Introduction
Late watergrass [Echinochloa phyllopogon (Stapf) Koso-Pol.] is a persistent weed that has been reported to have developed resistance to acetolactate synthase (ALS) inhibitors in the United States (Fischer et al. Reference Fischer, Ateh, Bayer and Hill2000), Greece (Kaloumenos et al. Reference Kaloumenos, Chatzilazaridou, Mylona, Polidoros and Eleftherohorinos2013), South Korea (Lee et al. Reference Lee, Kim and Lee2017), and most recently, northeastern China. The ability to rapidly develop resistance to herbicides indicates that E. phyllopogon may become a great agricultural challenge in the future. Historically, the use of herbicides, including penoxsulam, an ALS inhibitor, was the most important means to control E. phyllopogon, but their effectiveness has waned in recent years.
ALS, also known as acetohydroxyacid synthase, is the first enzyme in the biosynthesis pathway controlling the synthesis of three branched-chain amino acids: Leu, Ile, and Val (Duggleby et al. Reference Duggleby, McCourt and Guddat2008; Ray Reference Ray1984). Therefore, ALS has been the target of multiple classes of chemical herbicides, including pyrimidinylthiobenzoates (PTBs), sulfonylureas (SUs), imidazolinones (IMIs), sulfonylaminocarbonyltriazolinones (SCTs), and triazolopyrimidines (TPs). Penoxsulam, an ALS inhibitor belonging to the TP family, has been one of the most effective herbicides in rice fields in China since 2008; however, continued use has resulted in the rapid evolution of resistance to this compound in E. phyllopogon.
ALS-inhibiting herbicides have been widely used since their first introduction in 1982. The first resistance to them was reported in 1987 in prickly lettuce (Lactuca serriola L.) (Guttieri et al. Reference Guttieri, Eberlein, Malllory-Smith and Thill1992) in the United States, 5 years after its first use, and resistance cases have continued to be reported. Globally, 160 biotypes of weed species have evolved resistance to ALS-inhibiting herbicides (Tranel et al. Reference Tranel, Wright and Heap2019 ).
There are two mechanisms of herbicide resistance, one is non–target site resistance (NTSR), and the other is target-site resistance (TSR) (Powles and Yu Reference Powles and Yu2010). Previous studies on E. phyllopogon’s mechanisms of resistance to ALS inhibitors have generally concluded that resistance is mostly due to enhanced herbicide metabolism, possibly via cytochrome P450 mono-oxidation (Iwakami et al. Reference Iwakami, Endo, Saika, Okuno, Nakamura, Yokoyama, Watanabe, Toki, Uchino and Inamura2014a, Reference Iwakami, Uchino, Kataoka, Shibaike, Watanabe and Inamura2014b; Yasuor et al. Reference Yasuor, Osuna, Ortiz, Saldain, Eckert and Fischer2009; Yun et al. Reference Yun, Yogo, Miura, Yamasue and Fischer2005).
The most common cause of TSR is amino acid substitution due to a genetic mutation in a target enzyme, which alters the effects of herbicide binding (Yu and Powles Reference Yu and Powles2014). In terms of ALS inhibitors, 26 types of amino acid substitutions at eight conserved positions of the ALS gene have been reported in various weed species (Yu and Powles Reference Yu and Powles2014). The eight conserved positions are Ala-122 (Han et al. Reference Han, Yu, Purba, Li, Walsh, Friesen and Powles2012; Trucco et al. Reference Trucco, Hager and Tranel2006), Pro-197 (Bi et al. Reference Bi, Liu, Li, Yuan, Jin and Wang2013; Cui et al. Reference Cui, Li, Wang, Wang, Wei and Cao2012; Krysiak et al. Reference Krysiak, Gawronski, Adamczewski and Kierzek2011), Ala-205 (Ashigh and Tardif Reference Ashigh and Tardif2007), Asp-376 (Feng et al. Reference Feng, Gao, Zhang, Dong and Li2016; Huang et al. Reference Huang, Chen, Zhang, Huang, Wei, Zhou, Chen and Wang2016), Arg-377 (Massa et al. Reference Massa, Krenz and Gerhards2011), Trp-574 (Riar et al. Reference Riar, Tehranchian, Norsworthy, Nandula, McElroy, Srivastava, Chen, Bond and Scott2015), Ser-653 (Whaley et al. Reference Whaley, Wilson and Westwood2006), and Gly-654 (Laplante et al. Reference Laplante, Rajcan and Tardif2009; Shivrain et al. Reference Shivrain, Burgos, Sales and Kuk2010) (numbering refers to the Arabidopsis thaliana ALS gene sequence). Substitutions at Ala-122, Ser-653, and Trp-574 have been reported to confer resistance to ALS-inhibiting herbicides in barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.] and other Echinochloa species (Kaloumenos et al. Reference Kaloumenos, Chatzilazaridou, Mylona, Polidoros and Eleftherohorinos2013; Matzenbacher et al. Reference Matzenbacher, Bortoly, Kalsing and Merotto2015; Panozzo et al. Reference Panozzo, Scarabel, Tranel and Sattin2013). However, the mutations in the complete ALS gene of E. phyllopogon have not yet been reported, except for a Trp-574-Leu mutation found in an ALS gene fragment of E. phyllopogon (Kaloumenos et al. Reference Kaloumenos, Chatzilazaridou, Mylona, Polidoros and Eleftherohorinos2013).
This investigation aimed to (1) identify the penoxsulam resistance in E. phyllopogon populations, (2) explore the target-site basis for penoxsulam resistance, and (3) characterize the cross-resistance profiles of E. phyllopogon populations.
Materials and Methods
Plant Materials
In 2015, seeds from 30 E. phyllopogon populations were collected from the rice fields of several large farms in Heilongjiang Province, China, including Hongwei Farm, 856 Farm, and Qianjin Farm, where penoxsulam applied at the recommended dose failed to control this weed. All seeds were collected by hand, air-dried in the shade, and stored in paper bags at 4 C until used. Populations were propagated annually to ensure a continual supply of viable seed. Seed lots were numbered according to their collection sites.
