Windmillgrass is a warm-season, C4, annual or short-lived perennial grass native to Australia (Mitchell Reference Mitchell1994; Nightingale et al. Reference Nightingale, Lazarides and Weiller2005). In Australia, some native grass species have the ability to establish in disturbed ecosystems that have regular cultivation, input of fertilizer, ruminant grazing, or crop competition (Kloot Reference Kloot1985; Michael et al. Reference Michael, Yeoh and Scott2012a). This group includes windmillgrass, which has been a significant weed in cotton (Gossypium hirsutum L.) and grain crops in the subtropical region of Australia, particularly in no-till agriculture cropping systems (Werth et al. Reference Werth, Boucher, Thornby, Walker and Charles2013). In the Western Australian grain belt, this species was found at 12% of the sites in a field survey of summer fallow sites (Michael et al. Reference Michael, Borger, MacLeod and Payne2010a). It was projected that windmillgrass could become one of the five most threatening weed species to agriculture in the southwest of Western Australia (Michael et al. Reference Michael, Yeoh and Scott2010b). More recently, this species has been ranked as the seventh most important weed of summer fallow by yield loss (36,871,000kg) and revenue loss (A$9.1 million) in Australia (Llewellyn et al. Reference Llewellyn, Ronning, Ouzman, Walker, Mayfield and Clarke2016).
As a result of an overreliance on glyphosate for weed control, glyphosate resistance occurred in windmillgrass in chemical fallow sites and roadsides in Australia (Malone et al. Reference Malone, Cook, Wu, Hashem and Preston2012; Preston Reference Preston2016), making control of this weed in summer fallow situations more difficult. The dominance of reduced-tillage systems combined with the overreliance on glyphosate for weed control in the fallow phase of crop rotations has also favored this grass in northern Australia (Werth et al. Reference Werth, Boucher, Thornby, Walker and Charles2013; Widderick et al. Reference Widderick, Cook, McLean, Churchett, Keenan, Miller and Davidson2014). As a summer annual grass species, windmillgrass will reduce the potential yield of winter crops by taking up moisture and nutrients that would otherwise be available for crops and will delay sowing due to the time needed for weed control in the autumn (Osten et al. Reference Osten, Hashem, Koetz, Lemerle, Pathan and Wright2006). For example, the absence of management of windmillgrass over summer fallow reduced yield of the following wheat (Triticum aestivum L.) crop in Merredin, WA, by 0.3 ton ha−1 (Borger et al. Reference Borger, Riethmuller and Hashem2010).
Seed germination, a process regulated by several environmental factors such as temperature, light, moisture, and soil salinity, is a key event determining the success of a weed species in an agroecosystem (Chachalis and Reddy Reference Chachalis and Reddy2000; Koger et al. Reference Koger, Reddy and Poston2004). The timing of germination and emergence is critical for the survival of annual plants, with temperature and light among the most important environmental signals that regulate germination and emergence of a plant species (Baskin and Baskin Reference Baskin and Baskin1998; Saatkamp et al. Reference Saatkamp, Affre, Dutoit and Poschlod2009; Schutte et al. Reference Schutte, Tomasek, Davis, Andersson, Benoit, Cirujeda, Dekker, Forcella, Gonzalez‐Andujar and Graziani2014). For seeds in the soil, temperature and light are important cues for initiating germination, providing a sense of position in the soil profile and reflecting the occurrence of soil disturbance (Batlla and Benech-Arnold Reference Batlla and Benech‐Arnold2014). In Australia, there have been several studies of the effect of temperature and light on germination of windmillgrass in New South Wales (Lodge and Whalley Reference Lodge and Whalley1981; Maze et al. Reference Maze, Koen and Watt1993). However, there is currently no information available on the base temperature required for germination of windmillgrass in Australia. In addition, seeds that respond to environmental conditions and alter their germination behavior are more likely to survive and establish successfully. The effect of burial depth and other factors that may impact germination of windmillgrass in southern Australia are poorly understood.
