Introduction
Weeds are one of the major factors restricting and endangering agricultural production. Chemical herbicides have become an indispensable part of modern agricultural production because they provide economic and efficient weed control. Unfortunately, long-term abuse or overuse of chemical herbicides has resulted in herbicide resistance in weeds.
Herbicide resistance is a result of adaptation and selection pressure and is classified as target-site resistance (TSR) and non–target site resistance (NTSR) (Beckie and Tardif Reference Beckie and Tardif2012). TSR is usually caused by mutations in the gene that controls the target enzyme, resulting in decreased herbicide binding capacity to the site of action or overexpression of the target enzyme gene. There are many mechanisms responsible for NTSR, including reduced absorption or translocation or increased metabolism and sequestration (Powles and Yu Reference Powles and Yu2010). NTSR mechanisms confer unpredictable resistant phenotypes that may be resistant to other unrelated herbicides. Applying herbicides to a weed population whose resistance status is unknown runs the risk of resistance selection. Thus, herbicide resistance is a major threat to the continued efficacy of available herbicides (Délye Reference Délye2013).
Acetolactate synthase (ALS) or acetyl hydroxybutyric acid synthase, the target site of ALS-inhibiting herbicides, catalyzes one of the vital steps in the biosynthesis of the branched-chain amino acids valine, leucine, and isoleucine in plants and microorganisms (Durner et al. Reference Durner, Gailus and Boger1991). These herbicides are grouped into five chemical families: sulfonylureas (SUs), imidazolinones (IMIs), triazolopyrimidines (TPs), pyrimidinyl-thiobenzoates (PTBs), and sulfonyl-aminocarbonyl-triazolinone (SCTs). Mesosulfuron-methyl, a POST SU herbicide, is the most important ALS inhibitor for selective control of grass weeds in winter wheat (Triticum aestivum L.) fields in China. Other ALS inhibitors such as imazethapyr (IMI family) and bispyribac-sodium (PTB family) are registered to selectively control annual weeds in soybean [Glycine max (L.) Merr.] fields and grass weeds in rice (Oryza sativa L.) fields in China. Pyroxsulam (TP family) and flucarbazone (SCT family), which target Japanese foxtail (Alopecurus japonicus Steudel) and Japanese brome (Bromus japonicus Houtt.), are also used to control weeds in winter wheat fields in China.
The TSR mechanisms in ALS-inhibitor resistance have been clearly identified. The mutation of any one of eight amino acid sites in ALS—Ala-122, Pro-197, Ala-205, Asp-376, Arg-377, Trp-574, Ser-653, and Gly-654—will lead to TSR and cause varying degrees of cross-resistance to different herbicides (Yu and Powles Reference Yu and Powles2014). In addition to the resistance caused by amino acid mutations, the upregulation of target enzyme gene expression has also been identified in many resistant weeds. Target-gene duplication is a common glyphosate-resistance mechanism and could become a fundamental process for developing any resistance trait (Sammons and Gaines Reference Sammons and Gaines2014).
Cytochrome P450 monooxygenases (CYTP450s) play an important role in the metabolic detoxification of herbicides (Kwon and Pennner Reference Kwon and Pennner1995; Xu et al. Reference Xu, Wang and Guo2015; Yuan et al. Reference Yuan, Tranel and Stewart2007). Didierjean et al. (Reference Didierjean, Gondet, Perkins, Lau, Schaller, O’Keefe and Werck-Reichhart2002) determined that increased herbicide metabolism and tolerance can be achieved by ectopic constitutive expression of CYP76B1 in tobacco (Nicotiana tabacum L.) and Arabidopsis.
American sloughgrass [Beckmannia syzigachne (Steud.) Fernald], a winter annual gramineous plant, is distributed in most parts of China, but mainly in the middle and lower reaches of the Yangtze River (Li et al. Reference Li, Liu, Chi, Guo, Luo and Wang2015). It has same growth habit as winter wheat and has become one of the most predominant and troublesome weeds in wheat fields rotated with rice after 20 yr of reduced- and no-tillage practices (Li et al. Reference Li, Zhang, Zhao, Guo, Liu, Li and Wang2017). Mesosulfuron-methyl, an SU ALS inhibitor, is one of the main herbicides used to selectively control B. syzigachne in winter wheat fields in China. After continuous application over several years, the efficacy of mesosulfuron-methyl has decreased. In 2011, mesosulfuron-methyl failed to control B. syzigachne in Yutai County, Shandong Province after previous years of success. The objectives of this research were to (1) investigate susceptibility of B. syzigachne to mesosulfuron-methyl; (2) characterize the cross-resistance to other ALS-inhibiting herbicides; and (3) determine the mechanisms responsible for the resistance to mesosulfuron-methyl in a resistant population.