Whole-Plant Response Experiment with Penoxsulam
After bioassay screening of 30 E. phyllopogon populations with penoxsulam, sensitive (S) and resistant (R) populations were identified and named HSRH-538 (S) (location coordinates are 47.301°N, 133.287°E) and HSRH-520 (R) (location coordinates are 47.301°N, 133.287°E). Plastic pots (9-cm diameter by 10-cm height) were filled with a soil:fertilizer mixture 1:1 (v/v), and 25 seeds from either the HSRH-538 (S) or HSRH-520 (R) population were placed on the soil surface of each pot and covered with 1 cm of the same mixture. During the growth period, plastic pots were placed in incubators at 30 C/25 C (light/dark temperature) with a 12-h light/12-h dark cycle, light intensity of 12,000 lux, and 85% relative humidity. There were 15 plants per pot before herbicide treatment. When E. phyllopogon grew to the 2- to 3-leaf stage, herbicides were applied to the plants using a laboratory sprayer equipped with a flat-fan nozzle delivering 280 L ha−1 at 230 kPa. Penoxsulam was applied at 0, 3.75, 7.5, 15, 30, and 60 g ai ha−1 to the HSRH-520 (R) population and at 0, 1.88, 3.75, 7.5, 15 and 30 g ai ha−1 to the HSRH-538 (S) population. Treated plants were returned to the incubators and cultured as described above. The amount of fresh aboveground biomass was determined at 3 wk after penoxsulam application. The experiment was repeated twice, with four replicates per dose per experiment.
Sensitivity to Other ALS Inhibitors
To define the cross-resistance relationships of the penoxsulam-resistant E. phyllopogon populations, activities of ALS-inhibiting herbicides on HSRH-520 (R) were determined through whole-plant bioassays as described in the previous section. The ALS inhibitors selected for the bioassays included all types of inhibitors currently used and those newly registered for use in rice fields across China (Table 1). For the HSRH-538 (S) population, the highest dose given was the recommended dose (30 g ha−1), while the highest dose for the HSRH-520 (R) population was 60 g ha−1.
Table 1. Herbicides used in this study

Gene Cloning and Sequencing
Young shoot tissues were taken from individual plants at the 3- to 4-leaf stage for DNA extraction with the Plant Genomic DNA kit (Tiangen Biotech, Beijing, China), according to the manufacturer’s instructions. According to the two ALS gene sequences of E. phyllopogon (accessions AB636580 and AB636581) retrieved from the National Center for Biotechnology Information (NCBI) GenBank database, two specific pairs of primers were designed, as shown in Table 2, in Primer Premier v. 5.0 (Premier Biosoft International, Palo Alto, CA, USA) and used to amplify the complete sequence of ALS in E. phyllopogon. The PCR mixture contained 2.5 ng of template DNA, 2 μl of each primer (10 μM), 25 μl of 2× Phanta Max Buffer containing Mg2+ at a final concentration of 2 mM (Vazyme Biotech, Nanjing, China), 1 μl of Phanta Max Super-Fidelity DNA Polymerase (Vazyme Biotech), 2 μl of dNTP Mix (10 mM; Vazyme Biotech), and ddH2O to a final volume of 50 μl. In addition, as PCR additives, 5% v/v glycerin and 5% v/v dimethyl sulfoxide were added. To amplify full-length ALS gene sequences of E. phyllopogon, the following touchdown PCR program was set: 5 min at 95 C for DNA denaturation, followed by 20 touchdown cycles of denaturation at 95 C for 15 s, annealing at 60 C (−0.5 C per cycle) for 15 s, and elongation at 72 C for 75 s; then, 15 standard amplification cycles of denaturation at 95 C for 15 s, annealing at 50 C for 15 s, and elongation at 72 C for 75 s; and a final 10 min at 72 C for further DNA elongation. The amplification products were purified using TaKaRa MiniBEST agarose gel DNA extraction kit (TaKaRa Biotechnology, Dalian, China) and then cloned into a pMD19-T vector (TaKaRa Biotechnology). The plasmids containing the fragment insertions were bidirectionally sequenced by GenScript Biotechnology (Nanjing, China). Ten plants from each of the two populations were selected for gene cloning. At least eight transformed clones of each plant were sequenced to obtain complete ALS sequences that were aligned and compared in BioEdit Sequence Alignment Editor v. 7.2.5, (Tom Hall, Carlsbad, CA, USA). The BLAST procedure within the NCBI database was used to verify the accuracy of the sequences obtained.
Table 2. Primers used for amplification of Echinochloa phyllopogon ALS gene

a Melting temperature (Tm) was calculated using Primer Premier v. 5.0 (Premier Biosoft International, Palo Alto, CA, USA).
b Numbers indicate the corresponding cDNA region of ALS1 (AB636580) and ALS2 (AB636581).
In Vitro ALS Activity Assay
The response of the ALS enzyme to penoxsulam was determined using crude enzyme extracts. Young leaves from HSRH-520 and HSRH-538 at the 3- to 4-leaf stage were taken separately for in vitro assays of ALS activity as described by Yu et al. (Reference Yu, Friesen, Zhang and Powles2004), with slight modifications detailed below. Leaf blades were harvested from each population (3 g), powdered in liquid nitrogen, and suspended in enzyme extraction buffer (4 ml) containing 100 mM potassium phosphate buffer (pH 7.5), 10 mM sodium pyruvate, 1 mM MgCl2, 1 mM thiamine pyrophosphate, and 10 mM flavine adenine dinucleotide (FAD). Each dark reaction contained 100 μl of protein extract, 200 μl of enzyme assay buffer (100 mM potassium phosphate buffer, pH 7.5, 200 mM sodium pyruvate, 20 mM MgCl2, 2 mM thiamine pyrophosphate, 20 μM FAD, and 1 mM dithiothreitol) and 100 μl ALS inhibitor (penoxsulam at 0.005, 0.05, 0.5, 5, 50, 500, and 5,000 μM). A nontreated (no penoxsulam applied) control was included in each assay for comparison. Acetoin was formed by incubating the mixture with 100 μl creatine solution (0.55%) and 100 μl α-naphthol solution (5.5% in 5 N NaOH) at 60 C for 30 min. ALS activity was monitored colorimetrically (530 nm) on a microplate photometer (Thermo Fisher, Waltham, MA, USA) by measuring acetoin production. The assay was performed twice with independent extractions, each with three replications per herbicide concentration.
