Introduction
Barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.], an allohexaploid grass species, is considered the most troublesome weed in rice (Oryza sativa L.) paddy fields, causing significant crop yield losses (Ni et al. Reference Ni, Moody, Robles, Paller, Lales and Cosico1996). At present, the application of herbicides is the most effective strategy for weed control, and herbicides with different sites of action, such as acetolactate synthase (ALS) and acetyl-coenzyme A carboxylase (ACCase) inhibitors, have been developed and widely used in rice fields to control E. crus-galli.
ALS, also known as acetohydroxyacid synthase, is the first enzyme in the biosynthesis pathway of three essential branched-chain amino acids, namely leucine, isoleucine, and valine (Duggleby et al. Reference Duggleby, McCourt and Guddat2008; Ray Reference Ray1984). It is the target site of many commercial herbicides, including sulfonylureas (SUs), imidazolinones (IMIs), pyrimidinylthiobenzoates (PTBs), triazolopyrimidines (TPs), and sulfonylaminocarbonyltriazolinones (SCTs) (Yu and Powles Reference Yu and Powles2014). These herbicides are widely applied in weed control because of their low use rates, high efficiency, multicrop selectivity, broad spectrum of action, and season-long efficacy. Since 2008, penoxsulam, an ALS inhibitor, has been one of the most extensively used herbicides in rice fields in China; however, the continued use of penoxsulam has resulted in the rapid evolution of resistance in E. crus-galli. We have previously reported that, in certain locations in China, E. crus-galli developed resistance to penoxsulam in 2016 (Chen et al. Reference Chen, Wang, Yao, Zhu and Dong2016). This species, as well as other species of Echinochloa, is also reported to have developed resistance to penoxsulam in Turkey (Altop et al. Reference Altop, Mennan, Streibig, Budak and Ritz2014), the United States (Norsworthy et al. Reference Norsworthy, Wilson, Scott and Gbur2014), Italy (Panozzo et al. Reference Panozzo, Scarabel, Tranel and Sattin2013), Greece (Kaloumenos et al. Reference Kaloumenos, Chatzilazaridou, Mylona, Polidoros and Eleftherohorinos2013), Japan (Iwakami et al. Reference Iwakami, Hashimoto, Matsushima, Watanabe, Hamamura and Uchino2015), and South Korea (Song et al. Reference Song, Lim, Yook, Kim and Kim2017).
Mechanisms of herbicide resistance can be divided into target-site resistance (TSR) and non–target site resistance (Powles and Yu Reference Powles and Yu2010). TSR is generally associated with relevant gene mutations, resulting in amino acid changes in a target enzyme that prevent or reduce herbicide binding (Yu and Powles Reference Yu and Powles2014). With regard to ALS inhibitors, 28 amino acid substitutions at eight conserved positions in the ALS gene have been reported to date in various weed species (Tranel et al. Reference Tranel, Wright and Heap2018). These positions are Ala-122, Pro-197, Ala-205, Asp-376, Arg-377, Trp-574, Ser-653, and Gly-654 (numbered on the basis of the corresponding sequence of Arabidopsis thaliana L.) (Yu and Powles Reference Yu and Powles2014). Substitutions at Ala-122, Ser-653, and Trp-574 have been reported to confer resistance to ALS-inhibiting herbicides in E. crus-galli and other Echinochloa species (Kaloumenos et al. Reference Kaloumenos, Chatzilazaridou, Mylona, Polidoros and Eleftherohorinos2013; Matzenbacher et al. Reference Matzenbacher, Bortoly, Kalsing and Merotto2015; Panozzo et al. Reference Panozzo, Scarabel, Tranel and Sattin2013; Riar et al. Reference Riar, Norsworthy, Srivastava, Nandula, Bond and Scott2013). Given that E. crus-galli is an allohexaploid grass species, multiple copies of genes encoding ALS may exist. For example, two complete ALS genes and the carboxyltransferase domain of four ACCase genes have been isolated in rice barnyardgrass [Echinochloa phyllopogon (Stapf) Koso-Pol.] (Iwakami et al. Reference Iwakami, Uchino, Watanabe, Yamasue and Inamura2012), and the hexaploid wild oat (Avena fatua L.) has three ACCase gene copies, each associated with a different resistance mutation (Yu et al. Reference Yu, Ahmad-Hamdani, Han, Christoffers and Powles2013). These findings prompted us to look for multiple copies of the mutant target gene in E. crus-galli.