To effectively manage a weed, an understanding of its basic biology is critical. This includes germination conditions, dormancy, seedbank dynamics, and plant development (Bhowmik Reference Bhowmik1997; Cousens and Mortimer Reference Cousens and Mortimer1995; Mennan and Ngouajio Reference Mennan and Ngouajio2006). An understanding of the particular seed-dormancy mechanisms involved can also be useful for the development of control strategies for weeds (Adkins et al. Reference Adkins, Bellairs and Loch2002). Simpson (Reference Simpson1990) listed 89 species of C4 grasses from 24 genera, including Chloris, as having one or more forms of dormancy. In many species, the embryo has the ability to germinate, but dormancy is caused by embryo covering structures. The mechanisms within the covering structures may involve permeability (preventing water uptake or gaseous exchange), mechanical (preventing embryo expansion), and chemical barriers to germination (Adkins et al. Reference Adkins, Bellairs and Loch2002). Furthermore, it is important to manage the soil seedbank of herbicide-resistant weed populations such as windmillgrass (Kleemann et al. Reference Kleemann, Preston and Gill2016). Persistence of the soil seedbank determines the length of time management strategies are required to reduce the population to minimal levels (Matthews Reference Matthews1994). A preliminary report showed that windmillgrass seed had short field persistence (12 mo) in the northern cropping region of Australia (Widderick et al. Reference Widderick, Cook, McLean, Churchett, Keenan, Miller and Davidson2014). However, the seedbank persistence of windmillgrass in cooler Mediterranean conditions of the southern and western grain regions of Australia has not been reported.
The objectives of this study were to determine (1) plant development under field and controlled conditions (time required to reach panicle emergence and seed maturity); (2) seed dormancy; (3) effect of physical environmental factors (temperature, light, salt stress, osmotic potential and burial depth) on germination and seedling emergence; and (4) seedbank persistence under field conditions.
Materials and Methods
Seed Sources
Mature seeds were collected in June 2013 from two windmillgrass populations in Buchfelde (CT2) and Smithfield (CT3) in South Australia, Australia. Harvested seeds were cleaned and stored in paper bags at room temperature (~20 C). Seeds of these populations were grown at a common site (Waite Campus, University of Adelaide, SA, Australia), and the seeds collected from these plants were used for further studies.
Plant Development in an Irrigated Field and Pots
A total of 200 seeds (more than 6 mo old, average germination of 77% for lab tests) from each of the CT2 and CT3 populations were mixed with sand and surface scattered in 1-m2 plots with four replicates in randomized complete blocks at Roseworthy, SA, Australia (34.524807°S, 138.686362°E), on January 24, 2014. The field soil was a clay loam. Before this experiment was conducted, windmillgrass had never been observed at the experimental site, which is regularly used for crop production. After sowing, irrigation was applied weekly to prevent water stress. Ten tagged seedlings in each replicate were used to assess the time taken to reach tillering, panicle emergence, and seed production.
Field-collected seeds of CT2 and CT3 populations were germinated in an incubator set at 25 C to produce seedlings. These windmillgrass seedlings were transplanted into pots eight different times at 3- to 4-week intervals from October 3, 2013, to March 8, 2014, to determine the influence of growing season on the timing of panicle emergence. Ten seedlings (1-leaf) of each population (CT2 and CT3) were grown in four pots (25-cm deep by 18-cm diameter) containing standard potting mix (Boutsalis et al. Reference Boutsalis, Gill and Preston2012) and kept outdoors at Waite Campus, University of Adelaide, SA, Australia (34.970302°S, 138.639700°E). Plants were watered as required to maintain adequate soil moisture. The time taken to reach panicle emergence was recorded.