Computational Analysis of ALS Binding with Penoxsulam and other ALS Inhibitors
Because the crystal structure of ALS in E. phyllopogon had not been reported, homology modeling of the structure was required before molecular-docking verification. Using the BLAST function in NCBI to search for template protein sequences, 5WJ1 was selected as a template for homology modeling; sequence consistency with HSRH-520 and HSRH-538 was 74.7% and 74.5%, respectively. The 3D structure was constructed using Modeller v. 9.1.4 (laboratory of Andrej Sali, University of California, San Francisco, USA). The 500 template images were generated by single template model, and the 50 top-ranked constellations were screened by DOPE. Then, the 50 top-ranked constellations were screened by PROCHECK. The intersections of the two were taken and used to screen out the optimal conformation as a model of E. phyllopogon ALS, the docking receptor. The software used for docking was AutoDockTools v. 1.5.6, and the size of the docking box was 40 Å by 40 Å by 40 Å. Flexible docking was adopted using a Lamarckian genetic algorithm.
Data Analysis
The data of whole-plant response experiment and in vitro ALS activity assay were subjected to ANOVA in R v. 3.1.3 (R Foundation for Statistical Computing, Vienna, Austria). ANOVA results showed no significant difference between assay repetitions, and results were therefore averaged. Data were then pooled and fit to a four-parameter nonlinear logistic-regression model (Equation 1) and calculated in SigmaPlot v. 13.0 (SigmaPlot Software, Chicago, IL, USA) to get the effective dose of herbicide causing a 50% reduction in fresh weight (ED50):
where Y denotes fresh weight, expressed as a percentage of the nontreated control at dose x of the herbicide, b is the slope, c is the lower limit, d is the upper limit, and g is the herbicide dose at the point of inflection, halfway between the upper and lower limits. The same analysis was used to calculate the herbicide concentrations required to inhibit 50% of ALS activity (IC50) in enzymatic assays.
Results and Discussion
Sensitivity to Penoxsulam
The whole-plant response experiments established that the ED50 (the effective dose of the herbicide that can reduce growth or survival of weeds by 50%) of HSRH-520 (29.7 g ha−1) was very close to the recommended dose (30 g ha−1), while that of HSRH-538 (1.17 g ha−1) was much lower than the recommended dose. The resistance index (RI) for the resistant population HSRH-520 was 25.4, indicating a high level of resistance to penoxsulam, according to Beckie and Tardif (Reference Beckie and Tardif2012). The specific data are shown in Table 3. The results of this whole-plant response experiment confirmed that E. phyllopogon populations in China have developed resistance to penoxsulam.
Table 3. ED50 and IC50 values of penoxsulam for two Echinochloa phyllopogon populations.a

a ED50 refers to the effective dose of herbicide causing 50% inhibition in fresh weight and is indicated in grams of active ingredient per hectare (g ai ha−1). Within a row, ED50 or IC50 values with different letters are significantly different at P < 0.05. Data are the mean ± SE of two experiments. IC50 is the herbicide concentration inhibiting ALS activity by 50%.
b RI (resistance index) = ED50 of resistant biotype divided by ED50 of sensitive biotype.
c RR (resistance ratio) = IC50 value of resistant biotype divided by IC50 value of sensitive biotype.
d R, resistant population; S, sensitive population.
Sensitivity to Other ALS Inhibitors
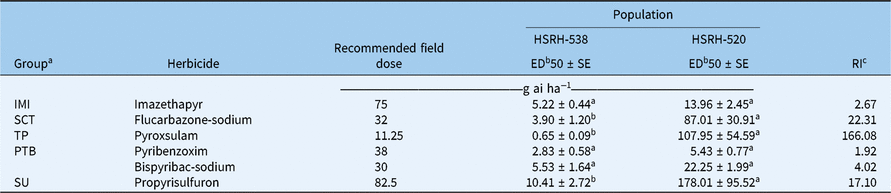
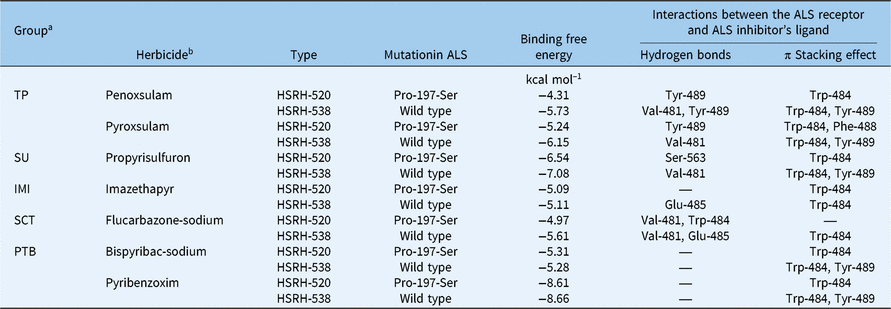
Penoxsulam-resistant E. phyllopogon exhibits cross-resistance to other, but not all, ALS-inhibiting herbicides. More specifically, there is almost no difference between the responses of the two populations examined to either the IMI (imazethapyr) or PTB (pyribenzoxim, bispyribac-sodium) families of herbicides, with the RI ranging from 1.92 to 4.02; on the contrary, HSRH-520 has obvious resistances to the SCT (flucarbazone-sodium), TP (pyroxsulam), and SU (propyrisulfuron) families, as the RIs are 22.3, 166, and 17.1, respectively. Detailed data are listed in Table 4. These results illustrate the complexity of cross-resistance to ALS-inhibiting herbicides in E. phyllopogon.
