Rigid ryegrass is one of the most problematic weeds in Australian winter cropping (Jones et al. Reference Jones, Vere, Alemseged and Medd2005; Llewellyn et al. Reference Llewellyn, Ronning, Ouzman, Walker, Mayfield and Clarke2016). Globally, rigid ryegrass has evolved resistance to 12 herbicide mode-of-action groups, the most for any species (Heap Reference Heap2016); most of these cases of resistance have evolved in Australia. The success of rigid ryegrass in rapidly evolving herbicide resistance is related to its widespread distribution, high genetic variation, and diploid genome (Walsh and Powles Reference Walsh and Powles2004). Apart from being very well adapted to the Mediterranean climate of southern Australia, rigid ryegrass is widespread due to it being sown and nurtured as a pasture species in the period between 1820 and 1960 (Walsh and Powles Reference Walsh and Powles2004). The species has the potential to set a large amount of seed, allowing any resistant survivors to significantly increase the resistance status of a population. The species also has a short-lived innate dormancy, which results in a large amount of the seedbank germinating in the next season (Gill Reference Gill1996). The ability of rigid ryegrass to rapidly evolve resistance to herbicides makes it a very difficult weed to control, particularly in cereal crops, where herbicide options are more limited.
In 1982, the first case of herbicide resistance in rigid ryegrass was identified only 4 yr after the acetyl CoA carboxylase (ACCase)-inhibiting herbicide diclofop-methyl became commercially available (Heap and Knight Reference Heap and Knight1982). Similarly, resistance in rigid ryegrass to the acetolactate synthase (ALS)-inhibiting herbicide chlorsulfuron was confirmed 4 yr after its release in 1982 (Heap and Knight Reference Heap and Knight1986). Over the past three decades, many rigid ryegrass populations have been confirmed resistant not only to ACCase- and ALS-inhibiting herbicides, but also to other mechanisms of action (MOAs) such as triazine (inhibition of photosynthesis at photosystem II) and dinitroaniline (inhibition of microtubule assembly) herbicides (Boutsalis and Broster Reference Boutsalis and Broster2006; Broster and Pratley Reference Broster and Pratley2006; Broster et al. Reference Broster, Koetz and Wu2011; McAlister et al. Reference McAlister, Holtum and Powles1995; Owen et al. Reference Owen, Walsh, Llewellyn and Powles2007; Pratley et al. Reference Pratley, Graham and Leys1993). A number of rigid ryegrass populations in Australia have evolved resistance to six different herbicide MOAs, although such populations are not common (Boutsalis et al. Reference Boutsalis, Gill and Preston2012; Preston et al. Reference Preston, Tardif, Christopher and Powles1996).
Trifluralin is a dinitroaniline (3(K1)) herbicide that inhibits microtubule assembly (Chambers Reference Chambers1999). Preplant soil incorporation of dinitroaniline herbicides is used for selective weed control for many grasses and some broadleaf weeds (Ashton and Crafts Reference Ashton and Crafts1973; Smeda and Vaughn Reference Smeda and Vaughn1994). Despite their continued use over a long period of time, there are surprisingly few cases of resistance to dinitroanilines (Heap Reference Heap1997; Smeda and Vaughn Reference Smeda and Vaughn1994). According to Heap (Reference Heap2016), dinitroaniline resistance makes up less than 3% of total cases of herbicide resistance worldwide.
Trifluralin resistance in rigid ryegrass in Australia was first reported more than 20 yr ago (McAlister et al. Reference McAlister, Holtum and Powles1995). However, these early trifluralin-resistant populations only showed moderate levels of resistance. More recently there has been a surge in the number of trifluralin-resistant populations reported from South Australian farms, and the expression of resistance also appears to be much stronger (Boutsalis et al. Reference Boutsalis, Gill and Preston2012). In a random survey, trifluralin resistance was found in 33% of South Australian cropping fields but in less than 5% of cropping fields in Victoria (Boutsalis et al. Reference Boutsalis, Gill and Preston2012). Clearly there is a need to better understand the mechanisms of resistance to dinitroaniline herbicides and to develop management strategies for the control of these resistant populations. This study documents the first known case of field-evolved target-site resistance to dinitroaniline herbicides in a population of rigid ryegrass.