Cross-resistance patterns to ALS-inhibiting herbicides depend on the position and specificity of mutations. Mutations at Ala-122, Ala-205, Ser-653, and Gly-654 generally provide resistance to IMIs, whereas mutations at Pro-197 lead to resistance to SUs, and mutations at Trp-574 confer resistance to both SU and IMI herbicides (Tranel et al. Reference Tranel, Wright and Heap2018). Moreover, substitutions at Asp-376 and Arg-377 can result in cross-resistance to one or more IMI, PTB, SCT, SU, and TP herbicides (Tranel et al. Reference Tranel, Wright and Heap2018). The discovery of multiple mutations at the same position revealed that not only the position, but also the mutation type can affect cross-resistance patterns. For instance, at position 122, substitution of alanine with threonine causes resistance to IMI herbicides only, whereas substitution of alanine with tyrosine imparts a high level of resistance to IMI, SU, and TP herbicides (Han et al. Reference Han, Yu, Purba, Li, Walsh, Friesen and Powles2012; Tranel et al. Reference Tranel, Wright and Heap2018), and substitution of alanine with valine confers partial resistance to some SU herbicides (Krysiak et al. Reference Krysiak, Gawroński, Adamczewski and Kierzek2011). Furthermore, the novel amino acid substitution Ala-205-Phe in annual bluegrass (Poa annua L.) confers broad-spectrum (IMI, PTB, SCT, SU, and TP) resistance to ALS-inhibiting herbicides (Brosnan et al. Reference Brosnan, Vargas, Breeden, Grier, Aponte, Tresch and Laforest2016). However, the patterns and levels of cross-resistance to ALS-inhibiting herbicides conferred by specific ALS mutations cannot be based on the response to one or two herbicides from a particular ALS-inhibitor chemical group (Han et al. Reference Han, Yu, Purba, Li, Walsh, Friesen and Powles2012), that is, there is no absolute connection between cross-resistance patterns and specific ALS mutations.
Therefore, in the present study, we aimed to (1) identify E. crus-galli populations resistant to penoxsulam, (2) explore the target-site basis of this resistance, and (3) characterize the cross-resistance observed in some populations to provide information for improved management.
Materials and methods
Plant materials
In 2012, seeds of suspected resistant E. crus-galli populations (Table 1) were collected from rice fields in Jiangsu (JNRG-2) and Anhui (AXXZ-2) provinces (China), where the application of penoxsulam at the recommended dose during that year had failed to control this weed. Seeds of a susceptible population (JLGY-3) were collected from a recreational field that has never been exposed to herbicide treatment. All seeds were collected by hand, air-dried in the shade, and stored in paper bags at 4 C until use. For the purposes of the present study, we renamed these populations, which we have used in a previous study (Chen et al. Reference Chen, Wang, Yao, Zhu and Dong2016).
Table 1. Source locations of the three Echinochloa crus-galli populations examined in this study

Whole-plant dose response
Twenty seeds from each of the three populations were sown in plastic pots (9-cm diameter by 10-cm height) filled with a 2:1 (w/w) mixture of sand and pH 5.6 organic matter and planted in growth chamber at 30 C/25 C (light/dark temperature) with a 12-h light/12-h dark cycle, light intensity of 8,000 lx, and 85% relative humidity. Before herbicide treatment, seedlings were thinned to 12 plants pot−1. At the 3- to 4-leaf stage, herbicides were applied using a laboratory sprayer (machine model: 3WP-2000, Nanjing Research Institute for Agricultural Mechanization, Nanjing, National Ministry of Agriculture of China) equipped with a flat-fan nozzle, delivering 280 L ha−1 at 230 kPa. On the basis of the findings of a preliminary experiment (unpublished data), penoxsulam was applied at 0, 3.75, 7.5, 15, 30, and 60 g ai ha−1 to the JNRG-2 population; at 0, 7.5, 15, 30, 60, and 120 g ai ha−1 to the AXXZ-2 population; and at 0, 0.94, 1.88, 3.75, 7.5, and 15 g ai ha−1 to the JLGY-3 population. Treated plants were returned to the incubators and cultured as described above. At 2 wk after penoxsulam application, the amount of fresh aboveground biomass was determined. This experiment was conducted twice in a completely randomized design with four replications.