Table 4. Sensitivity of the two Echinochloa phyllopogon populations to other ALS-inhibiting herbicides

a IMI, imidazolinone; PTB, pyrimidinylthiobenzoate; SCT, sulfonylaminocarbonyltriazolinone; SU, sulfonylurea; TP, triazolopyrimidine.
b ED50 refers to the effective dose of herbicide causing 50% inhibition in fresh weight and is indicated in grams of active ingredient per hectare (g ai ha−1). ED50 values with different letters are significantly different at P < 0.05. Data are the mean ± SE of two experiments.
c RI, resistance index.
It was previously thought that target-site ALS cross-resistance patterns depend on the mutation position. For example, mutations at Ala-122 provide resistance to IMI but not to SU herbicides, mutations at Pro-197 lead to resistance to SU but not to IMI herbicides, and mutations at Trp-574 confer resistance to both SU and IMI herbicides (Han et al. Reference Han, Yu, Purba, Li, Walsh, Friesen and Powles2012). Now, with multiple mutations found at a given ALS site and different cross-resistances detected in various species, it is recognized that cross-resistance is not only dependent on the position of the mutation but is also affected by the specific mutation, ALS inhibitor, and weed species. This means that there is no absolute connection between cross-resistance patterns and specific ALS mutations. For instance, at position 122, substitution of alanine to threonine causes resistance to IMI herbicides only, whereas substitution of alanine to tyrosine imparts a high level of resistance to IMI, SU, and TP herbicides (Han et al. Reference Han, Yu, Purba, Li, Walsh, Friesen and Powles2012); substitution of alanine to valine confers partial resistance to some SU herbicides (Krysiak et al. Reference Krysiak, Gawronski, Adamczewski and Kierzek2011). Additionally, Pro-197 is found at an α-helix at the entrance to active-site access channels. Pro-197 mutations only prevent access of SU herbicides and have no effect on the access of IMI herbicides (McCourt et al. Reference McCourt, Pang, King-Scott, Guddat and Duggleby2006), unless there are substitutions of a bulky amino acid such as Pro-197-Leu (Sibony et al. Reference Sibony, Michel, Haas, Rubin and Hurle2001). In this study, the resistant population HSRH-520, which has the Pro-197-Ser mutation, showed cross-resistance to SU, TP, and SCT herbicides but was still sensitive to IMI and PTB herbicides; this is consistent with previous reports on the same mutation in other weeds. For example, Pro-197-Ser mutations in A. thaliana and sugar beet (Beta vulgaris L.) were demonstrated to result in resistance to SU and TP but not to IMI herbicides (Haughn and Somerville Reference Haughn and Somerville1986; Mourad and King Reference Mourad and King1992; Wright and Penner Reference Wright and Penner1998); the resistance to SU caused by the Pro-197-Ser mutation has been confirmed in many other weeds, including rigid ryegrass (Lolium rigidum Gaudin) (Yu et al. Reference Yu, Han, Vila-Aiub and Powles2010), hare barley [Hordeum murinum L. ssp. leporinum (Link) Arcang] (Yu et al. Reference Yu, Nelson, Zheng, Jackson and Powles2007), annual sedge (Cyperus compressus L.) (McCullough et al. Reference McCullough, Yu, McElroy, Chen, Zhang, Grey and Czarnota2016), and flixweed [Descurainia sophia (L.) Webb. Ex Prantl] (Yang et al. Reference Yang, Deng, Wang, Liu, Li and Zheng2018). In general, there is a trend of cross-resistance between SU and TP herbicides and between IMI and PTB herbicides. (Devine and Eberlein Reference Devine, Eberlein, Roe, Burton and Kuhr1997) High-level resistance to SU and TP herbicides is generally conferred by the Pro-197 substitutions, and that is reflected in this study too.
Gene Cloning and Sequence Analysis
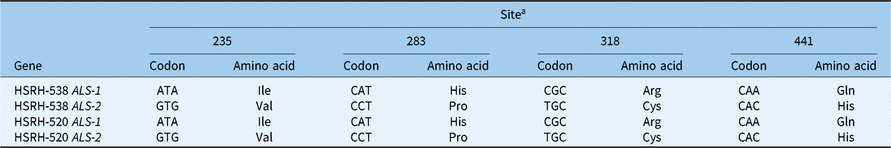
According to the work of Satoshi Iwakami (Iwakami et al. Reference Iwakami, Uchino, Watanabe, Yamasue and Inamura2012), we amplified two copies of the complete coding sequence (CDS) of ALS in E. phyllopogon using two pairs of primers. Then, two ALS sequences of HSRH-520 and HSRH-538 were isolated. The BLAST procedure conducted for the ALS sequences showed high similarity (98% to 99%) with E. phyllopogon (AB636580 and AB636581), indicating that the correct ALS sequences from E. phyllopogon were amplified. The single-nucleotide polymorphisms (SNPs) detected in the copies of the ALS sequences from HSRH-520 and HSRH-538 (Table 5) confirm the presence of two different copies of ALS sequences.
Table 5. The single-nucleotide polymorphisms in multiple sites of ALS sequences in two Echinochloa phyllopogon populations

a Numbered on the basis of the corresponding sequence of Arabidopsis thaliana.
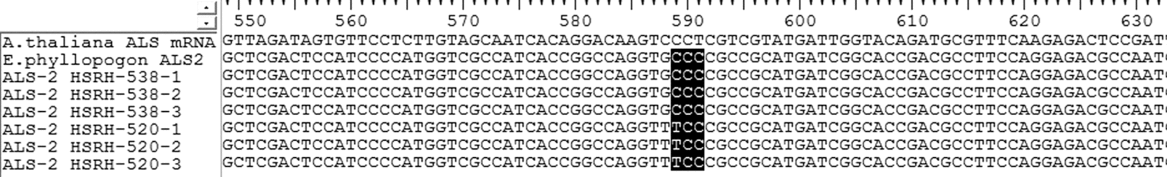
A nucleotide mutation (CCC to TCC) was found in ALS-2 when HSRH-520 was compared with HSRH-538, resulting in the substitution of proline for serine at position 197 (Figure 1); a mutation site previously identified in other weeds but not in E. phyllopogon.