Standard Germination Test Protocols
Effects of seed dormancy, temperature, light, and salt or osmotic potential on seed germination were evaluated. Germination was evaluated by evenly placing seeds of each population in a 15-cm-diameter petri dish containing two layers of Whatman No. 1 filter paper moistened with 9 ml of distilled water or a treatment solution (e.g., polyethylene glycol and salt). There were four replicates of each treatment. Dishes were sealed with parafilm and placed in an incubator (cat. no. R1235D, ser. no. SO 17, S.E.M., Magill, SA, Australia) set at 25 C, the temperature previously determined to be in the optimum range for germination of windmillgrass (Lodge and Whalley Reference Lodge and Whalley1981; Maze et al. Reference Maze, Koen and Watt1993; Michael et al. Reference Michael, Yeoh and Scott2012b). The photoperiod was set at 12 h with fluorescent lamps used to produce light intensity of 43 µmolm−2s−1. Germinated seeds (emerged coleoptile, radical >5-mm long) were counted every 2 d for 14 d.
Seed Dormancy
Covering structures (lemma and palea) of windmillgrass seeds turn black at maturation. Mature seeds were collected, and experiments were started within a week after seed collection and repeated at approximately monthly intervals for 12 mo. The tetrazolium seed viability test is considered unreliable for windmillgrass (Farley et al. Reference Farley, Bellairs and Adkins2013) and was not used in this study. Germination was calculated as a percentage of total seeds used in each test. The experiment was conducted with the original field-collected seeds of CT2 and CT3 in June 2013 (25 seeds each replicate). As windmillgrass can produce seeds over several weeks, the experiment was also conducted with seeds that were freshly harvested in November and December 2014 to determine the influence of the timing of seed maturity on germination response.
Effect of Temperature on Germination
Seeds (9 mo old, 25 seeds each replicate) of CT2 and CT3 populations were used to examine the effect of temperature on germination. Seeds were incubated at six different constant temperature regimes (10, 15, 20, 25, 30, and 40 C). Germinated seeds were counted and recorded daily, and germination tests were terminated when no further germination occurred for 7 d. The maximum germination (Gmax) was expressed as the percentage of total seeds used.
Effect of Light on Germination
The effect of two light regimes (12-h alternating light/dark and 24-h dark) on germination of CT2 and CT3 populations (5 to 7 mo old, 50 seeds each replicate) was investigated in an incubator set at 25 C. The 24-h dark treatment was achieved by wrapping each petri dish in two layers of aluminum foil. The petri dishes of both treatments were only opened after 14 d, and the number of germinated seeds were counted. The experiment was repeated.
Effect of Salt Stress and Osmotic Potential on Germination
Four replicates of 50 seeds (≥8 mo old) each of CT2 and CT3 were used to investigate the effect of salt stress (or osmotic potential) on germination. Salt-stress treatments were applied by using sodium chloride (NaCl) solutions of 0, 20, 40, 80, 160, 250, and 320 mM. This selected range represents the level of salinity occurring in some soils in southern Australia (Chauhan et al. Reference Chauhan, Gill and Preston2006b). Solutions with osmotic potentials of 0, −0.1, −0.2, −0.4, −0.6, −0.8, and −1.0 MPa were prepared by dissolving polyethylene glycol 8000 (BioUltra, 8,000, Sigma-Aldrich, Castle Hill, NSW, Australia) in distilled water as described by Michel (Reference Michel1983). The tests were terminated when no further germination occurred for 7d. The experiments were repeated.
Seedling Emergence from Different Burial Depths
A pot experiment was conducted by placing seed 0, 1, 2, and 5 cm below the soil surface to assess the impact of seed burial on emergence. Fifty seeds of each population (CT2 and CT3) were placed at a single depth in each pot (15-cm deep by 12-cm diameter) with four replicates. The pots were filled with field soil (clay loam) sieved through a 2-mm-diameter sieve to 1.5 cm below the rim and randomly placed on a bench. The pots were lightly watered as needed to maintain adequate soil moisture. Four pots without seeds were used as controls to check whether there was any contamination of the test soil with seeds of this grass species. There was no emergence of windmillgrass from the control pots during the course of the experiment, confirming that there was no contamination. The number of emerged seedlings was counted after 14 d and was expressed as the percentage of total seed input. The experiment was conducted in a greenhouse with natural light and a temperature of 25 C and was repeated in a growth room set at a 12-h photoperiod at 708 μmol m−2 s−1 and a temperature of 25 C.