Materials and methods
Plant materials and growth conditions
The putative resistant population (SD-04, R) was collected in 2011 from Yuntai County, Shandong Province. A known susceptible population (SD-12, S) was collected in 2013 from Taishan District, Shandong Province. Mature seeds were collected and bulked in the field. The seeds were then air-dried at room temperature and stored at 4 C until used. Seeds of B. syzigachne were placed in petri dishes containing two layers of filter paper and moistened with 8 ml of distilled water. All petri dishes were placed in an incubator (GXZ -500, Ningbo Jiangnan Instrument Factory, Ningbo, Zhejiang, China; 20/10 C day/night temperature and 12/12-h photoperiod). The distilled water in the petri dish was changed every day. When the shoot grew to 1 cm, 10 plants were transplanted into 11-cm-diameter plastic pots containing loam soil and were watered. Pots were transferred to a greenhouse on the Qingdao Agricultural University campus (temperature maintained at approximately 15 to 25 C and natural sunlight) and watered as needed (Li et al. Reference Li, Bi, Liu, Yuan and Wang2013). Ten days later, seedlings were thinned to 5 evenly sized plants per pot.
Herbicide treatment and data collection
To determine the resistance level to mesosulfuron-methyl and other ALS inhibitors in the R and S populations, a series of herbicide concentrations (Table 1) were sprayed on the seedlings at the 3- to 4-leaf stage using a moving-nozzle cabinet sprayer equipped with one TeeJet® 9503EVS flat-fan nozzle at 0.28 MPa (3WP-2000, Nanjing Mechanization Research Institute of the Ministry of Agriculture, China). Water was used as a control. The sprayer was calibrated to deliver 450 L ha−1. Treated plants were put back into the greenhouse and cultured as described earlier. After 21 d, the seedlings were cut at the soil surface, and fresh weight was determined. Water was used as a control. There were three replications per concentration. The experiment was conducted twice.
Table 1. Herbicides used in dose–response experiment.

a Mesosulfuron-methyl, pyroxsulam, flucarbazone, imazethapyr, and bispyribac-sodium belong to the sulfonylurea, triazolopyrimidine, pyrimidinyl-thiobenzoate, imidazolinone, and sulfonyl-aminocarbonyl-triazolinone families, respectively.
b R, resistant; S, susceptible.
ALS sensitivity in vitro
The ALS enzyme was extracted following the method developed by Ray (Reference Ray1986). Plants were grown as described earlier. Approximately 0.3 g of shoots from each population were ground in liquid nitrogen with a precooled mortar and pestle and then homogenized in 30 ml of enzyme extract buffer (0.1 M pH 7.0 phosphate buffer containing 1 mM sodium pyruvate, 0.5 mM MgCl2, 0.5 mM thiamine pyrophosphate [TPP], 10 μM flavin adenine dinucleotide [FAD], and 10 μM phenylmethylsulphonyl fluoride). The homogenate was incubated on ice for 30 min and then centrifuged at 12,000 rpm for 30 min at 4 C (CR21N, Hitachi Koki, Tokyo Japan). The supernatant was precipitated by dropwise addition of an equal volume of saturated (NH4)2SO4 and centrifuged at 12,000 rpm for 30 min at 4 C. The supernatant was discarded, and the pellet was dissolved with 30 ml of enzyme solution (0.1 M pH 7.0 phosphoric acid buffer containing 20 mM sodium pyruvate and 0.5 Mm MgCl2). Soluble protein content was determined colorimetrically (595 nm) with a UV spectrophotometer (TU-1810, Beijing General Instruments) (Bradford Reference Bradford1976).
The ALS activity was determined according to the method published by Yu et al. (Reference Yu, Friesen, Zhang and Powles2004). The reaction system consisted of 0.99 ml of enzyme, 1 ml of reaction solution (0.1 M pH 7.0 phosphoric acid buffer containing 20 mM sodium pyruvate, 0.5 M MgCl2, 0.5 mM TPP, and 10 μM FAD), and 10 μl of mesosulfuron-methyl solution, the final concentration of mesosulfuron-methyl was adjusted to 0, 0.001, 0.01, 0.1, 1, 10, and 100 μM. The mixture was maintained at 35 C for 1 h. Then the reaction was ended with 200 μl of 3M H2SO4 followed by incubation at 60 C for 15 min. Next, 1 ml of 0.5% creatine and 1 ml of 5.5% methylene naphthol (dissolved in 2.5 M NaOH) were added and held at 60 C for 15 min. The ALS activity was determined at 525 nm by measuring acetoin production with a UV spectrophotometer. The herbicide concentrations required to inhibit 50% of the ALS activity (I50) were calculated using nonlinear regression analyses. The assay was performed twice with independent extractions, each with three replications per herbicide concentration.