Cross-resistance of the penoxsulam-resistant populations JNRG-2 and AXXZ-2 was determined using the methods described above. With the exceptions of imazapic, pyroxsulam, and flucarbazone-sodium, all of the ALS inhibitors selected for the bioassays are used in Chinese rice fields. Application doses were based on the results of a preliminary experiment (unpublished data) and are listed in Table 2. Other procedures were identical to those described above.
Table 2. Herbicide doses applied in dose–response tests

a Abbreviations: IMI, imidazolinone; PTB, pyrimidinylthiobenzoate; SCT, sulfonylaminocarbonyltriazolinone; SU, sulfonylurea; TP, triazolopyrimidine.
b Pyribenzoxim (5% emulsifiable concentrate; Korea’s LG Life Science Co. Ltd., Shanghai, China); imazapic (240 g L−1 aqueous suspension; Rotam, Suzhou, China); flucarbazone-sodium (70% water-dispersible granules [WDG]; Arysta LifeScience, Shanghai, China]; pyroxsulam (7.5% WDG; Dow AgroSciences, Beijing, China); penoxsulam (25g L−1 oil dispersion; Dow AgroSciences, Beijing, China); flucetosulfuron (10% wettable powder; FMC, Suzhou, China); propyrisulfuron (100 g L−1 suspension concentrate; Sumitomo Chemical Co. Ltd., Japan); rimsulfuron (25% WDG; Jiangsu Futian Agrochemical Co., Ltd., China).
ALS activity assay
The response of the ALS enzyme to penoxsulam was determined using crude enzyme extracts. Seedlings at the 3- to 4-leaf stage from JNRG-2, AXXZ-2, and JLGY-3 were used for in vitro assays of ALS activity as described by Yu et al. (Reference Yu, Friesen, Zhang and Powles2004), with slight modifications as follows. Leaf blades were harvested from each population (3 g), powdered in liquid nitrogen, and suspended in enzyme extraction buffer (4.5 ml) containing 100 mM potassium phosphate buffer (pH 7.5), 10 mM sodium pyruvate, 1 mM MgCl2, 1 mM thiamine pyrophosphate, and 10 mM flavine adenine dinucleotide (FAD). Each reaction contained 100 μl of protein extract, 200 μl of enzyme assay buffer (100 mM potassium phosphate buffer pH 7.5, 200 mM sodium pyruvate, 20 mM MgCl2, 2 mM thiamine pyrophosphate, 20 μM FAD, 1 mM dithiothreitol), and 100 μl of ALS inhibitor (penoxsulam at 0.005, 0.05, 0.5, 5, and 50 μM). A nontreated (no herbicide applied) control was included in each assay for comparison. Acetoin was formed by incubation at 37 C for 60 min. The reaction was stopped by the addition of 8 μl of 6 N H2SO4, and the mixture was maintained at 60 C for 30 min before the addition of 100 μl of creatine solution (0.55%) and 100 μl of α-naphthol solution (5.5% in 5 N NaOH). ALS activity was monitored colorimetrically (530 nm) using a microplate photometer (Thermo Fisher, Waltham, MA, USA) by measuring acetoin production (pure acetoin was used as the standard). The concentration of protein in the extracts was measured using the Bradford method (Bradford Reference Bradford1976), with bovine serum albumin used as a standard. The assay was performed twice with independent extracts, each with three replications per concentration.