Seedbank Persistence under Field Conditions
A field experiment was conducted at Roseworthy, SA, Australia (34.525252°S, 138.686388°E), to determine seedbank persistence of windmillgrass. The experiment was a split-plot design with main plot treatments (two burial depths at 0 and 5 cm) randomly assigned among the four replicates. Within each main plot, subplots (populations) were randomly assigned. A total of 25 (Experiment 1: July 2013 to July 2014; Experiment 2: August 2014 to September 2015) or 50 (Experiment 3: April 2015 to June 2016) seeds from each population were mixed with soil (clay loam) and placed in a permeable nylon bag (10 by 5 cm). As previously described, there was no contamination of the test soil with windmillgrass seeds.
Seed bags from two burial depths in four replicates were removed at 0, 2, 4, 6, 8, 10, and 12 mo following burial. The exhumed seeds were germinated in an incubator set at 25 C, 12 h photoperiod cycle for 14 d. In addition, seeds of the same populations stored in the lab were germinated at the same time as the seeds exhumed from the field. Seed viability (%) was expressed as germination count of exhumed seeds buried at 0 or 5 cm in the field relative to the maximum germination count (as maximum viability) of seeds stored in the lab during the course of the experiment. The experiment was conducted three times in time and space.
Statistical Analyses. Estimation of Base Temperature
Among several methods used to estimate the minimum temperature thresholds for seed germination, the reciprocal time to 50% of germination has been shown to be the most statistically robust and biologically relevant method (Steinmaus et al. Reference Steinmaus, Prather and Holt2000). A logistic function was used to analyze germination response of each replicate (GraphPad Prism v. 6.00, LaJolla, CA):
where Y is the percentage of cumulative germination, X is the time (h), germination rate (T50) is the time required for the germination of half the total germinated seeds, and HillSlope describes the steepness of the family of curves.
A linear regression was performed with the reciprocal of the T50 against incubation temperature. The base temperature (Tb) was estimated as the intercept of the specific regression line with the temperature axis.
The estimated Tb value was used to calculate the growing degree days (GDD) to tillering, panicle emergence, and seed production by the following equation:
where GDD is degree days (Cd), Tmax is the daily maximum air temperature, Tmin is the daily minimum air temperature, and Tb is the estimated base temperature for seed germination of each population.
There was no population by treatment interaction for the plant development (pot study), seed dormancy, and seedbank persistence experiments, so data were pooled across populations. There was no experiment by treatment interaction for the salt-stress experiments, so data were pooled across experiments. There was no experiment and population by treatment interaction for the osmotic potential experiments, so data were pooled across experiments and populations. Logistic models were fit to data of germination at different concentrations of NaCl and different osmotic potentials (GraphPad Prism v. 6.00, LaJolla, CA).
The Shapiro-Wilk test for normality was conducted to investigate the distribution of percentage data. As the percentage data (effect of temperature: Gmax and effect of light) was not normally distributed (P<0.05), the original percentage values were arcsine transformed before ANOVA analysis. Fisher’s protected LSD multiple comparisons were employed to differentiate between predicted means, and means were presented as back-transformed data (GenStat 17, VSN International, Herts, UK).
Results and Discussion
Plant Development in an Irrigated Field and Pots
Under irrigated field conditions, windmillgrass required 272 (21 to 23), 518 to 522 (43 to 45), and 748 to 786 growing degree days (Cd) (74 to 75 d) from seedling emergence to reach tillering, panicle emergence, and mature seed stages, respectively (Table 1). The thermal time to panicle emergence of windmillgrass is similar to that of southern sandbur (Cenchrus echinatus L.) in a well-watered environment with 518 Cd (Machado et al. Reference Machado, Lima, Silva, Marques, Goncalves and Carvalho2014). However, windmillgrass in another study conducted in Merredin, WA, Australia, under greenhouse conditions required a greater thermal time from emergence to the start of seed production (970 Cd) (Michael et al. Reference Michael, Yeoh and Scott2012b) than did the CT2 and CT3 populations in this study. The observed differences between the two studies may be related to the differences between populations or environments for this parameter.