ALS gene analysis
To determine the molecular basis of resistance, the ALS gene was cloned, sequenced, and compared between the R and S populations. About 50 mg of shoot tissue from an individual plant was cut and immediately frozen in liquid nitrogen at the 3- to 4-leaf stage. Then the seedlings were treated with mesosulfuron-methyl at a dose of 15.75 g ai ha−1(recommended rate). Mortality rates were determined at 21 DAT. Twenty-five individuals from each population were sequenced and sprayed.
The genomic DNA was isolated from 0.2 g of fresh leaf tissues of each plant using the cetyltrimethylammonium bromide method (Doyle and Doyle Reference Doyle and Doyle1990). A forward primer s1 (CACCAACCACCTCTTCCG) and a reverse primer r1 (TCCTGCCATCACCTTCCA) were designed by Primer Premier 5.0 according to the published ALS gene sequence of B. syzigachne (NCBI accession number: KR809881.1). Reactions were completed in a 25-μl system consisting of 2.5 μl of 10×EasyTaq Buffer (Mg2+ Plus), 2 μl of dNTPs (2.5 mM), 0.25 μl of EasyTaq DNA Polymerase (5 U/μl), 1 μl of template, 1 μl of forward primer (100 μM), 1 μl of reverse prime (100 μM), with DNase-free water added to bring the final volume up to 25 μl. The polymerase chain reaction (PCR) was completed using the T100™ Thermal Cycler (Bio-Rad Laboratories). The PCR program was as follows: initial denaturation step for 4 min at 95 C; three-step cycles consisting of denaturation 40 s at 95 C, annealing 40 s at 63 C, and extension 90 s at 72 C, repeated for 35 cycles; and a final extension step for 10 min at 72 C. The 1,793-bp amplified fragment contained the intermediate conserved sequence of ALS. The PCR products were detected and purified by 1% agarose gel electrophoresis. Then the target band was sequenced by Sangon Biotech (Shanghai, China). Finally, the ALS gene sequences were aligned and compared by DNAman v. 9 software (Lynnon, San Ramon, CA).
Determination of relative expression level of ALS gene in the R population
Twenty individuals from each population were harvested and bulked to extract total RNA for this experiment. The total RNA was extracted from 50 mg of fresh shoot using the TaKaRa MiniBEST Plant RNA Extraction Kit (Takara Biomedical Technology, Beijing, China) according to the manufacturer’s protocol. The integrity of each sample was determined by 1% agarose gel electrophoresis. Using the total RNA as a template, the first strand cDNA was synthesized using Trans EasyScript First-Strand cDNA Synthesis SuperMix (TransGen Biotech) according to the procedure described on the kit.
In the real-time quantitative reverse transcriptase PCR (qRT-PCR) experiment, the forward primer Y2 (5′GGGTGCTACCAACCTCGTC3′) and reverse primer G2 (5′GGAGCGGGTGACCTCTACT3′) were designed according to the published ALS gene sequence of B. syzigachne (NCBI accession number: KR809881.1) and used to amplify the ALS gene. The 18S rRNA was selected as the reference (Wrzesińska et al. Reference Wrzesińska, Kierzek and Obrępalska-Stęplowska2016). The forward primer N1 (5′AGAAACGGCTACCACATC3′) and the reverse primer C1 (5′CCAAGTCCAACTACGAG3′) were designed according to the 18S rRNA gene sequence of maize (Zea mays L.) (NCBI accession number: XR _002750193.1) to amplify the reference gene.
The two-step method qRT-PCR reactions were completed in a 20-μl solution consisting of 10 μl of 2×SG Fast qPCR Master Mix, 0.4 μl of 10 μM forward primer, 0.4 μl of 10 μM reverse primer, 1 μl of template cDNA, and 8.2 μl of PCR-grade water. The reaction was completed on a LightCycler® 96 (Roche Diagnostic Products, Shanghai, China) with an initial denaturation step at 95 C for 180 s, followed by 40 cycles of 95 C for 3s, and then 60 C for 25 s. Dissociation was generated according to instrument guidelines. Dates from each reaction were compiled using software available on the thermal cycler. The expression level of ALS gene in the R and S populations was analyzed using the 2−△△Ct method (Schmittgen and Livak Reference Schmittgen and Livak2008).