Materials and Methods
Plant Material
Rigid ryegrass populations (17) used in the resistance-screening experiment were supplied by agricultural advisors from several broadacre farms in South Australia (including TR1, TR2, TR3, and TR4) where growers had reported control failure with trifluralin (Table 1). Rigid ryegrass populations TR1 to TR17 were all collected from farms within a 20-km radius of Laura. Population SLR31, included as a reference resistant population, has been well documented for its multiple resistance to several herbicides and moderate level of resistance to dinitroaniline herbicides (McAlister et al. Reference McAlister, Holtum and Powles1995; Tardif and Powles Reference Tardif and Powles1999). Susceptible population VLR1 has also been widely investigated and reported to be highly susceptible to different herbicides (Preston Reference Preston2003). SLR31 originates from Bordertown, SA, while VLR1 originates from Serviceton, VIC, from an area with no history of herbicide application, and is susceptible to all herbicides registered for controlling rigid ryegrass. Dose–response experiments included SLR31, VLR1, and the resistant population SLR74, which originates from Kadina, SA. Four of the most resistant populations from the resistance-screening experiment (TR1, TR2, TR3, and TR4), known susceptible populations VLR1 and SLR4, and resistant populations SLR31 and SLR74 were selected for α-tubulin gene sequencing. From the trifluralin-resistant populations, survivors treated with 800 gha−1 trifluralin were sampled for α-tubulin gene sequencing; susceptible populations were not prescreened with trifluralin. The location of the rigid ryegrass populations used in this study are summarized in Table 1.
Table 1 Location of collection sites for rigid ryegrass populations investigated for trifluralin resistance.
Seed Germination and Plant Growth
Rigid ryegrass plants were grown in 2.2-L round plastic pots (Masrac Plastics, Dry Creek, SA, Australia) containing pasteurized potting soil based on sand and peat (McAlister et al. Reference McAlister, Holtum and Powles1995). Potting soil was watered and leveled 10 mm below the top of the pot, and 100 seeds per pot of each population were placed on soil surface. Pots and seed were then covered in plastic film for 24 h before spraying occurred. Seed and chemical were incorporated immediately after herbicide spraying by adding 10 mm of potting soil.
In the resistance-screening experiment, pots were maintained in the greenhouse for 21 d after herbicide treatment. In the dose–response experiments, pots were maintained in a growth room (Phoenix Systems, SA, Australia) maintained at 12-h and 24 C light, 12-h and 12 C dark, with a light intensity of 237 µmolm2s−1. In all experiments, pots were positioned randomly on the benches and rerandomized every 5 to 7 d. Pots were watered as needed to maintain potting mix near field capacity; care was taken to prevent drainage of water.
Resistance Screening and Dose Response to Trifluralin herbicide
A commercial formulation of trifluralin (Trifluralin 480®, 480 gL−1, Crop Care Australasia, Pinkenba, Queensland, Australia), was applied directly onto the seeds placed on the surface of the potting mix. An experimental track sprayer (De Vries Manufacturing, Hollandale, MN) was used to apply trifluralin, delivering 103.5 Lha−1 spray solution through a single flat-fan nozzle (TeeJet® 8002E) at a speed of 3.6 kmh−1. Trifluralin was applied at 0, 200, 400, 800, and 1,600 gaiha−1 in the resistance-screening experiment (March 2007). In dose–response Experiment 1 (September 2007), trifluralin was applied at 0, 50, 100, 200, 400, 800, and 1,600 gha−1, with an additional 25 gha−1 rate used for the susceptible population. In dose–response Experiment 2 (April 2008), 0, 12.5, 25, 50, 100, and 200 gha−1 trifluralin was applied to the susceptible population VLR1; 0, 50, 100, 200, 400, 800, and 1,600 gha−1 was applied to the resistant population SLR31 and 0, 100, 200, 400, 800, 1,600, and 3,200 gha−1 was applied to resistant population SLR74. The recommended field rate for rigid ryegrass control with trifluralin in South Australia is 384 gha−1 for the conventional tillage cropping