Introduction
Waterhemp [Amaranthus tuberculatus (Moq.) J. D. Sauer var. rudis (Sauer) Costea & Tardif, or syn. A. rudis Sauer] (Costea et al. Reference Costea, Weaver and Tardif2005; Pratt and Clark Reference Pratt and Clark2001) is a small-seeded dicot species that has become a major problem in corn (Zea mays L.) and soybean [Glycine max (L.) Merr.] production in North America (Steckel Reference Steckel2007). Amaranthus tuberculatus can reduce corn yields by up to 74% (Steckel and Sprague Reference Steckel and Sprague2004) and soybean yields more than 40% (Hager et al. Reference Hager, Wax, Stoller and Bollero2002). Amaranthus tuberculatus is a dioecious species with obligate outcrossing (Murray Reference Murray1940) and, as a result, possesses a large degree of intraspecific genetic diversity (Trucco et al. Reference Trucco, Tatum, Rayburn and Tranel2009). Amaranthus tuberculatus control is crucial, because outcrossing leads to the rapid spread of herbicide-resistance events via pollen between Amaranthus species and/or resistant populations (Wetzel et al. Reference Wetzel, Horak, Skinner and Kulakow1999). Additionally, A. tuberculatus produces up to 1 million seeds per female plant (Hartzler et al. Reference Hartzler, Battles and Nordby2004; Steckel et al. Reference Steckel, Sprague, Hager, Simmons and Bollero2003) that can remain dormant and viable on the soil surface layer for up to 4 yr (Buhler and Hartzler Reference Buhler and Hartzler2001; Burnside et al. Reference Burnside, Wilson, Weisberg and Hubbard1996) and germinate and emerge well into the summer growing season (Hartzler et al. Reference Hartzler, Buhler and Stoltenberg1999; Reference Hartzler, Battles and Nordby2004). The combination of increased implementation of reduced- or no-till systems (Horowitz et al. Reference Horowitz, Ebel and Uexda2010), variability in herbicide responses (Patzoldt et al. Reference Patzoldt, Tranel and Hager2002), and the evolution of herbicide resistances (Heap Reference Heap2018) led to a dramatic increase in A. tuberculatus infestations in Illinois cropping systems within the last 20 yr (Hager et al. Reference Hager, Wax, Simmons and Stoller1997; Steckel Reference Steckel2007). Together, these biological factors and management practices have made this species a significant problem for farmers across the Midwest (Steckel Reference Steckel2007).
Herbicides that inhibit 4-hydroxyphenylpyruvate dioxygenase (HPPD) are commonly applied in corn and other cereal crops mainly for dicot weed management. These herbicides competitively inhibit the HPPD enzyme (Ndikuryayo et al. Reference Ndikuryayo, Moosavi, Yang and Yang2017), which is a key enzyme in the biosynthesis of plastoquinone (PQ) and tocopherols. These herbicides also indirectly inhibit carotenoid biosynthesis, because PQ is an electron acceptor required for phytoene desaturase (PDS) activity in the carotenoid biosynthetic pathway (Lee et al. Reference Lee, Knudsen, Michaely, Chin, Nguyen, Carter, Cromartie, Lake, Shribbs and Fraser1998; Pallett et al. Reference Pallett, Cramp, Little, Veerasekaran, Crudace and Slater2001). Sensitive plants die from loss of carotenoids, which leads to the distinctive white color or “bleaching” of treated plants due to the oxidation of chlorophyll in the presence of light (Hess Reference Hess2000; Pallett et al. Reference Pallett, Cramp, Little, Veerasekaran, Crudace and Slater2001). Triplet chlorophyll and singlet oxygen are also produced, leading to further chlorophyll destruction and subsequent membrane damage in the chloroplast and thylakoids (Pallett et al. Reference Pallett, Cramp, Little, Veerasekaran, Crudace and Slater2001). New leaves and meristems are primarily affected due to the systemic nature of these herbicides.
PQ also serves an important role as a membrane-soluble electron carrier in photosystem II (PSII) electron transport (Hess Reference Hess2000). PSII-inhibiting herbicides such as atrazine or metribuzin inhibit the light reactions of photosynthesis by reversibly competing with PQ for the Qb binding site of the D1 protein (Fuerst and Norman Reference Fuerst and Norman1991; Hess Reference Hess2000). This inhibition blocks the flow of electrons to cytochrome b6f, subsequently triggering the rapid formation of triplet chlorophyll, followed by singlet oxygen, in the presence of light in sensitive plants (Hess Reference Hess2000). A synergistic interaction has been determined for POST tank mixtures of mesotrione (MES) and PSII inhibitors, in which the combination of these two herbicides produced greater activity than the sum of either herbicide applied alone (Abendroth et al. Reference Abendroth, Martin and Roeth2006; Sutton et al. Reference Sutton, Richards, Buren and Glasgow2002). Synergistic herbicidal activity has the potential to reduce both costs for farmers and the amount of herbicides entering the environment (Kudsk and Mathiassen Reference Kudsk and Mathiassen2004; Streibig and Jensen Reference Streibig and Jensen2000).