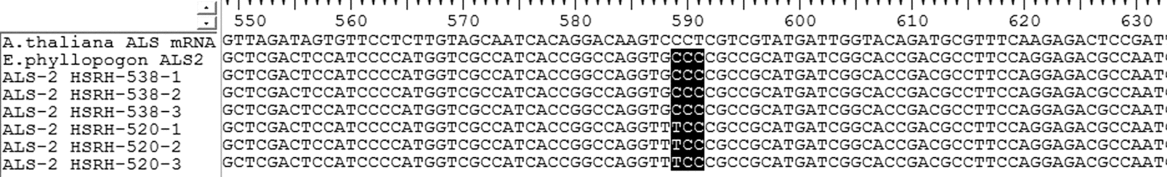
Figure 1. Nucleotide sequence alignment of acetolactate synthase (ALS) gene fragments originating from resistant (R) (HSRH-520) and sensitive (S) (HSRH-538) Echinochloa phyllopogon populations. Each population is listed with three single plant repeats. The observed base sequence mutation (CCC to TCC) is marked in bold. The codon positions refer to the ALS gene in Arabidopsis thaliana.
This is the first report on the Pro-197-Ser mutation in the complete ALS gene of E. phyllopogon. The difficulty in obtaining the CDS of ALS is related to the extremely high GC content at its 5′ region (approximately 78% in 400 bp of the 5′ region), just as with other Poaceae genes (Tatarinova et al. Reference Tatarinova, Alexandrov, Bouck and Feldmann2010; Wong et al. Reference Wong, Wang, Tao, Tan, Zhang, Passey and Yu2002). Multiple copies of genes encoding ALS were reported in several polyploid weed species. For example, two copies of active ALS genes were also observed in rock bulrush [Schoenoplectus juncoides (Roxb.) Lye], a sedge weed often found in rice fields (Uchino et al. Reference Uchino, Ogata, Kohara, Yoshida, Yoshioka and Watanabe2010). These previous studies highlight the necessity of obtaining a single copy of the target gene in E. phyllopogon, a heterotetraploid plant, and why a gene cloning experiment was performed after amplification of the target gene. Considering that SNP variations have been found between the two ALS gene copies and the mutation site is located in Domain A (197), a highly conserved gene fragment between different species, we can be sure that the nucleotide changes found at position 197 (CCC to TCC) are a genetic mutation rather than differences between different copies. The same mutation, Pro-197-Ser, has been reported in many other plants, such as in wild radish (Raphanus raphanistrum L.) (Yu et al. Reference Yu, Zhang, Hashem, Walsh and Powles2003), L. rigidum (Yu et al. Reference Yu, Han, Vila-Aiub and Powles2010), D. sophia (Yang et al. Reference Yang, Deng, Wang, Liu, Li and Zheng2018), A. thaliana (Haughn et al. Reference Haughn, Smith, Mazur and Somerville1988), and C. compressus (McCullough et al. Reference McCullough, Yu, McElroy, Chen, Zhang, Grey and Czarnota2016), and this is the most frequent mutation of ALS genes in different weeds. This mutation may lead to ALS herbicide resistance without major adverse impacts on ALS functionality and, therefore, there are probably no plant fitness costs (Yu et al. Reference Yu, Han, Vila-Aiub and Powles2010). In comparison with the other Pro-197 amino acid substitutions (e.g., Ile, Lys, Met, and Trp), which require two nucleotide changes, whereas Pro-197-Ser requires only one in E. phyllopogon.
In Vitro ALS Inhibition Assays
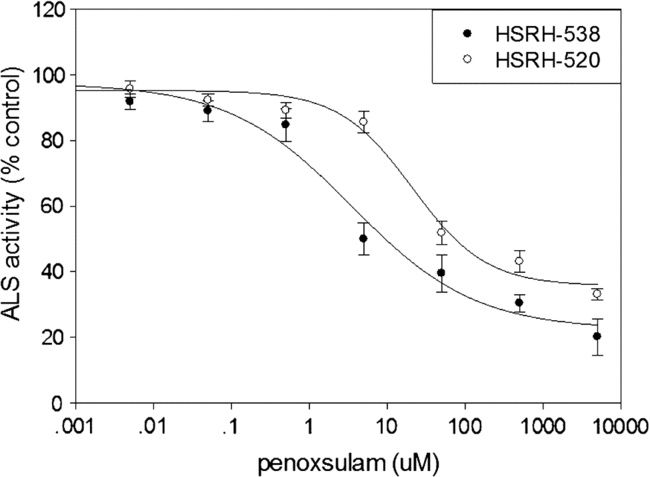
The activity of ALS in HSRH-538 and HSRH-520 was relatively stable when treated with penoxsulam from 0 to 0.5 µM. The inhibition of the activity of ALS by penoxsulam in HSRH-520 was greater than in HSRH-538 (Figure 2). The IC50 value found for HSRH-520 was 229 μM (Table 3), which was 13.7-fold higher than that of HSRH-538 (16.7 μM), suggesting that high ALS activity in the presence of herbicide conferred resistance to penoxsulam in E. phyllopogon populations. It is worth noting that the IC50 ratio (13.73) of enzyme activity for R and S was much lower than the ED50 ratio (25.40) of R and S for whole-plant dose response. This result indicates that target enzyme changes might not be the only mechanism that causes the resistance of E. phyllopogon to penoxsulam, non–target site based resistance is also very likely to exist.
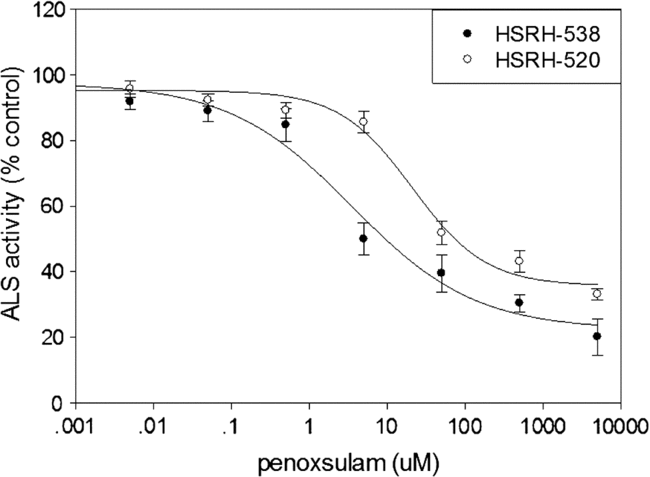
Figure 2. Acetolactate synthase (ALS) activity in vitro inhibited by penoxsulam for two Echinochloa phyllopogon populations: HSRH-538, sensitive to penoxsulam; and HSRH-520, resistant to penoxsulam. Each point is the mean ± SE of two experiments, and each experiment contained three replicates. The ALS activity of HSRH-538 was significantly inhibited under the treatment of penoxsulam at 5 μM, while for HSRH-520 it was 50 μM.