systems. Directly after herbicide treatment, the soil surface was covered with 10 mm of soil; pots were watered lightly and returned to either the greenhouse (resistance screening) or the growth room (dose response). At 21 d (resistance screening) and 28 d (dose response) after treatment, survival assessments were made and aboveground plant biomass was harvested. Rigid ryegrass plants that had reached the Z12 growth stage at assessment were considered survivors (Zadoks et al. Reference Zadoks, Chang and Konzak1974). The harvested plants were dried in an oven at 60 C for 72 h and weighed. The mean dry weight of all plants was calculated for each population and expressed as a percentage of the untreated controls for that population. In the resistance-screening experiment, populations were classed as resistant if seedling emergence after trifluralin application was at least 20% of the emergence in the control pots (Boutsalis et al. Reference Boutsalis, Gill and Preston2012). Dose–response experiments had four replications, and pots were arranged in a completely randomized design; experiments were run twice in September 2007 and April 2008, respectively. As there was no population by experimental run interaction, data from the two runs were pooled. In the dose–response experiments, LD50 (dose of herbicide required to kill 50% of the plants) and GR50 (dose of herbicide required to reduce shoot biomass per pot by 50%) were obtained by log-logistic analysis (GraphPad Prism v. 7, GraphPad Software, San Diego, CA, USA). Resistance indices (RIs) were calculated as the ratio between the LD50 (or GR50) of each population and the LD50 (or GR50) of the susceptible control (VLR1). The model fitted to pooled data was
where y is the plant survival (%) or biomass reduction (%), x is the log dose of the herbicide used, IC50 is the dose of herbicide required to produce 50% reduction in plant survival or biomass, and b is the slope of the curve.
Sequencing of α-Tubulin Gene
DNA was extracted from five plants of each rigid ryegrass population using the DNeasy Plant Mini Kit (Qiagen, Doncaster, Victoria, Australia) according to the manufacturer’s directions. The concentration of nucleic acids was determined spectrophotometrically on a NanoDrop ND-1000 (Thermo Fisher Scientific, Norwood, SA, Australia) at 260 nm.
Standard PCR conditions, and primers designed against the goosegrass (accession number AJ005599.1) α-tubulin gene sequence (Table 2) were used to amplify a 746-bp fragment covering the equivalent of aa 112–360 in goosegrass.
Table 2 Primer sequences used for amplification and sequencing of the α-tubulin gene in rigid ryegrass from genomic DNA.

a Primers amplify a 746-bp fragment covering the equivalent of aa 112–360 in goosegrass.
For PCR amplification, ~50 ng DNA was added to a standard 25 µl PCR reaction mix containing 1× High Fidelity buffer [60 mM Tris-SO4, pH 8.9, 18 mM (NH4)2SO4], 2 mM MgSO4, 0.4 mM dNTPs, 0.4 µM of each specific primer AW08-Fwd and AW05-Rev (Table 2), and 1 U Platinum Taq High Fidelity DNA Polymerase enzyme mix (Invitrogen, Mt. Waverley, Victoria, Australia). Amplification was carried out in an automated DNA thermal cycler (Eppendorf Mastercycler Gradient, Hamburg, Germany) with PCR conditions as follows: 3-min denaturing at 94 C; 35 cycles of 30-s denaturation at 94 C, 30-s annealing at 55 C, and 45-s elongation at 68 C, and final extension for 7 min at 68 C.
PCR products were prepared with 1× Ficoll loading dye (15% [w/v] Ficoll 4000, 0.25% [w/v] bromophenol blue, 0.25% [w/v] xylene cyanol FF) and visualized on ethidium bromide–stained (1 mgml−1) 1.4% agarose gels. Samples were electrophoresed in 1× TAE Buffer (40 mM Trizma base, 1 mM Na2EDTA, pH to 8 with glacial acetic acid) at 100 V and photographed under UV light (λ302 nm). DNA fragment sizes were estimated by comparing their mobility to bands of known sizes of a low mass molecular weight marker (Invitrogen, Australia). DNA sequencing was conducted by the Australian Genome Research Facility using forward primer α-TubF and reverse primer α-TubR (Table 2). Sequence data were analyzed using ContigExpress and Align X from the Vector-NTI Suite 6 programs (Invitrogen, Australia).