One proposed method for delaying the development of resistance is tank mixing herbicides with different sites of action, rather than applying each herbicide alone or in rotation (Diggle et al. Reference Diggle, Neve and Smith2003; Evans et al. Reference Evans, Tranel, Hager, Schutte, Wu, Chatham and Davis2016). Unfortunately, both A. tuberculatus and Palmer amaranth (Amaranthus palmeri S. Watson) have developed multiple resistances to PSII and HPPD inhibitors (Heap Reference Heap2018), creating uncertainty as to whether a tank mix of herbicides with these sites of action would be effective on these species. Previous quantifications of synergistic, additive, or antagonistic effects for combinations of HPPD and PSII inhibitors relative to each herbicide applied alone have been performed using the Colby equation (Colby Reference Colby1967). For example, previous research demonstrated synergistic activity on broadleaf weeds (Abendroth et al. Reference Abendroth, Martin and Roeth2006; Sutton et al. Reference Sutton, Richards, Buren and Glasgow2002), including both triazine-sensitive and site of action–based triazine-resistant (TR) redroot pigweed (Amaranthus retroflexus L.) biotypes (Hugie et al. Reference Hugie, Bollero, Tranel and Riechers2008; Woodyard et al. Reference Woodyard, Hugie and Riechers2009b). Additionally, a synergistic interaction was documented when atrazine and MES were applied in a POST tank mixture to metabolism-based, atrazine-resistant velvetleaf (Abutilon theophrasti Medik.) plants (Woodyard et al. Reference Woodyard, Hugie and Riechers2009b).
The synergism between PSII and HPPD inhibitors thus plays an important role for weed control in corn, even for TR weed populations. In contrast, HPPD inhibitors applied to a known HPPD- and symmetrical (s)-triazine–resistant A. tuberculatus population (named MCR for McLean County, IL, resistant) provided only partial control (Hausman et al. Reference Hausman, Singh, Tranel, Riechers, Kaundun, Polge, Thomas and Hager2011, Reference Hausman, Tranel, Riechers, Maxwell, Gonzini and Hager2013), and s-triazine–treated plots (PRE) were similar to nontreated plots. However, the potential for POST synergism between HPPD inhibitors and metribuzin, an asymmetrical (as)-triazine or triazinone (Fuerst and Norman Reference Fuerst and Norman1991), in metabolism-based HPPD- and atrazine-resistant populations has not been examined. With the anticipated introduction of HPPD-resistant soybean technology (Siehl et al. Reference Siehl, Tao, Albert, Dong, Heckert, Madrigal, Lincoln-Cabatu, Lu, Fenwick, Bermudez, Sandoval, Horn, Green, Hale, Pagano, Clark, Udranszky, Rizzo, Bourett, Howard, Johnson, Vogt, Akinsola and Castle2014), this topic warrants further investigation due to the possibility of combining HPPD inhibitors with metribuzin early POST (EPOST) in HPPD-resistant soybean varieties or in corn, when the herbicide safener cyprosulfamide (formulated with isoxaflutole [IFT]) is added.
The research described herein explores potential control options for two HPPD- and s-triazine–resistant A. tuberculatus populations: SIR (from Stanford, IL; sampled from the same field site as the MCR population described in Hausman et al. Reference Hausman, Singh, Tranel, Riechers, Kaundun, Polge, Thomas and Hager2011) and NEB (from Nebraska, described in Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and Mcindoe2017). Both populations demonstrated enhanced oxidative metabolism of MES, presumably via cytochrome P450 monooxygenases (P450s) (Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and Mcindoe2017; Ma et al. Reference Ma, Kaundun, Tranel, Riggins, McGinness, Hager, Hawkes, McIndoe and Riechers2013), relative to sensitive populations. The first objective of this research was to determine fold-resistance levels to two HPPD-inhibiting herbicides; specifically, a herbicide that had previously been applied to both populations (MES) and another that had not been applied to either population (IFT), at two plant heights. For the second objective, joint activity of either MES or IFT at varying rates combined with a single rate of metribuzin POST was evaluated in SIR and NEB. This objective was designed to test two interconnected hypotheses: (1) HPPD-inhibitor activity contributes to synergism in a tank mix with metribuzin, and (2) metabolic atrazine resistance can be overcome with a different PSII inhibitor (e.g., metribuzin). This hypothesis is based on the assumption that a single glutathione S-transferase (GST) enzyme, AtuGSTF2, is most likely responsible for detoxifying atrazine in resistant A. tuberculatus (Evans et al. Reference Evans, O’Brien, Ma, Hager, Riggins, Lambert and Riechers2017). GST enzymes in A. tuberculatus may only recognize specific herbicide substrates within the triazine family (i.e., s-triazines), and thus expected herbicide activity might be observed in multiple herbicide-resistant plants.
Materials and Methods
Amaranthus tuberculatus Populations, Plant Culture, and Greenhouse Conditions
Seeds from each population were stratified in 0.1% agarose solution at 4 C for 30 d (Bell et al. Reference Bell, Hager and Tranel2013). A listing of all populations and their corresponding documented resistances are shown in Table 1. All plants used in these experiments were germinated from seeds sown in 12 by 12 cm flats containing a commercial potting medium (LC1, Sun Gro Horticulture, 15831 NE 8th Street, Bellevue, WA 98008). Emerged seedlings (2 cm) were transplanted into 950 cm3 pots (1 seedling pot−1) containing a 3:1:1:1 mixture of potting mix (LC1):soil:peat:sand that included a slow-release fertilizer (Osmocote® 13-13-13, Scotts, 14111 Scottslawn Road, Marysville, OH 43041). The soil component contained 3.5% organic matter with a pH of 6.8. Greenhouse conditions were maintained at 28/22 C day/night with a 16:8-h photoperiod. Natural sunlight was supplemented with mercury-halide lamps to provide 800 μmol m−2 s−1 photon flux at the plant canopy.
