. The Asp-376-Glu substitution, resulting from a GAT to GAA nucleotide change, was found in the HN1 and HN5 populations. The Trp-574-Leu substitution caused by a nucleotide mutation (TGG to TTG) was observed in the HN3 and HN6 populations. Furthermore, the Pro-197-Ser and Trp-574-Leu mutations were identified in a single plant of the HN6 population (Table 5).
Table 5 Nucleotide mutations related to ALS-inhibiting herbicides resistance at sites Pro-197, Asp-376, and Trp-574 in the ALS gene in Galium aparine populations.
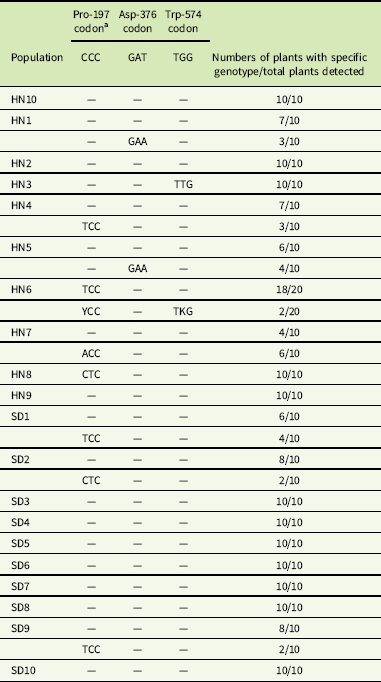
a Y: T or C; K: T or G.
ALS mutation diversity has been confirmed in many ALS inhibitor–resistant weeds. For example, amino acid substitution at Pro-197, Asp-376, and Trp-574 were found in D. sophia (Yang et al. Reference Yang, Deng, Wang, Liu, Li and Zheng2018a), kochia [Bassia scoparia (L.) A. J. Scott] (Warwick et al. Reference Warwick, Xu, Sauder and Beckie2008), and wild mustard (Sinapis arvensis L.) (Warwick et al. Reference Warwick, Sauder and Beckie2005; Whaley et al. Reference Whaley, Wilson and Westwood2007); Ala-122, Pro-197, Asp-376, and Trp-574 mutations were identified in wild radish (Raphanus raphanistrum L.) (Han et al. Reference Han, Yu, Purba, Li, Walsh, Friesen and Powles2012; Yu et al. Reference Yu, Han, Li, Purba, Walsh and Powles2012); and Ala-122, Pro-197, Ala-205, Asp-376, Trp-574, and Ser-653 mutations were observed in redroot pigweed (Amaranthus retroflexus L.) (Chen et al. Reference Chen, Huang, Zhang, Huang, Wei, Chen and Wang2015; Huang et al. Reference Huang, Chen, Zhang, Huang, Wei, Chen and Wang2016; McNaughton et al. Reference McNaughton, Letarte, Lee and Tardif2005). In this study, the molecular basis of ALS mutations has been well studied, and five different ALS mutations (Pro-197-Ser/Leu/His, Asp-376, and Trp-574) were established in tribenuron-methyl–resistant G. aprine. In addition, we noticed that two populations (HN9 and SD7) exhibited a low resistance level to tribenuron-methyl and had no resistance-conferring mutations in the ALS gene. This indicated that other resistance mechanisms, such as metabolic resistance, may play an important role in this case, and a non–target site based resistance mechanism could not be ruled out in ten other resistant populations with ALS mutations.