Results and Discussion
Trifluralin Resistance Screening and Dose Responses
Resistance-screening tests confirmed resistance in 15 out of 17 field populations of rigid ryegrass suspected to be trifluralin-resistant due to repeated control failures (Table 3). Survival in the resistant populations ranged from 23% to 100% at 400 gha−1 trifluralin (a little higher than the recommended field rate of 384 gha−1). SLR31 (64% survival) and SLR74 (80% survival) were also classified as being resistant to trifluralin. The known susceptible population VLR1 was completely controlled at the lowest rate of 200 g ha−1. Plant survival in the four most-resistant rigid ryegrass populations (TR1, TR2, TR3, and TR4) ranged from 38% to 45% at 1,600 g ha−1 trifluralin, which is more than 4-fold the registered field rate. These four populations along with SLR74 and SLR31 were selected for sequencing of the α-tubulin gene. Rigid ryegrass shoot biomass was also assessed in the screening experiment, and results supported survival data (unpublished data).
Table 3 Survival of rigid ryegrass populations treated with different rates of trifluralin.
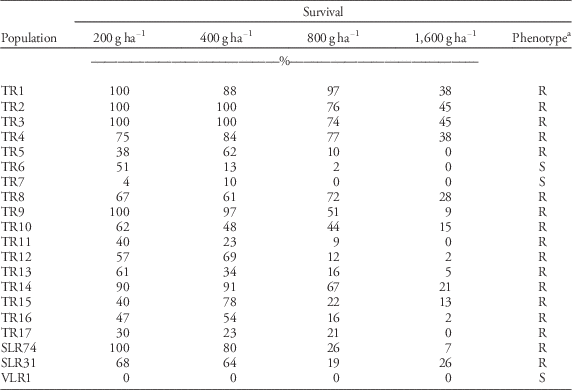
a Populations were classified as resistant (R) to trifluralin if plant survival at the label rate (400 g ha−1) was at least 20% (Boutsalis et al. Reference Boutsalis, Gill and Preston2012). S, susceptible.
In the dose–response experiments, the susceptible population VLR1 was completely controlled with trifluralin at 400 gha−1 (Figure 1). In contrast, plant survival at 400 gha−1 trifluralin for the resistant populations was 47% for SLR31 and 67% for SLR74 (Figure 1a). Shoot biomass data confirmed the trends observed for plant survival (Figure 1b). VLR1 was completely killed at 100 g ha−1 trifluralin. In contrast, shoot biomass reduction in rigid ryegrass at the field rate was 74% for SLR31 and 47% for SLR74 (Figure 1b).
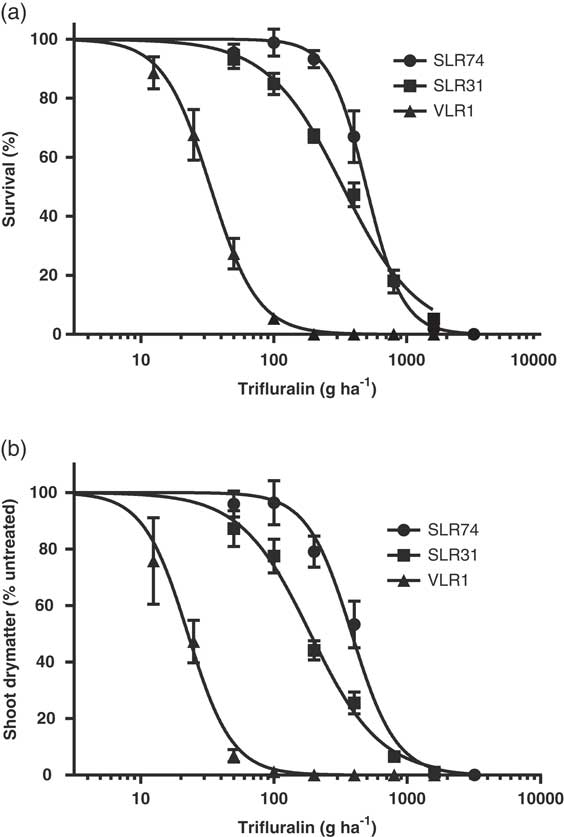
Figure 1 (a) Survival (% of untreated control) and (b) shoot dry matter (% of untreated control) of rigid ryegrass populations (susceptible VLR1 and resistant SLR31 and SLR74) treated with trifluralin. Data were pooled across two experiment runs; each data point is the mean of four replicates per experiment; and vertical bars are standard error of mean.