Table 1 Amaranthus tuberculatus populations used in this study and their known resistance to herbicides.

a Resistances listed in Table 1 are further described in Heap (Reference Heap2018). ALS, acetolactate synthase; HPPD, 4-hydroxyphenyl-pyruvate dioxygenase; PPO, protoporphyrinogen oxidase; PSII, photosystem II.
b Sampled from the same field site as the MCR (McLean County, IL, resistant) population described by Hausman et al. (Reference Hausman, Singh, Tranel, Riechers, Kaundun, Polge, Thomas and Hager2011).
c PSII resistance to s-triazines (i.e., atrazine or simazine) specifically.
d SEN, sensitive to ALS, HPPD, PPO, and PSII inhibitors.
Quantifying Resistance to Foliar-applied HPPD Inhibitors at Different Timings
Uniformly sized A. tuberculatus plants were treated with IFT or MES. EPOST and POST treatments were applied when plants reached 5 cm in height with 4 to 5 true leaves and 10 cm in height with 8 to 9 true leaves, respectively. HPPD inhibitors and their respective application rates included IFT ranging from 0.8 to 420 g ha−1 and MES from 1.6 to 840 g ha−1 on a base 2 logarithmic scale. The IFT formulation did not include the corn safener cyprosulfamide. Herbicides were applied using a compressed-air research sprayer (Generation III Research Sprayer, DeVries, 28081 870th Avenue, Hollandale, MN 56045) fitted with a TeeJet® 80015 EVS nozzle (TeeJet® 80015EVS, TeeJet Technologies, P.O. Box 7900, Wheaton, IL 60187) calibrated to deliver 185 L ha−1 at 275 kPa. All treatments included methylated seed oil (MSO, 1% v/v) and liquid ammonium sulfate (AMS, 2.5% v/v).
Experimental Design and Statistical Analysis
Following application, plants were placed on greenhouse benches in a randomized complete block design. Each treatment had three replications, and the experiment was independently conducted three times. Visual assessment of plant responses was recorded at 7, 14, and 21 d after treatment (DAT) using a scale ranging from 0 (no plant injury) to 100 (plant mortality). At 21 DAT, all aboveground plant tissue was harvested and dried at 65 C for 7 d. Dry weights were recorded and converted to a percentage of the nontreated control for each population. Dry-weight data were analyzed with nonlinear regression using the ‘drc’ (dose–response curve) package (Knezevic et al. Reference Knezevic, Streibig and Ritz2007) in the R statistical computing environment (R Development Core Team 2017). The dose–response model was fit using a four-parameter logistic function, as shown in Equation 1:
where b is a shape parameter, c is the lower limit, d is the upper limit, and GR50 is a herbicide rate causing 50% reduction in dry weight. Dose–response curves were fit for each experimental replicate of population and treatment, allowing for the direct estimation of variation in GR50 values. The GR50 values were then compared using linear mixed-effects models in the ‘nlme’ package of R v. 3.3.1.
Response to Foliar-applied HPPD Inhibitors Combined with Metribuzin
Amaranthus tuberculatus plants from the SIR and NEB populations were grown under the same greenhouse conditions described previously. Amaranthus tuberculatus plants 10 cm in height (8 to 9 true leaves) were treated with IFT, MES, or metribuzin alone or in a tank-mix combination with an HPPD inhibitor plus metribuzin. HPPD inhibitors and their respective application rates were IFT at 26.3, 52.5, and 105 g ha−1 and MES at 52.5, 105, and 210 g ha−1. Although IFT is typically applied preplant or PRE, the label states it can be applied early POST in corn (with cyprosulfamide). MES can also be applied PRE or POST. Metribuzin was applied alone at 191 g ha−1, which resulted in an approximately 20% biomass reduction during initial dose–response analysis (Figure 1). Herbicides were applied using the compressed-air research spray system described earlier. All treatments included MSO at 1% v/v and AMS at 2.5% v/v.

Figure 1 Dose–response study of metribuzin (1% v/v methylated seed oil plus 2.5% v/v liquid ammonium sulfate) applied POST. Amaranthus tuberculatus plants were treated at 10 cm in height (8 to 9 true leaves). (A) SIR (Stanford, IL, HPPD- and atrazine-resistant) population at 7 d after treatment (DAT), (B) NEB (Nebraska HPPD- and atrazine-resistant) population at 7 DAT. U, plant on the far left is the nontreated control (adjuvants only).
Experimental Design and Statistical Analysis
Following application, plants were placed on greenhouse benches in a randomized complete block design (by experimental replicate and position on the bench) with seven blocks of a full factorial of the experimental treatments described earlier: A. tuberculatus population, herbicide, application type (single herbicide or mixture), and HPPD inhibitor rate. The experiment was independently conducted three times, using a different randomization scheme each time. Visual assessment of plant responses was recorded at 7 and 14 DAT using a scale ranging from 0 to 100 as described previously. At 14 DAT, all aboveground plant tissue was harvested and dried at 65 C for 7 d.
The assessment of joint activity of herbicides with independent modes of action is most commonly calculated using variants of the multiplicative survival model (MSM) (Flint et al. Reference Flint, Cornelius and Barrett1988; Green et al. Reference Green, Jensen and Streibig1997; Kelly and Chapman Reference Kelly and Chapman1995; Streibig et al. Reference Streibig, Kudsk and Jensen1998), which applies Colby’s equation to determine herbicide joint action through analysis of various quantitative observations, including plant biomass (fresh or dry weight), growth suppression, or percent survival (Colby Reference Colby1967; Gowing Reference Gowing1960). Colby’s equation (Equation 2) is expressed as:
where ε is the expected growth (dry weights as a percent of control) with application of herbicides A + B in combination, and X and Y are the growth observed with herbicide A or B, respectively, applied at specific rates.