Table 1 Growing degree days and days from emergence to tillering, panicle emergence, and first mature seed of two windmillgrass populations (CT2 and CT3) in an irrigated field (2014).

a P, probability of t-test of null hypothesis that mean±SE of CT2 is equal to mean±SE of CT3.
In the pot studies, the thermal time to panicle emergence increased from 442 Cd for plants transplanted on October 3, 2013, to 629 Cd for plants transplanted on January 8, 2014, whereas the number of days to panicle emergence decreased from 55 to 35 d over this period (Figure 1). However, the thermal time to panicle emergence increased from 621 to 964 Cd for plants transplanted from February 5 to March 8, 2014 with a corresponding increase in days to panicle emergence from 52 to 191 over this period. Plants transplanted on March 8, 2014, when day length was less than 12 h and temperatures were cooler, remained vegetative over the winter and did not produce panicles until September 15, 2014 (the following spring). This indicates that windmillgrass can behave as an annual or a short-lived perennial in South Australia.

Figure 1 Growing degree days and number of days from 1-leaf stage to panicle emergence of windmillgrass populations at different transplant dates.
Seed Dormancy
Freshly harvested seed had low dormancy, with 16% to 40% germination at the optimum temperature (Figure 2). Lower germination (9%) was observed for freshly harvested seed in a study of windmillgrass in New South Wales, Australia (Lodge and Whalley Reference Lodge and Whalley1981). In the present study, germination response was not affected by the timing of seed maturity. Seeds that had matured in June 2013, November 2014, and December 2014 had similar germination patterns. Seed required approximately 1 mo after maturity to achieve 43% to 56% germination. Germination reached a maximum (73% to 83%) at 5 to 7 mo after maturity (Figure 2). Other windmillgrass populations in Western Australia and New South Wales, Australia also took more than 6 mo after seed production to achieve a maximum germination of 70% and 80%, respectively (Borger et al. Reference Borger, Riethmuller and Hashem2011; Maze et al. Reference Maze, Koen and Watt1993). These findings indicate that windmillgrass can germinate soon after seed maturity whenever temperature and moisture are suitable, but some seed will not germinate for several months, allowing it to avoid complete depletion of the seedbank in adverse summer conditions.

Figure 2 Germination response of CT2 and CT3 populations of windmillgrass from matured seed collected in June 2013, November 2014, and December 2014. Each data point represents the mean of two populations pooled with four replicates. Vertical bars are SE of the mean.
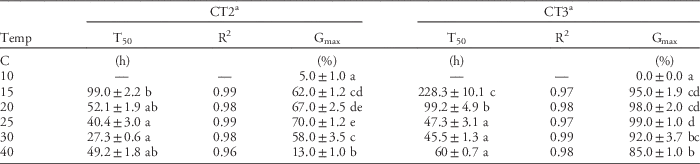
Effect of Temperature on Germination
Windmillgrass seed could germinate across a wide range of temperatures from 10 to 40 C. The optimum temperatures for maximum germination (Gmax) ranged from 15 to 30 C, with highest Gmax of 70% (CT2) and 99% (CT3) at 25 C. Gmax was significantly inhibited to 0% (CT3) and 5% (CT2) at 10 C, and to 13% (CT2) at 40 C (Table 2). This is consistent with previous studies that reported the optimum range for germination of windmillgrass was 15 to 35 C (Lodge and Whalley Reference Lodge and Whalley1981; Maze et al. Reference Maze, Koen and Watt1993; Michael et al. Reference Michael, Yeoh and Scott2012b). Our results also show large variation in the tolerance of some populations (CT3) to germinate under high-temperature conditions (Gmax of 85% at 40 C) (Table 2).