Some resistant plants survived and grew normally even at two to four times the recommended herbicide rate. The LD50 for trifluralin for the susceptible population VLR1 was 33 g ha−1, which is only 9% of the recommended field rate (Table 4). The resistant population SLR31 had an LD50 of 331 gha−1, this is 9.9-fold greater than that of VLR1 (Table 4). A similar level of trifluralin resistance in this population was previously reported by McAlister et al. (Reference McAlister, Holtum and Powles1995). The resistant population SLR74 had an LD50 of 496 g ha−1. The LD50 of SLR74 was 14.9-fold higher than that of the susceptible population (Table 4). For the resistant population SLR31, the dose of trifluralin required to reduce 50% of shoot biomass (GR50) was 188 gha−1, which was 8.3-fold greater than that of the susceptible population (Table 4). GR50 for SLR74 was 384 gha−1, which was 17-fold greater than VLR1. As there was no overlap of confidence intervals for LD50 or GR50 between SLR74 and SLR31, it can be concluded that SLR74 had a higher level of trifluralin resistance than SLR31 (Table 4). Based on the results of plant survival, SLR74 was 1.5-fold more resistant than SLR31 and 2-fold more resistant in terms of plant shoot biomass.
Table 4 Estimated LD50, GR50, slope values (b), and resistance index (RI) values for rigid ryegrass populations treated with trifluralin.Footnote a

a Values in parentheses are 95% confidence intervals; data were pooled across both dose–response experiments.
While resistance to dinitroaniline herbicides such as trifluralin only makes up as little as 3% of total herbicide resistance worldwide, resistance to this herbicide has been confirmed in several grass weed species. This includes rigid ryegrass in Australia (McAlister et al. Reference McAlister, Holtum and Powles1995), goosegrass in the United States (Mudge et al. Reference Mudge, Gossett and Murphy1984), blackgrass (Alopecurus myosuroides Huds.) in England (Moss Reference Moss1990), green foxtail [Setaria viridis (L.) Beauv.] in Canada (Morrison et al. Reference Morrison, Beckie and Nawolsky1991), annual bluegrass (Poa annua L.) from the United States (Isrigg et al. Reference Isrigg, Yelverton, Brownie and Warren2002), shortawn foxtail (Alopecurus aequalis Sobol.) from Japan (Hashim et al. Reference Hashim, Jan, Sunohara, Hachinoche, Ohdan and Matsumoto2011), and American sloughgrass [Beckmannia syzigachne (Steud.) Fernald] from Japan (Heap Reference Heap2016).
Long-term exposure of rigid ryegrass populations to trifluralin in South Australia has resulted in the evolution of resistance to this herbicide. Increased incidence of rigid ryegrass populations with resistance to POST herbicides during the 1990s saw an increased emphasis on PRE herbicides such as trifluralin for rigid ryegrass control in South Australia (Boutsalis et al. Reference Boutsalis, Gill and Preston2012). It is likely that this increased dependence on trifluralin increased the selection pressure for resistance to this herbicide. This study has confirmed the presence of field-evolved resistance to trifluralin in local rigid ryegrass populations.