Although Colby’s equation is a commonly used method for calculating herbicide joint activity within the MSM, a statistical test for analyzing herbicide joint activity is still necessary. A model proposed by Flint et al. (Reference Flint, Cornelius and Barrett1988) applied a statistical test to Colby’s equation by using slope comparisons. In this test, a slope estimate significantly less than 0 indicates a synergistic interaction, a slope significantly greater than 0 indicates antagonism, and a slope that is not significantly different from 0 indicates an additive effect. With regard to determining increased herbicidal activity when combining MES or IFT with metribuzin POST in this study, experiments were designed for application to a wide range of growth data and to fit this statistical method (Flint et al. Reference Flint, Cornelius and Barrett1988), which follows previous work on MES–atrazine interactions (Hugie et al. Reference Hugie, Bollero, Tranel and Riechers2008; Woodyard et al. Reference Woodyard, Bollero and Riechers2009a, Reference Woodyard, Hugie and Riechers2009b). Plant dry-weight biomass data were transformed via log-transformation to account for heterogeneity of variances, allow for slope comparisons (Flint et al. Reference Flint, Cornelius and Barrett1988), and describe nonlinear effects of herbicide joint action (Hugie et al. Reference Hugie, Bollero, Tranel and Riechers2008; Woodyard et al. Reference Woodyard, Bollero and Riechers2009a, Reference Woodyard, Hugie and Riechers2009b). Determining herbicide joint action in HPPD-resistant populations involved comparing the effects of MES, IFT, and metribuzin applied alone and in combination with those quantified in control (HPPD-sensitive) plants. Herbicide joint action analysis was performed using the mixed procedure in SAS software for slope comparisons (SAS Institute 2004).
Results and Discussion
Quantifying Resistance to Foliar-applied HPPD Inhibitors at Different Timings
Treatment of SIR, NEB, ACR (HPPD sensitive; metabolic atrazine-resistant), and SEN (sensitive to HPPD and photosystem II [PSII] inhibitors) plants with a range of IFT rates resulted in typical dose–response curves, with decreasing dry weights in response to increasing rates applied EPOST (Figure 2) and POST (Figure 3). After calculating and comparing GR50 values (described in “Materials and Methods”), population, treatment, and the interaction of population and treatment were determined to be significant; F(3, 144)=21.5, P<0.001.
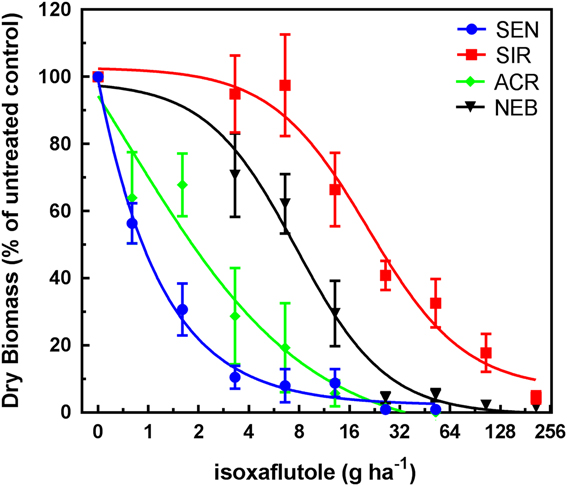
Figure 2 Isoxaflutole dose–response curves (early POST [EPOST] timing) for 4-hydroxyphenyl-pyruvate dioxygenase (HPPD) inhibitor–sensitive populations SEN (sensitive to HPPD and PSII inhibitors) and ACR (Adams County, IL, HPPD sensitive but atrazine-resistant) and the HPPD inhibitor–resistant populations SIR (Stanford, IL, HPPD and atrazine resistant) and NEB (Nebraska, HPPD and atrazine resistant). Plant dry weights were obtained 21 d after treatment. Plants were treated EPOST at 5 cm in height (4 to 5 true leaves). Vertical bars represent the standard error of the mean.
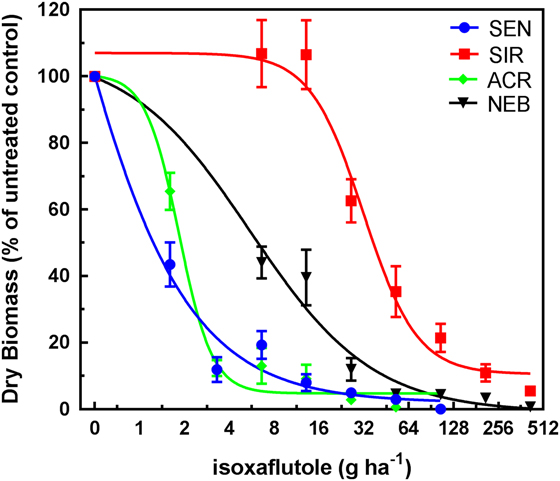
Figure 3 Isoxaflutole dose–response curves (POST timing) for 4-hydroxyphenyl-pyruvate dioxygenase (HPPD) inhibitor–sensitive populations SEN (sensitive to HPPD and PSII inhibitors) and ACR (Adams County, IL, HPPD sensitive but atrazine-resistant) and the HPPD inhibitor–resistant populations SIR (Stanford, IL, HPPD and atrazine resistant) and NEB (Nebraska, HPPD and atrazine resistant). Plant dry weights were obtained 21 d after treatment. Plants were treated POST at 10 cm in height (8 to 9 true leaves). Vertical bars represent the standard error of the mean.