In the dose–response test of the resistant (HN6) population, we found that the growth of some plants was not significantly affected or only slightly inhibited by tribenuron-methyl at a dose of 900 g ha−1, while the growth of other plants was significantly inhibited (Figure 1). ALS gene sequencing identified that the individuals with better survival were heterozygous for both the Pro-197-Ser and Trp-574-Leu mutations, while the other plants were homozygous for the Pro-197-Ser mutation alone (Table 5). This finding suggested that plants with two heterozygous mutations (Pro-197-Ser and Trp-574-Leu) showed a higher resistance level than plants with a single homozygous mutation (Pro-197-Ser) to tribenuron-methyl. Two different resistance alleles in a single plant were also reported in other weeds, including rigid ryegrass (Lolium rigidum Gaudin) (Yu et al. Reference Yu, Han and Powles2008), C. bursa-pastoris (Zhang et al. Reference Zhang, Guo, Li, Wu, Zhao, Liu and Wang2017), R. raphanistrum (Yu et al. Reference Yu, Zhang, Hashem, Walsh and Powles2003), and D. sophia (Deng et al. Reference Deng, Yang, Zhang, Jiao, Mei, Li and Zheng2017) This combination of different resistance alleles may have two causes. The first is cross-pollination between resistant weeds. The second cause may be a stronger target-site resistance mechanism evolved under the continuous selection pressure of the herbicide. Regardless, the plants with two mutated alleles had a stronger resistance to herbicides, which poses a serious challenge for resistant weed management.

Figure 1 The growth of the susceptible (HN10) and resistant (HN6) Galium aparine populations at 21 d after treatment with tribenuron-methyl. Red arrows identify plants that were heterozygous for Pro-197-Ser and Trp-574-Leu mutations.
Cross-resistance Patterns to ALS-inhibiting Herbicides
The purified populations homozygous for Pro-197-Ser, Pro-197-Leu, Pro-197-His, Asp-376-Glu, and Trp-574-Leu mutations were generated by self-pollination and were used to evaluate the cross-resistance to ALS-inhibiting herbicides in the discriminating dose test. The results showed that pHN4 (Pro-197-Ser), pHN7 (Pro-197-His), pHN8 (Pro-197-Leu), pHN5 (Asp-376-Glu), and pHN3 (Trp-574-Leu) plants were resistant to pyrazosulfuron-methyl, flumetsulam, flucarbazone-sodium, pyribenzoxim, and imazethapyr. All plants of the pHN4, pHN7, pHN8, pHN5, and pHN3 populations survived when treated with 1-, 2-, and 4-fold discriminating rates of five different ALS-inhibiting herbicides, while plants of the susceptible population died at the same discriminating rates.
Previous studies indicated that the Asp-376 or Trp-574 substitution in other weeds usually conferred broad cross-resistance to SU, TP, SCT, IMI, and PTB (Deng et al. Reference Deng, Yang, Jiao, Zhang, Li and Zheng2016, Reference Deng, Yang, Zhang, Jiao, Mei, Li and Zheng2017; Pandolfo et al. Reference Pandolfo, Presotto, Moreno, Dossou, Migasso, Sakima and Cantamutto2016; Whaley et al. Reference Whaley, Wilson and Westwood2007). In this study, plants with the Asp-376-Glu or Trp-574-Leu mutations all survived at a 4-fold discriminating dose of five different classes of ALS-inhibiting herbicides. These results were consistent with previous reports. The cross-resistance pattern conferred by the Pro-197 mutation varies, because it is determined by the specific amino acid substitution, individual weed species, and the herbicides being applied. The Pro-197-Ser mutation in eclipta [Eclipta prostrata (L.) L.] exhibited resistance to SU, TP, PTB, and IMI (Li et al. Reference Li, Li, Yu, Wang and Cui2017), while the same mutation in D. sophia only showed resistance to SU and TP (Deng et al. Reference Deng, Cao, Yang, Liu, Mei and Zheng2014). In addition, the Pro-197-Ser and Pro-197-Leu mutations in D. sophia displayed different cross-resistance to ALS-inhibiting herbicides (Deng et al. Reference Deng, Cao, Yang, Liu, Mei and Zheng2014). These results demonstrated that the pattern of cross-resistance is very complex. The cross-resistance to ALS-inhibiting herbicides determined in G. aprine. will be helpful for farmers to choose alternative herbicides for controlling this weed. Farmers should be encouraged to use herbicides with different modes of action in rotation and an integrated weed management system to delay herbicide resistance.
Acknowledgments
No conflicts of interest have been declared. This research received no specific grant from any funding agency or the commercial or not-for-profit sectors.