Sequencing of α-Tubulin Gene
A 746-bp fragment of the plastidic α-tubulin gene was sequenced from five individuals of six trifluralin-resistant and two susceptible populations. The nucleotide sequences of some individuals of the resistant populations (SLR74, TR2, and TR4) differed from that of the susceptible populations by a single nucleotide, predicting a single amino acid modification. In populations SLR74, TR2, and TR4, a single nucleotide change from ACT to ATT resulted in a predicted amino acid substitution of threonine to isoleucine at codon 239. In SLR74, all 5 individuals (4 of the 5 mutants were homozygous) sequenced contained threonine at position 239, whereas 4 out of 5 plants (all 4 mutants were homozygous) contained this mutation in TR2 and 3 out of 5 plants (all 3 mutants were heterozygous) in TR4. In populations TR2 and TR4, a single base change from GTC to TTC resulted in a predicted amino acid substitution of valine to phenylalanine at codon 202. From the 5 individuals sequenced from these two populations, 1 individual (heterozygous) from TR2 and 3 from TR4 (1 homozygous and 2 heterozygous of the 3 mutants) had the Val-202-Phe amino acid substitution. The individual from population TR2 that had the amino acid substitution Val-202-Phe was the only individual sequenced from that population that did not have the Thr-239-Ile amino acid substitution. Similarly, in population TR4 the 2 individuals that did not have the Thr-239-Ile did have the Val-202-Phe amino acid substitution; one individual from this population had both (heterozygous) amino acid substitutions (Table 5).
Table 5 Comparison of nucleotide sequence and derived amino acid sequence of a highly conserved region of the α-tubulin gene from susceptible (S) and resistant (R) rigid ryegrass populations.Footnote a
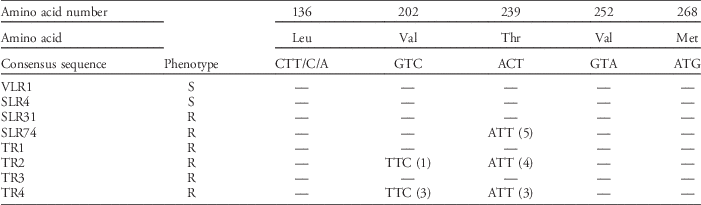
a Figures in parentheses are numbers of individuals in which specific mutation was identified.
The amino acid change from threonine to isoleucine at position 239, and the valine to phenylalanine at position 202 of the α-tubulin gene are both likely to be major mechanisms of resistance to dinitroaniline herbicides in some of the resistant rigid ryegrass populations investigated. Rigid ryegrass populations with the Thr-239-Ile mutation and Val-202-Phe exhibited high levels of trifluralin resistance (Tables 3 and 4; Figure 1). Trifluralin-resistant rigid ryegrass populations that did not exhibit either mutation (SLR31, TR1, and TR3), could possibly have non–target site mechanisms of resistance. McAlister et al. (Reference McAlister, Holtum and Powles1995) found that trifluralin resistance in SLR31 was not caused by reduced herbicide absorption and translocation. Further work by Tardif and Powles (Reference Tardif and Powles1999) found evidence that in SLR31, resistance to pendimethalin, another dinitroaniline herbicide, was most likely related to increased plant metabolism. The target-site mutation Thr-239-Ile identified in SLR74, TR2, and TR4 has previously been found to cause trifluralin resistance in goosegrass (Anthony et al. Reference Anthony, Waldin, Ray, Bright and Hussey1998; Yamamoto et al. Reference Yamamoto, Zeng and Baird1998) and also in green foxtail (Délye et al. Reference Délye, Menchari, Michel and Darmency2004). The target-site mutation Val-202-Phe identified in TR2 and TR4 has previously been found in trifluralin-resistant shortawn foxtail (Hashim et al. Reference Hashim, Jan, Sunohara, Hachinoche, Ohdan and Matsumoto2011). Two other previously reported mutations that confer trifluralin resistance were also sequenced but not found in rigid ryegrass populations. Yamamoto et al. (Reference Yamamoto, Zeng and Baird1998) reported the amino acid substitution of methionine to threonine at the 268 position to confer trifluralin resistance in goosegrass; however, this mutation was not found in any rigid ryegrass populations in this study. Another α-tubulin gene mutation leucine to phenylalanine at the 136 position has been found to cause trifluralin resistance in green foxtail (Délye et al. Reference Délye, Menchari, Michel and Darmency2004), but again this mutation was not found in rigid ryegrass populations in this study. While it is not a documented mutation conferring trifluralin resistance in plants, valine at position 252 has been recognized as a key binding site for dinitroaniline herbicides on plant α-tubulins (Délye et al. Reference Délye, Menchari, Michel and Darmency2004; Reference Nyporko and BlumeNyporko and Blume 2014); however, no mutation was found at this site in rigid ryegrass populations investigated here.