Changes in the ALS enzyme can affect binding of ALS-inhibiting herbicides, leading to resistance, and the Pro-197-Ser mutation causes structural changes that can be characterized in vitro. The results of in vitro ALS inhibition assays indicate that there is indeed a difference between the two populations studied here. Specifically, when the sensitive population HSRH-538 is compared with the resistant population HSRH-520 (which has the Pro-197-Ser mutation in the ALS-2 gene), the latter showed higher tolerance to ALS inhibitors, and the resistance ratio (RR; IC50 value for R biotype/IC50 value for S biotype) is 13.7, consistent with the results of the whole-plant bioassays; similar situations have been reported in L. rigidum (Yu et al. Reference Yu, Han, Vila-Aiub and Powles2010), H. murinum (Yu et al. Reference Yu, Nelson, Zheng, Jackson and Powles2007), and D. sophia (Yang et al. Reference Yang, Deng, Wang, Liu, Li and Zheng2018).
Computational Analysis of the Effects of Pro-197-Ser Mutation on ALS Binding Affinity for Penoxsulam and Other ALS Inhibitors
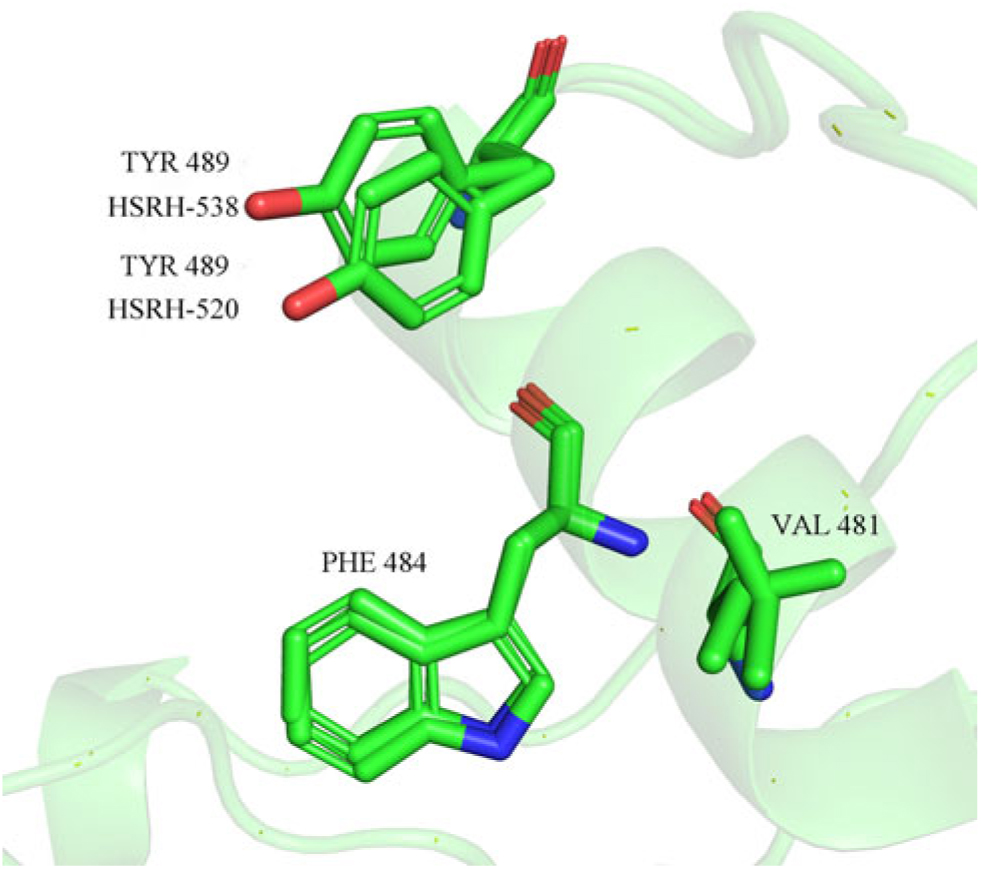
The results of molecular docking (Table 6) indicated that the binding free energy of some complex systems (ALS and ALS inhibitors) changed significantly because of the Pro-197-Ser amino acid mutation. To be specific, the mutation significantly increased the binding free energy of ALS in E. phyllopogon in response to certain types of ALS inhibitors, including TP, SU, and SCT herbicides. However, there was almost no change in the binding free energy for ALS in response to the IMI and PTB herbicides. Based on the molecular-docking results, ALS inhibitors bind to hydrophobic active centers composed of Met-400, Leu-420, Met-423, Val-481, Trp-484, Phe-488, and Tyr-489, and the main contributions to the binding come from the hydrogen bond and conjugate interactions of Trp-484, Phe-488, and Tyr-489 on ALS with ALS inhibitors. Although the amino acid at position 197 of the ALS active site is not located at the binding site of the substrate, the change in this active site affects the spatial structure of the substrate center and makes a certain positional offset of the side chain, especially in the case of the hydrophobic amino acid Tyr-489 in the hydrophobic cavity (Figure 3).