GR50 values calculated for each population by herbicide by treatment timing are shown in Table 2. ACR and SEN were not significantly different from each other, so only ACR was used as the HPPD-sensitive population to determine fold-resistance levels in these studies. Based on these estimated GR50 values, the relative levels of resistance to IFT in SIR were 10-fold EPOST and 17-fold POST, compared with 4-fold EPOST and 3-fold POST in NEB. Treatment of each population with MES resulted in decreasing dry weights in response to increasing rates EPOST (Figure 4) and POST (Figure 5). As stated previously for IFT experiments, ACR and SEN were not significantly different, so only ACR was used as the HPPD-sensitive population. The relative levels of resistance to MES in SIR were 32-fold EPOST and 21-fold POST, compared with 7-fold for both EPOST and POST for NEB. Visual assessments of plant injury at 21 DAT were consistent with dry-weight data, which revealed different rate responses among the populations for both herbicides (unpublished data).
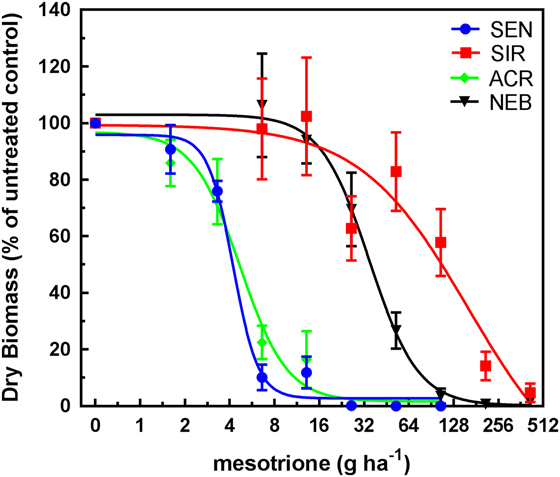
Figure 4 Mesotrione dose–response curves (early POST [EPOST] timing) for 4-hydroxyphenyl-pyruvate dioxygenase (HPPD) inhibitor–sensitive populations SEN (sensitive to HPPD and PSII inhibitors) and ACR (Adams County, IL, HPPD sensitive but atrazine-resistant) and the HPPD inhibitor–resistant populations SIR (Stanford, IL, HPPD and atrazine resistant) and NEB (Nebraska, HPPD and atrazine resistant). Plant dry weights were obtained 21 d after treatment. Plants were treated EPOST at 5 cm in height (4 to 5 true leaves). Vertical bars represent the standard error of the mean.
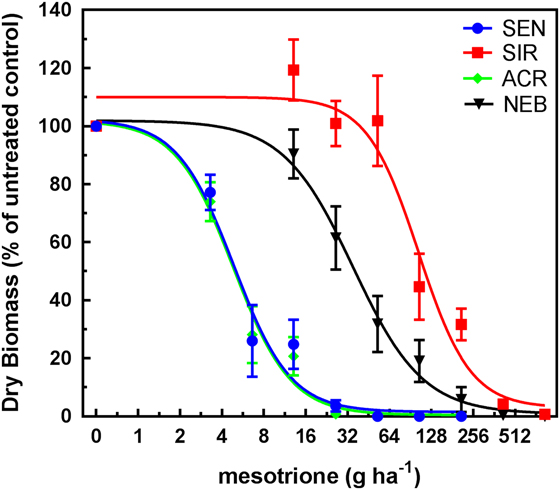
Figure 5 Mesotrione dose–response curves (POST timing) for 4-hydroxyphenyl-pyruvate dioxygenase (HPPD) inhibitor–sensitive populations SEN (sensitive to HPPD and PSII inhibitors) and ACR (Adams County, IL, HPPD sensitive but atrazine-resistant) and the HPPD inhibitor–resistant populations SIR (Stanford, IL, HPPD and atrazine resistant) and NEB (Nebraska, HPPD and atrazine resistant). Plant dry weights were obtained 21 d after treatment. Plants were treated POST at 10 cm in height (8 to 9 true leaves). Vertical bars represent the standard error of the mean.
Table 2 Herbicide rates that cause 50% reductions in plant growth (GR50) determined for each Amaranthus tuberculatus population by herbicide by treatment timing shown in Figures 2–5.
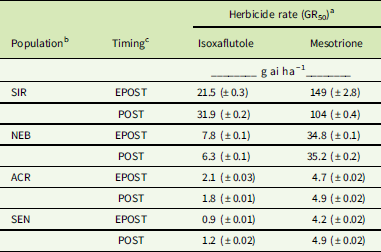
a Values represent the treatment mean ±SD (n=9). Amaranthus tuberculatus population, treatment, and the interaction of population and treatment were determined significant; F(3, 144)=21.5, P<0.001.
b ACR, Adams County, IL, 4-hydroxyphenyl-pyruvate dioxygenase (HPPD) sensitive, metabolic atrazine resistant; NEB, Nebraska HPPD and atrazine resistant; SEN, sensitive to HPPD and PSII inhibitors; SIR, Stanford, IL, HPPD and atrazine resistant.
c Plants were treated at 5 cm in height (4 to 5 true leaves) for the early POST (EPOST) timing, or 10 cm in height (8 to 9 true leaves) for the POST timing.