Table 2 Effect of temperature on germination rate (T50, R2 from Equation 1) and maximum germination (Gmax) of CT2 and CT3 populations of windmillgrass under 12 h alternating light/dark conditions.
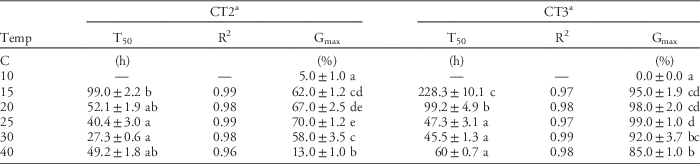
a Values (mean±SE) within a column followed by different letters are significantly different (Fisher’s protected LSD test: P≤0.05).
Windmillgrass germinated more rapidly as temperature increased. The optimum temperatures for both Gmax and germination rate (T50) of windmillgrass were 25 to 30 C. It required about 27.3 to 45.5 h for 50% germination at 25 to 30 C. (Table 2). By plotting temperature against the inverse of time to 50% germination, the base temperature (Tb) for germination was estimated to be 9.2 to 11.2 C (Figure 3). This estimated Tb value is consistent with previous findings that windmillgrass germination was near zero at 5 to 10 C (Lodge and Whalley Reference Lodge and Whalley1981; Maze et al. Reference Maze, Koen and Watt1993). The estimated Tb value of windmillgrass is similar to that of three other annual summer grass weeds: yellow foxtail [Setaria pumila (Poir.) Roemer & J. A. Schultes.], large crabgrass [Digitaria sanguinalis (L.) Scop.], and goosegrass [Eleusine indica (L.) Gaertn.], with Tb for germination of 8.3, 8.4, and 12.6 C, respectively; however, it is higher than that of green foxtail [Setaria viridis (L.) Beauv.], which has a Tb of 6.1 C (Masin et al. Reference Masin, Zuin, Archer, Forcella and Zanin2005). With its rapid germination (T50=27.3 to 45.5 h) and Tb of 9.2 to 11.2 C, windmillgrass is likely to germinate and emerge under field conditions after rainfall events that would maintain adequate soil moisture for only a few days in spring (6 to 29 C), summer (12 to 34 C), and autumn (9 to 29 C) (Figure 4) in South Australia. Germination in spring would make weed control difficult, as cereal crops are still growing in the fields at this time (Borger et al. Reference Borger, Riethmuller and Hashem2011).

Figure 3 Base temperature (Tb) estimation for windmillgrass seed germination. The lines are: CT2: Y=0.041*X−0.378 (R2=0.98), and CT3: Y=0.031*X−0.344 (R2=0.92). Each data point represents a replicate.

Figure 4 Monthly total rainfall and mean maximum and minimum temperatures from July 2013 to May 2016 at Roseworthy, SA, Australia, the period covered by the field experiments (http://www.bom.gov.au).
Effect of Light on Germination
Germination was strongly stimulated by light. In 2014, germination increased from 2% in the dark to 44% to 77% when seeds were exposed to light; and in 2015, germination increased from 0% to 0.5% in the dark to 63% to 83% in the light (Table 3). Maze et al. (Reference Maze, Koen and Watt1993) also reported that less than 5% of windmillgrass seeds germinated in the dark, while there was more than 80% germination in the light. Light requirement for germination is common among small-seeded species and warm-season grasses (Adkins et al. Reference Adkins, Bellairs and Loch2002; Grime et al. Reference Grime, Mason, Curtis, Rodman and Band1981; Milberg et al. Reference Milberg, Andersson and Thompson2000). Light may stimulate germination by altering the balance of germination promoters and inhibitors in the embryo (Adkins et al. Reference Adkins, Bellairs and Loch2002). The light requirement can ensure that germination takes place away from other vegetation and only on or near the soil surface (Adkins et al. Reference Adkins, Bellairs and Loch2002; Milberg et al. Reference Milberg, Andersson and Thompson2000), which would enhance the probability of seedling survival for small-seeded weed species such windmillgrass, which had average seed mass of 0.31 to 0.33 mg seed−1 in the present study. Management practices such as tillage (or use of a seed drill at planting time), narrow crop row spacing, and use of cover crops could reduce germination and emergence of windmillgrass under field conditions.