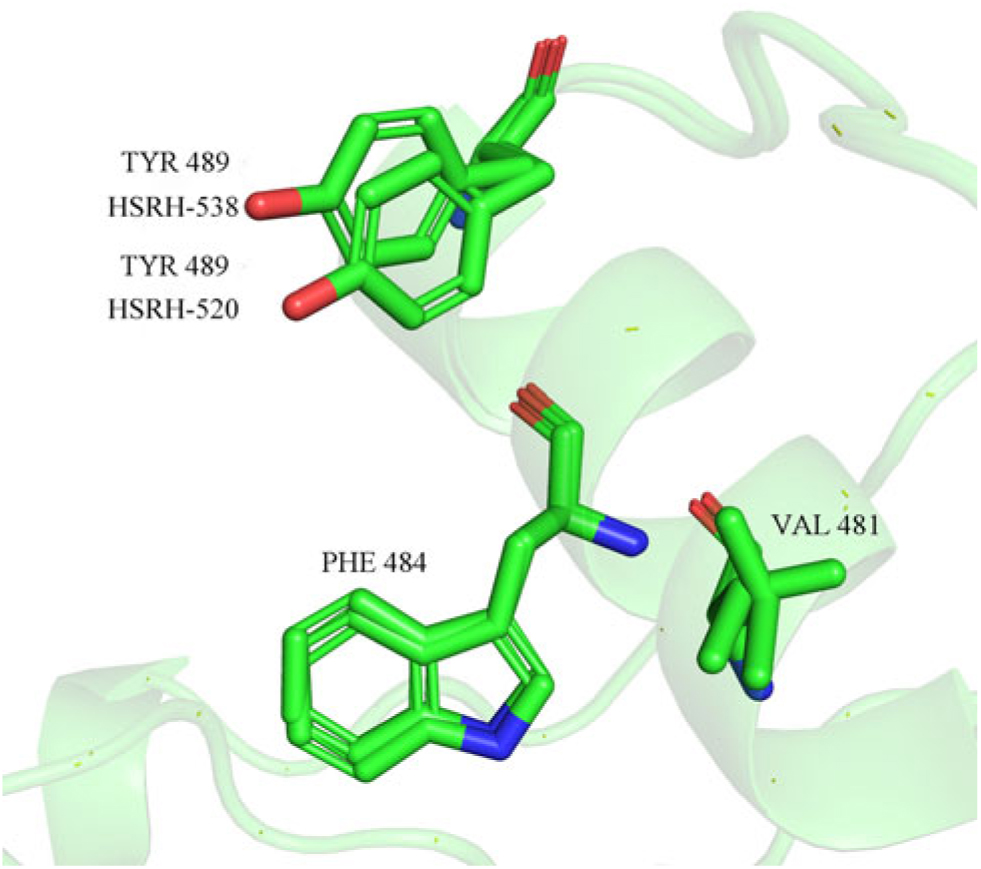
Figure 3. Positional shift of hydrophobic amino acid side chains in the hydrophobic cavity of the acetolactate synthase (ALS) substrate caused by the Pro-197-Ser mutation.
Table 6. Computational analysis of the binding free energy and the interactions between the ligand (different ALS inhibitors) and ALS receptor in sensitive and resistant Echinochloa phyllopogon populations
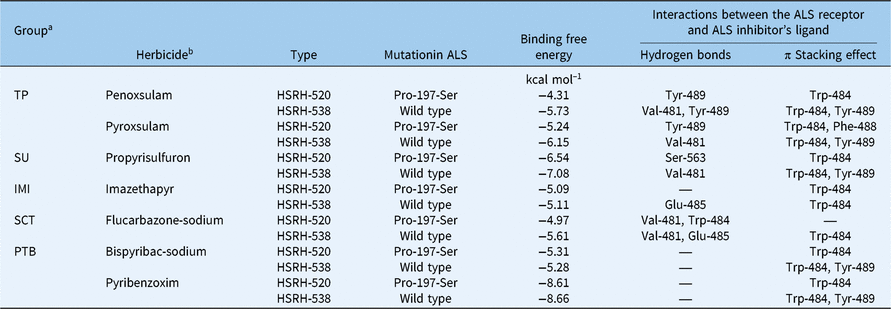
a IMI, imidazolinone; PTB, pyrimidinylthiobenzoate; SCT,
b sulfonylaminocarbonyltriazolinone; SU, sulfonylurea; TP, triazolopyrimidine.
The effects on the binding affinity between ALS and inhibitors because of this change are different for different types of inhibitor (Table 6). Specifically, there is almost no effect on binding affinity for ALS and IMI and PTB herbicides when the spatial configuration of the protein has been changed by a mutation at position 197 when ALS binds to IMI and PTB herbicides (Figure 4A–C). The reason for this result may be that compared with hydrogen bonding, π stacking effects play a more important role in the bonding. For example, in imazethapyr, although the 197 mutation caused a decrease in the number of hydrogen bonds at Glu-485 of the HSRH-520, due to changes in bond angle and bond distance, the π stacking effect at Trp-484 actually received a slight reinforcement, resulting in no significant change to binding affinity in general (Figure 4A). Even if the π stacking effect of one site is missing, the enhancement of the π stacking at the original site is also balanced overall, just as in pyribenzoxim (Figure 4B). These results also explain why HSRH-520, with its Pro-197-Ser mutation, will still be sensitive to IMI (imazethapyr) and PTB (pyribenzoxim and bispyribac-sodium).