Overall, these findings indicated greater fold resistance to MES relative to IFT at each timing. The SIR and NEB populations had been previously exposed only to MES, which may have selected for greater MES resistance relative to IFT (Hausman et al. Reference Hausman, Singh, Tranel, Riechers, Kaundun, Polge, Thomas and Hager2011; Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and Mcindoe2017). However, POST treatments to SIR showed contrasting effects on fold-resistance levels relative to EPOST. For example, the fold resistance in SIR to IFT increased, while the resistance levels in SIR to MES decreased. These opposite effects following IFT treatment result from the combination of a greater GR50 at the POST timing relative to EPOST in SIR and a lower GR50 at the POST timing relative to EPOST in ACR (Table 2). In contrast, the inverse pattern occurred following MES treatment in each population. The underlying basis for the different trends in R/S ratios between herbicide treatments as plant height increased may be related to several reasons: biokinetic factors such as herbicide uptake, translocation, and metabolism; P450 expression or enzyme activity differences between plant heights; or other physiological factors that warrant further study.
SIR is more resistant than NEB when compared with ACR at all treatments and timings (Figures 2–5). The mechanism of resistance in the MCR population (the site where SIR was also collected; Hausman et al. Reference Hausman, Singh, Tranel, Riechers, Kaundun, Polge, Thomas and Hager2011) was attributed to rapid oxidative metabolism via P450s (Ma et al. Reference Ma, Kaundun, Tranel, Riggins, McGinness, Hager, Hawkes, McIndoe and Riechers2013), which is similar to results with the NEB population (Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and Mcindoe2017). Tolerant corn also rapidly metabolizes MES via P450-catalyzed ring hydroxylation (Hawkes et al. Reference Hawkes, Holt, Andrews and Thomas2001; Ma et al. Reference Ma, Kaundun, Tranel, Riggins, McGinness, Hager, Hawkes, McIndoe and Riechers2013). However, it is still unknown how many P450s are responsible for rapid MES metabolism in HPPD-resistant A. tuberculatus. Based on our results, it is possible that the SIR population more rapidly metabolizes IFT and MES, which could be attributed to differences in P450 expression in the leaves or higher substrate specificity (lower Km) of herbicide-detoxifying P450(s) in SIR relative to NEB.
Surprisingly, the MCR population had a reported GR50 of 48.5 g ha−1 for MES (Hausman et al. Reference Hausman, Singh, Tranel, Riechers, Kaundun, Polge, Thomas and Hager2011), which is significantly lower than the GR50 of 162 g ha−1 for MES in the NEB population (Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and Mcindoe2017). However, differences exist between these studies. For example, MCR plants were treated with MES at 10 to 12 cm (Hausman et al. Reference Hausman, Singh, Tranel, Riechers, Kaundun, Polge, Thomas and Hager2011), whereas NEB plants were treated at 7 cm (Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and Mcindoe2017). Additionally, NEB plants were cultured under different growing conditions, with a light intensity of 180 µmol m−2s−1 and temperatures of 24/18 C day/night (Kaundun et al. Reference Kaundun, Hutchings, Dale, Howell, Morris, Kramer, Shivrain and Mcindoe2017). In our study, all populations were grown under 800 μmol m−2 s−1 photon flux at the plant canopy with 28/22 C day/night cycles (as in Hausman et al. Reference Hausman, Singh, Tranel, Riechers, Kaundun, Polge, Thomas and Hager2011).
These differences in growth parameters might account for varying results obtained between our studies and previous research. HPPD-inhibiting herbicides deplete PQ, which indirectly blocks carotenoid biosynthesis by inhibiting PDS activity (Hess Reference Hess2000). Carotenoids play several important roles in plants, including protection of chlorophyll from photodegradation under high light intensity by quenching excess energy released and scavenging free radicals (Ramel et al. Reference Ramel, Birtic, Cuiné, Triantaphylidés, Ravanat and Havaux2012). Because NEB and MCR plants were exposed to different light intensities and temperatures during these experiments, herbicide activity levels may have differed in treated plants, thus affecting fold-resistance levels.
Response to Foliar-applied HPPD Inhibitors Combined with Metribuzin
Synergism between MES and metribuzin was detected (i.e., the joint activity estimate showed a negative slope that was significantly different from 0) in SIR plants when applied in a tank mix of 52.5 g ai ha−1 MES and 191 g ai ha−1 metribuzin (Table 3). However, as the rate of MES increased in combination with a constant rate of metribuzin, the slope estimates were not significantly different from 0, indicating an additive effect. As the rate of MES increased, more biomass reduction occurred from MES alone; therefore, a significant interaction between the herbicides was not determined. Injury symptoms (Figure 6) indicated that 105 g ai ha−1 MES (Figure 6B) did not kill the meristem at 7 DAT, which is inconsistent with a systemic, weak-acid herbicide (Hess Reference Hess2000). The lack of meristem death is also evident with the combination of 105 g ai ha−1 MES with 191 g ai ha−1 metribuzin (Figure 6D). Consequently, the inability to kill the meristem may explain the lack of synergism in this tank mix.