Table 3 Effect of light on germination of CT2 and CT3 populations of windmillgrass at 25 C.

a Values (mean±SE) followed by different letters are significantly different (Fisher’s protected LSD test: P≤0.05).
Effect of Salt Stress and Osmotic Potential on Germination
A logistic model was fit to germination response to NaCl concentration (R2=0.94 to 0.95). Seed germination was greater than 50% up to a NaCl concentration of 40 mM. The concentration of NaCl required to inhibit germination by 50% was estimated to range from 51 to 73 mM (Figure 5). The NaCl concentration that completely inhibited germination of windmillgrass was 250 mM NaCl, similar to that of annual sowthistle (Sonchus oleraceus L.) (Chauhan et al. Reference Chauhan, Gill and Preston2006a) but slightly lower than that of rigid ryegrass in South Australia (Lolium rigidum Gaudin), which required 320 mM for complete inhibition of germination (Chauhan et al. Reference Chauhan, Gill and Preston2006b).

Figure 5 Effect of salt stress (NaCl) on germination of CT2 and CT3 populations of windmillgrass at 25 C and 12-h alternating fluorescent light/dark. Each data point represents the mean of two experiments pooled with four replicates. Vertical bars are SE of the mean. The fitted line represents a logistic response equation: Y=100/{1+10[(1.704−X)*−3.750]} (R2=0.95) for CT2; and Y=100/{1+10[(1.861−X)*−3.150]} (R2=0.94) for CT3.
A logistic model was fit to the germination response to osmotic potential of the germination medium (R2=0.95). Germination was inhibited by 50% at −0.27 MPa and completely at −0.8 MPa (Figure 6). Similarly, the base water potentials for germination of yellow foxtail, green foxtail, and large crabgrass were −0.7, −0.7, and −0.8 MPa, respectively (Masin et al. Reference Masin, Zuin, Archer, Forcella and Zanin2005). This indicates that windmillgrass has a base water potential similar to other summer grass weed species, is not drought tolerant at germination, and will only germinate when there is adequate soil moisture (osmotic potential less than −0.8 MPa).

Figure 6 Effect of osmotic potential on germination of pooled populations of windmillgrass (CT2 and CT3) at 25 C and 12-h alternating light/dark. Each data point represents the mean of four replicates from two experiments and two populations pooled. Vertical bars are SE of mean. The fitted line represents a logistic response equation: Y=100/{1+10[(2.425−X)*−4.587]} (R2=0.95), where X is log(osmotic potential) in kilopascals.
Seedling Emergence from Different Burial Depths
Seedling emergence was completely inhibited by burial regardless of depth. Emergence of seedlings from seeds on the soil surface was 57.3±5.7% to 69.8±4.3% for CT2 and 66.5±8.5% to 78.4±2.6% for CT3, while seedling emergence was zero for all seeds buried at depths of 0.5, 1, 2, or 5 cm (unpublished data). The lack of seedling emergence with burial is consistent with other findings in this study that windmillgrass seed required light for germination (Table 3). Light can penetrate only a few millimeters in soil (Benvenuti Reference Benvenuti1995; Cussans et al. Reference Cussans, Raudonius, Brain and Cumberworth1996), so there would be no light to stimulate germination of buried seed; therefore, seedling emergence was not observed, even at the shallowest burial depth (0.5 cm). Tillage that buries seed is likely to inhibit germination, and widespread adoption of no-till cropping in Australia is likely to favor windmillgrass invasion. These results are consistent with a previous study in which all types of tillage treatments reduced emergence of windmillgrass compared with the no-till treatment (Widderick et al. Reference Widderick, Cook, McLean, Churchett, Keenan, Miller and Davidson2014).