Gene cloning and sequencing
Young shoot tissues obtained from individual plants at the 3- to 4-leaf stage were used for DNA extraction using a Plant Genomic DNA kit (Tiangen Biotech, Beijing, China), according to the manufacturer’s instructions. A specific pair of primers (forward: TTGCCACCCTCCCCAAACCC; reverse: GCACCACTCGCTGAAATCCG) was designed using Primer Premier v. 5.0 (PREMIER Biosoft International, Palo Alto, CA, USA) based on the ALS gene sequences of Echinochloa crus-galli var. crus-galli (accession LC006061.1) and Echinochloa crus-galli var. formosensis (accession LC006063.1), retrieved from the National Center for Biotechnology Information (NCBI) GenBank database, and was used to amplify the complete sequence of ALS in E. crus-galli. The polymerase chain reaction (PCR) mixture contained 2.5 ng of template DNA, 2 μl of each primer (10 μM), 25 μl of 2× Phanta Max Master Mix (Vazyme Biotech, Nanjing, China), and ddH2O to a final volume of 50 μl. Amplification was conducted as follows: 5 min at 95 C for DNA denaturation; 35 cycles of 30 s at 95 C for DNA denaturation, 30 s at 58 C for annealing, and 90 s at 72 C for DNA elongation; and a final elongation for 7 min at 72 C. The PCR products were purified using a TaKaRa MiniBEST agarose gel DNA extraction kit (TaKaRa Biotechnology, Dalian, China). After addition of poly(A) using the TaKaRa Taq Kit (TaKaRa Biotechnology), the resulting productions were cloned into a pMD19-T vector (TaKaRa Biotechnology). Plasmids containing the fragment insertion were bidirectionally sequenced by GenScript Biotechnology (Nanjing, China). Ten plants from each of the three populations were selected for gene cloning. At least 12 transformed clones of each plant were sequenced to obtain complete ALS sequences, which were aligned and compared using BioEdit Sequence Alignment Editor v. 7.2.5 (Tom Hall, Carlsbad, CA, USA). The Basic Local Alignment Search Tool (BLAST) procedure within the NCBI database was used to verify the accuracy of the obtained sequences.
Data analysis
Whole-plant dose–response data were subjected to ANOVA using SPSS v. 21.0 (IBM, Armonk, NY). The ANOVA results showed no significant difference between assay repetitions, so the results for the repeat assays were averaged. Data were then pooled and fit to the four-parameter nonlinear logistic-regression model (Equation 1), calculated using SigmaPlot v. 10.0 (SigmaPlot Software, Chicago, IL, USA), to determine the effective dose of herbicide causing 50% inhibition of fresh weight (ED50):
where Y denotes fresh weight, expressed as a percentage of the nontreated control at dose x of the herbicide; b is the slope; c is the lower limit; d is the upper limit; and g is the herbicide dose at the point of inflection, halfway between the upper and lower limits (Xu et al. Reference Xu, Li, Zhang, Cheng, Jiang and Dong2014).
The same analysis was used to calculate the herbicide concentrations required to inhibit 50% of ALS activity (IC50) in enzymatic assays. Resistance indexes (RIs) were calculated by dividing the ED50 (or IC50) of the resistant population (R) by the ED50 (or IC50) of the susceptible population (S).
Results and discussion
Sensitivity to penoxsulam
The ED50 of AXXZ-2 (65.33 g ha−1) was considerably higher than the recommended application dose (15 to 30 g ha−1), whereas those of JNRG-2 (14.34 g ha−1) and JLGY-3 (1.96 g ha−1) were lower than the recommended dose (Table 3; Figure 1). The RIs of the resistant populations AXXZ-2 and JNRG-2 were 33 and 7.3, respectively, indicating a high and moderate resistance, respectively, to penoxsulam, according to Beckie and Tardif (Reference Beckie and Tardif2012).

Figure 1. Fresh weight of the aboveground parts of three populations of Echinochloa crus-galli treated with penoxsulam. Vertical bars represent the mean ± SE.
Table 3. Sensitivity of the three Echinochloa crus-galli populations to penoxsulam and other acetolactate synthase inhibitors

a ED50 refers to the effective dose of herbicide causing 50% inhibition of fresh weight and is indicated as grams of active ingredient per hectare (g ai ha−1).
b RI is the resistance index. Herbicide resistance was classified into five groups: no resistance (RI < 2); low resistance (RI = 2–5); moderate resistance (RI = 6–10); high resistance (RI = 11–100); and very high resistance (RI >100).
Gene cloning and sequence analysis
To our knowledge, this is the first study reporting the complete coding sequence (CDS) of ALS in E. crus-galli obtained using a single pair of primers. The difficulty in obtaining the complete CDS of ALS is related to the extremely high GC content in its 5′ region. Two ALS sequences of JNRG-2 and JLGY-3 and three ALS sequences of AXXZ-2 were isolated, and the mutant sequences were submitted to the NCBI database (GenBank accession numbers MH013500 and MH013488). The BLAST procedure conducted for ALS sequences revealed that these were highly similar (96% to 97%) to the reported sequences LC006061.1 (see Supplementary Table S1), indicating that we had amplified the correct ALS sequences from E. crus-galli. Thus, the primer pair designed for the present study might be useful for acquiring the complete sequences of ALS in other Echinochloa species. After further analysis of the obtained sequences, we identified single-nucleotide polymorphisms at multiple conserved sites between different copies (Table 4). Based on the amino acid residues at these positions, we can determine the copy numbers and infer the sequence of the ALS copy in E. crus-galli. The variation in ALS gene copy number observed in the present study is consistent with that previously reported in shortawn foxtail (Alopecurus aequalis Sobol.) (Iwakami et al. Reference Iwakami, Shimono, Manabe, Endo, Shibaike, Uchino and Tominaga2017) and Japanese foxtail (Alopecurus japonicus Steudel) (Feng et al. Reference Feng, Gao, Zhang, Dong and Li2017).