Figure 6 SIR (Stanford, IL, HPPD and atrazine resistant) Amaranthus tuberculatus plants 7 d after treatment with (A) control, adjuvants only (1% v/v methylated seed oil plus 2.5% v/v ammonium sulfate), (B) mesotrione at 105 g ai ha−1, (C) metribuzin at 191 g ai ha−1, and (D) mesotrione at 105 g ai ha−1 plus metribuzin at 191 g ai ha−1. Herbicide treatments contained the same adjuvants as in A and were applied when A. tuberculatus seedlings were 10 cm in height (8 to 9 true leaves).
Table 3 Herbicide joint activity of mesotrione and metribuzin applied in a tank mix in the Stanford, IL, resistant (SIR) Amaranthus tuberculatus population.
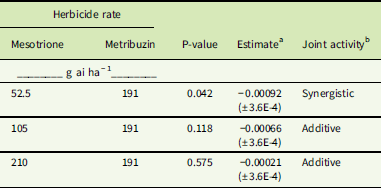
a Estimate, deviation of slope magnitude from parallel or assumed “additivity” determined from reduction in aboveground plant biomass (±SE). A significant negative slope indicates a synergistic interaction between the herbicides, whereas a significant positive slope would indicate antagonism.
b Joint activity was determined as described in “Materials and Methods.”
Antagonism was not detected with any MES or IFT rate plus metribuzin (Tables 3–6); that is, no mixture provided significantly less control than expected (determined by a positive estimate closer to 1). However, because herbicide rates were chosen based on initial dose–response data, we did not anticipate that stand-alone rates would result in complete death in these populations. Although combinations of MES or IFT with metribuzin did not result in synergistic activity in the NEB population, the results are additive (Tables 5 and 6). Additive activity was also observed in the SIR population in regard to each IFT and metribuzin combination (Table 4) and the 105 or 210 g ai ha−1 rates of MES combined with metribuzin (Table 3). Again, statistical analysis showed estimates close to 0 and associated P-values that are not significant (>0.05), which resulted in an additive response for these tank-mix combinations.
Table 4 Herbicide joint activity of isoxaflutole and metribuzin applied in a tank mix in the Stanford, IL, resistant (SIR) Amaranthus tuberculatus population.
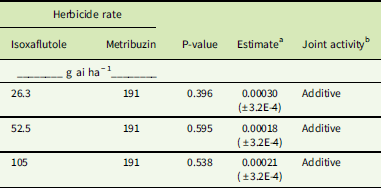
a Estimate, deviation of slope magnitude from parallel or assumed “additivity” determined from reduction in aboveground plant biomass (±SE). A significant negative slope indicates a synergistic interaction between the herbicides, whereas a significant positive slope would indicate antagonism.
b Joint activity was determined as described in “Materials and Methods.”
Table 5 Herbicide joint activity of mesotrione and metribuzin applied in a tank mix in the Nebraska resistant (NEB) Amaranthus tuberculatus population.
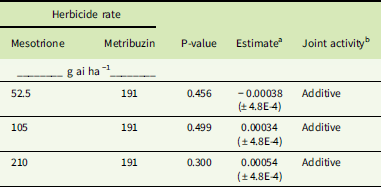
a Estimate, deviation of slope magnitude from parallel or assumed “additivity” determined from reduction in aboveground plant biomass (±SE). A significant negative slope indicates a synergistic interaction between the herbicides, whereas a significant positive slope would indicate antagonism.
b Joint activity was determined as described in “Materials and Methods.”
Table 6 Herbicide joint activity of isoxaflutole and metribuzin applied in a tank mix in the Nebraska resistant (NEB) Amaranthus tuberculatus population.

a Estimate, deviation of slope magnitude from parallel or assumed “additivity” determined from reduction in aboveground plant biomass (± SE). A significant negative slope indicates a synergistic interaction between the herbicides, whereas a significant positive slope would indicate antagonism.
b Joint activity was determined as described in “Materials and Methods.”
The lack of synergism detected with IFT plus metribuzin in either population may be attributable to IFT being a proherbicide (Pallett et al. Reference Pallett, Little, Sheekey and Veerasekaran1998). Proherbicides are inactive, nonphytotoxic chemicals metabolized by plant enzymes to yield an active, herbicidal metabolite (Jeschke Reference Jeschke2016). IFT is applied in an inactive form, but following plant uptake, it is converted to the diketonitrile (DKN) active metabolite (Pallett et al. Reference Pallett, Little, Sheekey and Veerasekaran1998, Reference Pallett, Cramp, Little, Veerasekaran, Crudace and Slater2001). DKN competitively inhibits the HPPD enzyme and prevents conversion of 4-hydroxyphenylpyruvate to homogentisate (Pallett et al. Reference Pallett, Little, Sheekey and Veerasekaran1998). We hypothesize that conversion of IFT to DKN could be a rate-limiting step in determining the timing of foliar activity of HPPD-inhibiting herbicides relative to metribuzin within these populations.
In contrast, MES is applied in its active triketone form and therefore does not require a bioactivation step, which may allow specific MES rates to directly interact and overlap with the phytotoxicity mechanism triggered by metribuzin in mature leaves (Hess Reference Hess2000). This finding supports previous work indicating that the timing of events leading to phytotoxicity is critical in generating a synergistic interaction between HPPD and PS II inhibitors. For example, synergism was not detected in a metabolism-based, atrazine-resistant A. theophrasti biotype when atrazine and MES applications were temporally separated (Woodyard et al. Reference Woodyard, Hugie and Riechers2009b). The underlying mechanism for lack of synergism was attributed to rapid atrazine detoxification (when applied PRE) in resistant A. theophrasti plants, thus preventing a synergistic interaction from occurring when MES was applied POST (Woodyard et al. Reference Woodyard, Hugie and Riechers2009b).