Target-site resistance to the dinitroaniline herbicides is inherited as a recessive trait (Jasieniuk et al. Reference Jasieniuk, Brule-Babel and Morrison1994; Tian et al. Reference Tian, Délye and Darmency2006; Zeng and Baird Reference Zeng and Baird1997) and has previously been identified in weed species that are either fully or partially self-pollinated. It is much easier to select for target-site resistance in self-pollinated species as two copies of the resistance allele are required for survival. In contrast, in obligate outcrossing species such as rigid ryegrass, non–target site resistance to these herbicides is much easier to select for than target-site resistance. However, we have demonstrated here that both target-site and non–target site resistance are present in trifluralin-resistant populations of this species (Table 5). While the resistance trait is recessive, it may still be possible to select for resistance to trifluralin, so long as there is a rate of herbicide where there is greater survival of the heterozygote compared with the homozygous susceptible individuals. Trifluralin activity in the soil decays over a long period of time in Australian farming systems (Johnstone et al. Reference Johnstone, Jolley, Code, Moerkerk and Corbett1998). While heterozygous resistant individuals may be killed by the full dose of the herbicide, later-germinating weeds will be exposed to a lower dose, and differential survival may occur.
Of the trifluralin-resistant populations for which α-tubulin was sequenced in this work, 50% did not carry a target-site mutation (Table 5) and resistance was due to one or more non–target site mechanisms. This is in contrast to other grass species with resistance to dinitroaniline in which target-site mutations have been observed (Anthony et al. Reference Anthony, Waldin, Ray, Bright and Hussey1998; Délye et al. Reference Délye, Menchari, Michel and Darmency2004; Hashim et al. Reference Hashim, Jan, Sunohara, Hachinoche, Ohdan and Matsumoto2011; Yamamoto et al. Reference Yamamoto, Zeng and Baird1998). SLR31 was the first example of trifluralin resistance identified in rigid ryegrass (McAlister et al. Reference McAlister, Holtum and Powles1995), and it does not have a mutation within α-tubulin (Table 5). It is probable that non–target site resistance would have evolved first and target-site resistance later in this species. As individuals carrying target-site mutations are more resistant to trifluralin (Table 4), once target-site mutations have been selected for, continued selection with trifluralin will tend to favor these individuals over those with non–target site resistance.
This study is the first documented case of field-evolved target-site resistance to dinitroaniline herbicides in populations of rigid ryegrass. Resistance was associated with amino acid substitutions (Thr-239-Ile or Val-202-Phe) in the CT domain of the α-tubulin gene. As a consequence of the presence of these target-site mutations in the field populations of rigid ryegrass, this herbicide group is becoming ineffective in South Australia, where up to one-third of rigid ryegrass populations are trifluralin resistant (Boutsalis et al. Reference Boutsalis, Gill and Preston2012). Even though some weed control can be achieved in these populations by increasing the trifluralin dose, this is unlikely to be effective, due to increased risk of crop damage. Herbicides with different sites of action are needed to provide effective control of dinitroaniline-resistant populations. The use of triallate and propyzamide and the introduction of pyroxasulfone and prosulfocarb+S-metalachlor herbicides has enabled growers to manage these populations (Boutsalis et al. Reference Boutsalis, Gill and Preston2014). Growers should also consider integration of hay production and grain legume crops, which allows use of mechanical (hay) methods and nonselective herbicides for preventing weed seed production. Greater diversity in weed management tactics is required for the long-term effective management of herbicide-resistant weed populations.
Acknowledgments
The authors are grateful for the financial support for the postgraduate scholarship from the Commonwealth of Australia and the CRC for Weed Management. They are also grateful to Angela Wakelin for her support and guidance in the early stages of the gene-sequencing work.