Table 4. The single-nucleotide polymorphisms in multiple sites of acetolactate synthase sequences in the E. crus-galli populations examined

A nucleotide mutation (GCC to GGC) was found in the ALS2;2 sequence of JNRG-2 when compared with the corresponding sequence in JLGY-3, which results in the substitution of alanine with glycine at position 122. Another nucleotide mutation (GCC to GTC) was detected in the ALS1;3 sequence of AXXZ-2, which results in the substitution of alanine with valine at position 205 (positions are numbered relative to the ALS of A. thaliana). However, none of the mutants known to confer resistance to ALS inhibitors in E. crus-galli or other Echinochloa species, namely Trp-574 and Ser-653 (Kaloumenos et al. Reference Kaloumenos, Chatzilazaridou, Mylona, Polidoros and Eleftherohorinos2013; Matzenbacher et al. Reference Matzenbacher, Bortoly, Kalsing and Merotto2015; Panozzo et al. Reference Panozzo, Scarabel, Tranel and Sattin2013), were detected in the present study. Target-gene mutation is responsible for most resistance to ALS-inhibiting herbicides (Yu and Powles Reference Yu and Powles2014). The Ala-205-Val substitution was documented for the first time in E. crus-galli, although previous studies have revealed that this mutation is the target-site basis for the resistance to ALS-inhibiting herbicides in eastern black nightshade (Solanum ptychanthum Dunal) (Ashigh and Tardif Reference Ashigh and Tardif2017), Erigeron spp. (Matzrafi et al. Reference Matzrafi, Lazar, Sibony and Rubin2015), and redroot pigweed (Amaranthus retroflexus L.) (McNaughton et al. Reference McNaughton, Letarte, Lee and Tardif2005). Amino acid mutations at position 122 have previously been reported to confer resistance to ALS inhibitors (Panozzo et al. Reference Panozzo, Scarabel, Rosan and Sattin2017; Riar et al. Reference Riar, Norsworthy, Srivastava, Nandula, Bond and Scott2013). In summary, our findings indicate that the amino acid mutations detected in the two penoxsulam-resistant populations are the main target-site basis for penoxsulam resistance in E. crus-galli.
In vitro ALS inhibition assays
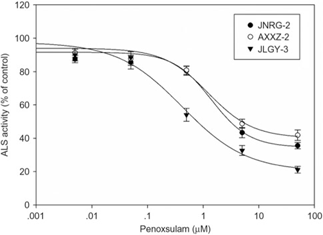
The inhibitory effect of penoxsulam on ALS activity was observed to be less pronounced in AXXZ-2 and JNRG-2 than in JLGY-3 (Figure 2). The IC50 values determined for AXX-2 and JNRG-2 were 13.02 and 5.89 μM, respectively, which were 11- and 5.2-fold greater than that for JLGY-3 (1.14 μM), suggesting that the low sensitivity of ALS occurs in conjunction with resistance to penoxsulam in E. crus-galli populations. In addition, this trend was consistent with our whole-plant dose–response analysis, although the RIs were not high compared with the whole-plant dose response. These results strongly support the hypothesis that a TSR mechanism is responsible for the resistance of E. crus-galli populations.

Figure 2. In vitro acetolactate synthase (ALS) activity of three Echinochloa crus-galli populations when treated with penoxsulam. Vertical bars represent the mean ± SE.