Metribuzin is an as-triazine herbicide, while atrazine is an s-triazine. Within the PSII-inhibitor class of herbicides, each herbicide subclass interacts with the D1 protein via overlapping, but not identical, Qb binding sites (Fuerst and Norman Reference Fuerst and Norman1991; Michel and Deisenhofer Reference Michel and Deisenhofer1988). However, triazine herbicides interact with binding sites in the D1 protein in a similar manner (Fuerst et al. Reference Fuerst, Arntzen, Pfister and Penner1986). By contrast, significant differences in metabolism for these two triazines exist among crops and weeds. Atrazine is typically applied in corn and grain sorghum [Sorghum bicolor (L.) Moench] because these crops possess high specific GST activities that rapidly detoxify atrazine via conjugation with reduced glutathione (GSH; Dixon et al. Reference Dixon, Lapthorn and Edwards2002). Metribuzin is typically applied in soybeans, tomatoes (Solanum lycopersicum L.), and potatoes (Solanum tuberosum L.) for dicot weed control, but can also applied PRE in corn. Metribuzin is detoxified via rapid GST-catalyzed conjugation with homoglutathione (hGSH) in soybeans (Colville et al. Reference Colville, Blanco Sáez, Lewis and Kranner2015; Flury et al. Reference Flury, Wagner and Kreuz1996), which is a metabolic reaction unique to certain legumes (Frear et al. Reference Frear, Swanson and Mansager1985). While the only difference is the form of GSH conjugated with the triazine substrate, it is possible that the GST(s) likely responsible for triazine metabolism in A. tuberculatus only recognize one subclass of the herbicide (i.e., s-triazines). Thus, the GST(s) responsible for metabolism-based atrazine resistance in A. tuberculatus (Evans et al. Reference Evans, O’Brien, Ma, Hager, Riggins, Lambert and Riechers2017) may only recognize atrazine for detoxification but not metribuzin as a substrate for GSH conjugation (Figure 1). This potential scenario differs from the resistance mechanism in site of action–based TR dicots, where specific mutations in psbA (encoding the D1 protein) typically confer cross-resistance to atrazine and metribuzin (Fuerst et al. Reference Fuerst, Arntzen, Pfister and Penner1986).
To our knowledge, the unique POST combination of IFT or MES with metribuzin has not been previously investigated. Our results demonstrate synergism between MES and metribuzin in SIR plants when 52.5 g ai ha−1 MES was paired with metribuzin at 191 g ai ha−1 in a tank mix (Table 3). Although previous research demonstrated the value of synergism between HPPD and PSII inhibitors, particularly in herbicide-resistant weeds (Hausman et al. Reference Hausman, Tranel, Riechers, Maxwell, Gonzini and Hager2013; Hugie et al. Reference Hugie, Bollero, Tranel and Riechers2008; Woodyard et al. Reference Woodyard, Hugie and Riechers2009b), our results shows that specific combinations of metribuzin and MES might be viable options for controlling atrazine- and HPPD-resistant A. tuberculatus in corn and HPPD-tolerant soybean varieties in the field. Importantly, the rates that elicited synergism in our study are below typical field-use rates for each herbicide, which is a critical consideration for the environment and sustainability of weed management in the future. However, previous interaction studies included many more combinations of an HPPD inhibitor and PSII inhibitor than our study, such as varying the rates of one herbicide while the holding the other constant (Hugie et al. Reference Hugie, Bollero, Tranel and Riechers2008). Including more rates in follow-up research, such as combining a GR50 rate of an HPPD inhibitor with various metribuzin rates, might assist with determining the “directionality” of synergism; that is, whether the HPPD inhibitor or metribuzin is more responsible for greater than expected biomass reductions (Hugie et al. Reference Hugie, Bollero, Tranel and Riechers2008). In addition, tank-mix combinations could be applied at different plant heights.
The evolution of herbicide resistance within the genus Amaranthus continues to increase rapidly (Heap Reference Heap2018), in stark contrast to the lack of commercial herbicides with novel sites of action during this period (Cole et al. Reference Cole, Pallett and Rodgers2000; Duke and Dayan Reference Duke and Dayan2015). Our results provide fundamental insight into management of metabolism-based, HPPD- and atrazine-resistant A. tuberculatus populations using chemical tools already available to growers. Tank mixing herbicides with different sites of action, rather than applying either herbicide alone or in rotation, is a proposed method for delaying resistance (Evans et al. Reference Evans, Tranel, Hager, Schutte, Wu, Chatham and Davis2016). It is important to note, however, that a rigid ryegrass biotype (Lolium rigidum Gaudin) in Australia was resistant to atrazine due to a target-site mutation, but upon further testing, was also found to be cross-resistant to metribuzin despite no reports of previous exposure to metribuzin (Burnet et al. Reference Burnet, Hildebrand, Holtum and Powles1991). It is therefore critical to know precisely which resistance mechanism (target site, non-target site, or both) is present in a weed population before prescribing a tank-mix recommendation or making management decisions.
Acknowledgments
We thank Shiv S. Kaundun, Syngenta Ltd., United Kingdom, for providing A. tuberculatus seed from each population for this research, as well as Charlie Mitsdarfer and Lisa Gonzini, University of Illinois–Urbana, for technical assistance. No conflicts of interest have been declared. This research received no specific grant from any funding agency or the commercial or not-for-profit sectors.