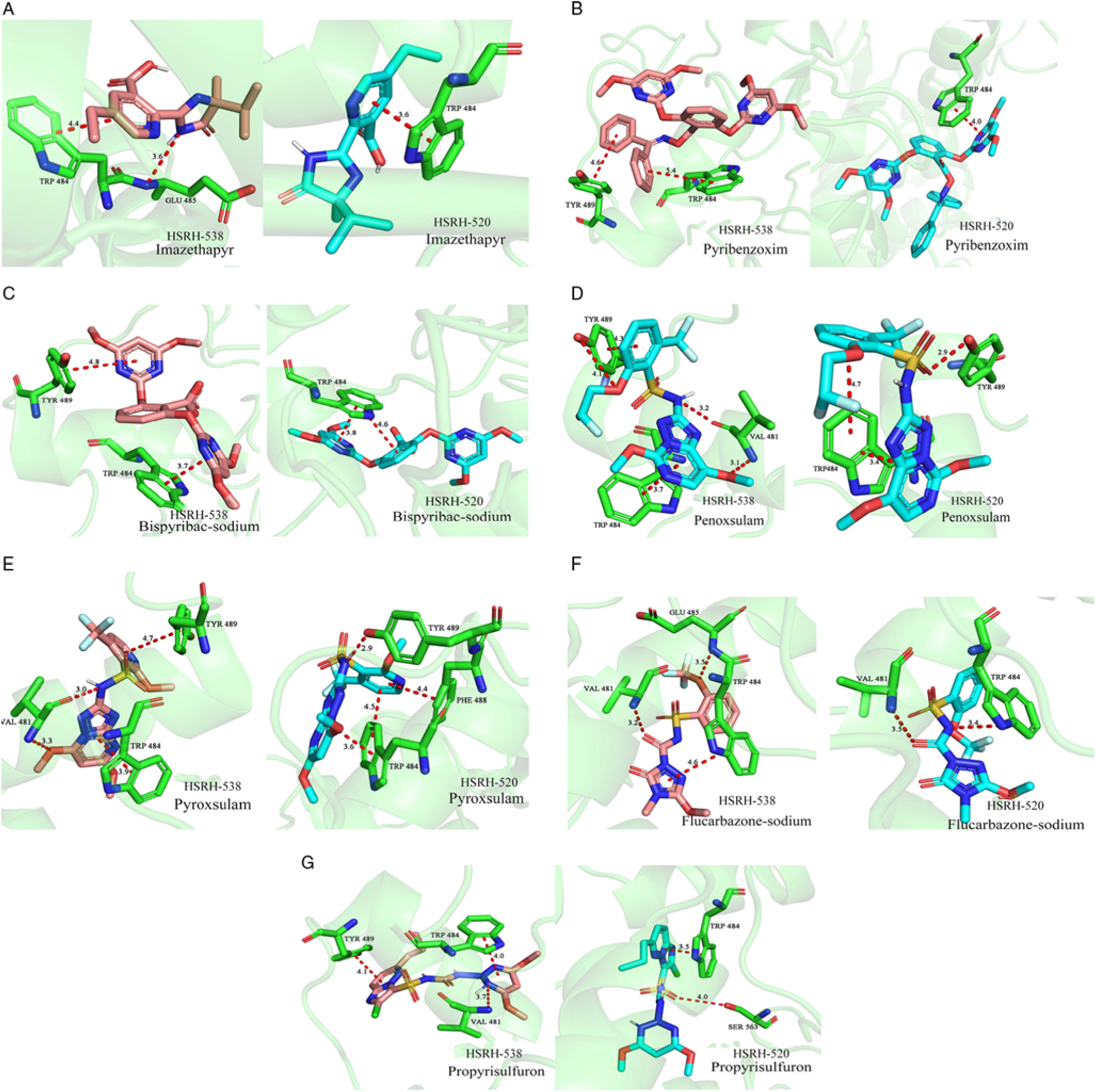
Figure 4. Molecular-docking simulation of acetolactate synthase (ALS) in sensitive (S) and resistant (R) Echinochloa phyllopogon populations with different groups of ALS inhibitors: (A) imazethapyr; (B) pyribenzoxim; (C) bispyribac-sodium; (D) penoxsulam; (E) pyroxsulam; (F) flucarbazone-sodium; and (G) propyrisulfuron.
On the contrary, a significant binding affinity change of ALS and inhibitors can be observed between the R and S populations when the types of ALS inhibitors are TP, SCT, and SU herbicides (Figure 4D–G). Perhaps the reason for this phenomenon is the change of Tyr-489, which is the most important binding residue. The disappearance of the π stacking effect or the transition to the hydrogen bonding at this position directly weakens the binding affinity of ALS and inhibitors, leading to a more unstable bonding in HSRH-520, which had the Pro-197-Ser mutation, compared with HSRH-538, which is the wild type, explaining why HSRH-520 shows relatively high resistance to the ALS inhibitors that belong to the TP, SCT, and SU families.
In general, Tyr-489 is the most critical residue site for the binding of inhibitors to ALS, and the π stacking effect is the most important intermolecular force in the process for different types of ALS inhibitors binding to the protein. The effect of the Pro-197-Ser mutation on these factors varies with the different types of inhibitors, explaining why HSRH-520 shows different resistances to different inhibitors.
In this investigation, the complete ALS gene of E. phyllopogon was amplified and the Pro-197-Ser mutation, which had been reported to lead to ALS resistance in other species, was identified. In vitro ALS activity assays found that the IC50 of the resistant population was 13 times that of the sensitive population; this was also confirmed by the results of the analysis of the sensitivity of E. phyllopogon to penoxsulam. After molecular-docking analysis, it was found that the Pro-197-Ser mutation had an effect on the binding affinity of ALS and its inhibitors, and the effects differed depending on the type of ALS inhibitor; specifically, the mutation reduced the binding affinity when the inhibitor was a member of the TP, SU, or SCT families, but there were no significant changes when the inhibitors were members of the PTB or IMI families, and this was consistent with previous cross-resistance patterns. In general, at least to some extent, the Pro-197-Ser mutation caused E. phyllopogon resistance to penoxsulam and determined the cross-resistance pattern. Echinochloa phyllopogon does have TSR to penoxsulam, but this is probably not the only cause of resistance, because we cannot rule out the possibility of NTSR, and there are many reports of this (Iwakami et al. Reference Iwakami, Endo, Saika, Okuno, Nakamura, Yokoyama, Watanabe, Toki, Uchino and Inamura2014a, Reference Iwakami, Uchino, Kataoka, Shibaike, Watanabe and Inamura2014b; Yasuor et al. Reference Yasuor, Osuna, Ortiz, Saldain, Eckert and Fischer2009; Yun et al. Reference Yun, Yogo, Miura, Yamasue and Fischer2005). Even so, this research is the first report of the typical TSR mechanism for the ALS inhibitors of E. phyllopogon. This investigation has filled a gap in our knowledge base regarding the target site–based resistance mechanisms of E. phyllopogon to ALS inhibitors.
Author ORCID
Liyao Dong https://orcid.org/0000-0002-4842-713X.
Acknowledgments
This research was funded by National Natural Science Foundation of China (31871993). No conflicts of interest have been declared.