Seedbank Persistence under Field Conditions
Under field conditions, seed viability was reduced with time (Figure 7) and was influenced by the amount of summer rainfall received over the 3 yr (Figure 4). In Experiment 1 (July 2013 to July 2014), viability of seeds on the soil surface and at 5-cm depth decreased by 50% after 6 mo, and was completely lost after 12 mo (Figure 7a). Seeds in Experiment 2 (August 2014 to September 2015) initially lost viability faster than those in Experiment 1. After 2 mo, seeds on the soil surface and at 5-cm depth lost 50% of viability in Experiment 2. In this year, viability of buried seed was lost completely after 12 mo, while there was 8% viable seed remaining on the soil surface (Figure 7b). Widderick et al. (Reference Widderick, Cook, McLean, Churchett, Keenan, Miller and Davidson2014) also found that less than 1% of windmillgrass seed persisted up to 12 mo after burial at 2 cm and 10 cm in the northern cropping region of Australia. As the windmillgrass seedbank was found to only persist for 12 mo, prevention of seed set in windmillgrass populations with herbicides, tillage, or mowing could deplete their seedbanks rapidly.

Figure 7 Changes in viability of seeds of CT2 and CT3 populations of windmillgrass at the soil surface compared with seeds at 5-cm burial depth. Experiment 1 (a): July 2013 to July 2014; Experiment 2 (b): August 2014 to September 2015; and Experiment 3 (c): April 2015 to June 2016. Each data point represents the mean of two populations pooled with four replicates. Vertical bars are SE of the mean.
Conversely, seedbank persistence in Experiment 3 (April 2015 to June 2016) was much greater than that in Experiment 1 and Experiment 2. Seeds buried at 5 cm in Experiment 3 had greater viability in all assessments than those placed on the soil surface. Viability of seeds exhumed at 8 mo after burial was 70% for those at 5-cm depth as compared with 56% for those on the soil surface. After 14 mo, buried seed still had 37% viability, whereas seed viability on the soil surface was almost completely lost (6% viable seed) (Figure 7c). An extremely dry spring and summer in 2015 to 2016 could have increased seedbank persistence compared with 2013 to 2014 and 2014 to 2015, when significant rainfall events occurred (Figure 4). These differences in seedbank persistence between years could be associated with less seed germination and less seed decay in dry and hot conditions than would occur in wet and warm conditions. Our results show that an adequate surface seedbank can be present in spring to summer (September to February) for seedling recruitment of this weed species in most seasons in southern Australia.
Windmillgrass has several characteristics that enable it to survive and persist in the Mediterranean environment of South Australia. It has rapid germination (27.3 to 45.5 h for 50% germination) and a base temperature of 9.2 to 11.2 C, so it can germinate and emerge under field conditions after rainfall events in spring, summer, and autumn in South Australia. It has a short period to maturity, requiring 780 Cd from emergence to mature seed stage. Germination of this small-seeded species required light, so seedling emergence occurred only for seeds present on the soil surface. Seed has low dormancy; however, seeds on the soil surface and buried at 5-cm depth remained viable for more than 11 mo. Low rainfall over spring and summer in the third year of this study extended seedbank persistence beyond 14 mo, especially for the seeds buried at 5-cm depth. These characteristics make this grass species ideally suited to the no-till farming system widely adopted in southern Australia, and it is likely this species will become a problem in such production systems. The demonstration that windmillgrass seeds persist for about 12 mo in an average season offers the opportunity to eradicate this weed species from a field, provided there is no further seed invasion. Control of new seed production for a period of 12 mo or more should exhaust the soil seedbank. Windmillgrass seed requires soil temperatures above 9C and sufficient moisture to germinate. This allows a focus on periods during the year when seedlings may establish and need to be controlled. Finally, seed germination in windmillgrass has an absolute requirement for light. Therefore, burial to depths greater than 0.5 cm, through strategic inversion tillage, for example, could completely inhibit germination.
Acknowledgments
This research was supported by the Grains Research and Development Corporation (project UA00149) and a Ph.D scholarship from the Vietnam Government and the University of Adelaide.