Sensitivity to other ALS inhibitors
On the basis of ED50 and RI values, we examined cross-resistance patterns in the resistant populations of E. crus-galli. Cross-resistance to ALS inhibitors was found in AXXZ-2 (Table 3; Figure 3), which was resistant to IMI, PTB, SCT, TP, and SU herbicides, whereas JNRG-2 was sensitive to other ALS inhibitors, and sometimes even more sensitive than JLGY-3, which was the susceptible population in the present study. With regard to the ALS Ala-122-Gly mutation found in the JNRG-2 population, we conjecture that there might be a negative interaction between the Ala-122-Gly mutation and other ALS inhibitors. Negative cross-resistance is not rare and has been reported in yeast (Duggleby et al. Reference Duggleby, Pang, Yu and Guddat2003) and tobacco (Nicotiana tabacum L.) (Le et al. Reference Le, Yoon, Kim and Choi2005). An Asp-376-Asn substitution in yeast ALS and an Asp-376-Ala substitution in tobacco ALS conferred higher resistance to SU herbicides, but rendered plants more sensitive to IMI herbicides. In the present study, we also observed that, in response to penoxsulam treatment at 30 g ha−1, the growth of the resistant population bearing the Ala-122-Gly mutation was strongly inhibited compared with that of the population harboring the Ala-205-Val substitution. This is similar to the response observed in resistant wild radish (Raphanus raphanistrum L.) homozygous for the Asp-376-Gln mutation and other mutations affecting plant growth (Yu and Powles Reference Yu and Powles2014). Given the cross-resistance patterns and plant growth, the ALS Ala-122 mutation found in resistant populations of E. crus-galli appears to be a TSR mechanism and similar to Asp-376.

Figure 3. Fresh weight of the aboveground parts of three populations of Echinochloa crus-galli treated with seven acetolactate synthase (ALS)-inhibiting herbicides. Vertical bars represent the mean ± SE.
In the present study, the AXXZ-2 population exhibited cross-resistance to five classes of ALS inhibitors, which might be due to the Ala-205-Val substitution. The growth of treated AXXZ-2 seedlings was virtually uninhibited by penoxsulam at 30 g. ha−1 and was not notably different from that of untreated seedlings. In addition, the AXXZ-2 population was resistant to IMI, PTB, SCT, TP, and SU herbicides, with RI values varying from 2.68 to 16.37. These observations indicated that AXXZ-2 showed different levels of resistance to other ALS inhibitors, ranging from low to high. Interestingly, the AXXZ-2 population was resistant to four herbicides to which it had never previously been exposed (pyroxsulam and flucarbazone-sodium are only used in wheat (Triticum aestivum L.) fields, imazapic has not been used in rice fields in China, and propyrisulfuron is a novel herbicide registered in China in 2015), which presents a considerable challenge in terms of weed control and management. The Ala-205 mutation has been reported in several weeds, but its cross-resistance pattern is less clear than those associated with Pro-197 and Trp-574. Overall, this mutation could cause resistance to IMI herbicides. Recently, an amino acid substitution (Ala-205-Phe) in ALS was shown to confer broad-spectrum resistance to ALS-inhibiting herbicides in P. annua (Brosnan et al. Reference Brosnan, Vargas, Breeden, Grier, Aponte, Tresch and Laforest2016), which in certain respects is consistent with the results of the present study.
Conclusions
In the E. crus-galli populations we studied, AXXZ-2 and JNRG-2 had evolved different levels of penoxsulam resistance confirmed by whole-plant dose response. After analysis of the ALS sequences of single plants in resistant populations, two novel mutations (Ala-122-Gly and Ala-205-Val) were detected. To our best knowledge, this is the first time these two mutations have been reported in resistant Echinochloa species, although the Ala-122-Val and Ala-122-Thr substitutions had been revealed in E. crus-galli resistant to ALS-inhibiting herbicides (Riar et al. Reference Riar, Norsworthy, Srivastava, Nandula, Bond and Scott2013). Lower sensitivity of ALS to penoxsulam was also revealed in the two resistant populations compared with the susceptible population. Overall, amino acid substitutions and the low sensitivity of ALS to penoxsulam might be the target-site bases for resistance to penoxsulam in E. crus-galli, as they are the typical target-site bases of resistance to ALS inhibitors in many other Echinochloa species. Moreover, the AXXZ-2 population was resistant to all ALS inhibitors tested, which might cause great difficulty in controlling this biotype, whereas JNRG-2 was not. The cross-resistance patterns in resistant populations harboring different mutations reported here highlight the need to apply herbicides scientifically and to effectively to manage E. crus-galli in rice fields.
Author ORCID
Liyao Dong https://orcid.org/0000-0002-4842-713X
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2019.5
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31871993). No conflicts of interest have been declared.