Effect of malathion pretreatment on resistant plants
Malathion, a CYTP450 inhibitor has been extensively used to detect resistance resulting from herbicide metabolism accelerated by CYTP450s (Beckie and Tardif Reference Beckie and Tardif2012; Preston et al. Reference Preston, Tardif, Christopher and Powles1996). In this experiment, R seedlings were sprayed with 2,000 g ai ha−1 malathion at the 3- to 4-leaf stage. Two hours later, the pretreated R seedlings were sprayed with mesosulfuron-methyl in a series of concentrations of 1.75, 5.25, 5.75, 47.25, 141.75, and 425.25 g ai ha−1. A no-malathion treatment was used as control. Simultaneously, S seedlings were sprayed with mesosulfuron-methyl in a series of concentrations of 0.58, 1.75, 5.25, 15.75, 47.25, and 141.75 g ai ha−1. After 21 d, the seedlings were cut at the soil surface, and fresh weight was determined. The assay was performed twice with independent extractions, each with three replications per herbicide concentration.
Metabolic impact of mesosulfuron-methyl
Beckmannia syzigachne seedlings were treated using mesosulfuron-methyl (30 g ai ha−1) at the 3- to 4-leaf stage. Shoots were harvested at 4 h, 1 d, 5 d, 10 d, 16 d, and 21 d after herbicide treatment and frozen at −20 C.
To analyze the metabolic differences of mesosulfuron-methyl in the R and S populations, plant samples were extracted and quantified following the QuEChERS method (AOAC Official Method 2007.01). The 0.2-g sample was ground in liquid nitrogen and extracted with 4 ml of acetonitrile containing 1% acetic acid (v/v) using an ultrasonic wave (480 W, 40 kHz) for 15 min. Then, 0.4 g of NaCl was added. After a 5-min incubation, the extract was centrifuged at 4,000 rpm for 10 min. One milliliter of supernatant was transferred into a 2-ml centrifuge tube, and 25 mg of primary secondary amine, 7 mg of graphitized carbon black, and 50 mg of MgSO4 were added. Then the extract was centrifuged at 12,000 rpm for 3 min. Finally, 1 ml of supernatant was filtered by passing it through a 0.22-μm syringe filter, and this was used in the ultraperformance liquid chromatography–tandem mass spectrometry (UPLC-MS-MS) experiment.
The separation and quantitation of mesosulfuron-methyl was conducted by UPLC-MS/MS with an Agilent 1290 Infinity/6460 equipped with an Agilent ZORBAX Eclipse Plus C18 column (150 mm × 2.1 mm × 1.8 µm) maintained at 30 C. The mobile phase was composed of 70% water with 0.2% HCOOH (v/v) and 30% acetonitrile, and the flow rate was 0.2 ml min−1. The MS was run at the conditions of DL temperature at 325 C, heat block temperature at 300 C, nebulizing gas flow of 8.0 L min−1, and drying gas flow of 3 L min−1. The MS conditions were as follows: (+) ESI, detection of molecular weight 504/282, voltage 213 V, and bombardment energy 13 eV.
To obtain a standard linear formula, technical-grade mesosulfuron-methyl (95%, Shandong Binnong Technology, China) was dissolved in acetonitrile containing 1% acetic acid (v/v) to prepare the standard solutions of 100, 50, 10, 5, 1, 0.5, and 0.1 μg L−1. The linear formula was determined using the concentration of herbicide and corresponding peak area provided by MS analysis. The assay was performed twice with independent extractions, each with three replications per herbicide concentration.
Statistical analysis
All data were processed by SigmaPlot v. 12.0 (Systat Software, Chicago, IL) bi-logistic nonlinear regression model, and the growth inhibition medium value (GR50 or I50) was calculated according to the following equation (Seefeldt et al. Reference Seefeldt, Jensen and Fuerst1995):
where Y was the response (fresh weight), C was the lower dose–response limit, D was the upper dose–response limit, X was the herbicide dosage, GR50 or I50 was the growth inhibition medium value, and b was the slope. Data sets from repeated experiments were analyzed by ANOVA (SPSS v. 20.0, IBM, Armonk, NY), and a t-test (P < 0.05) was used to test the significance of the regression parameters; where variance between repeated experiments was not significant, pooled data were used for subsequent analyses. Resistance index (RI) was calculated as the GR50 of the R population divided by the GR50 of the S population to indicate the level of resistance for the R population.
Results and discussion
Herbicide treatment
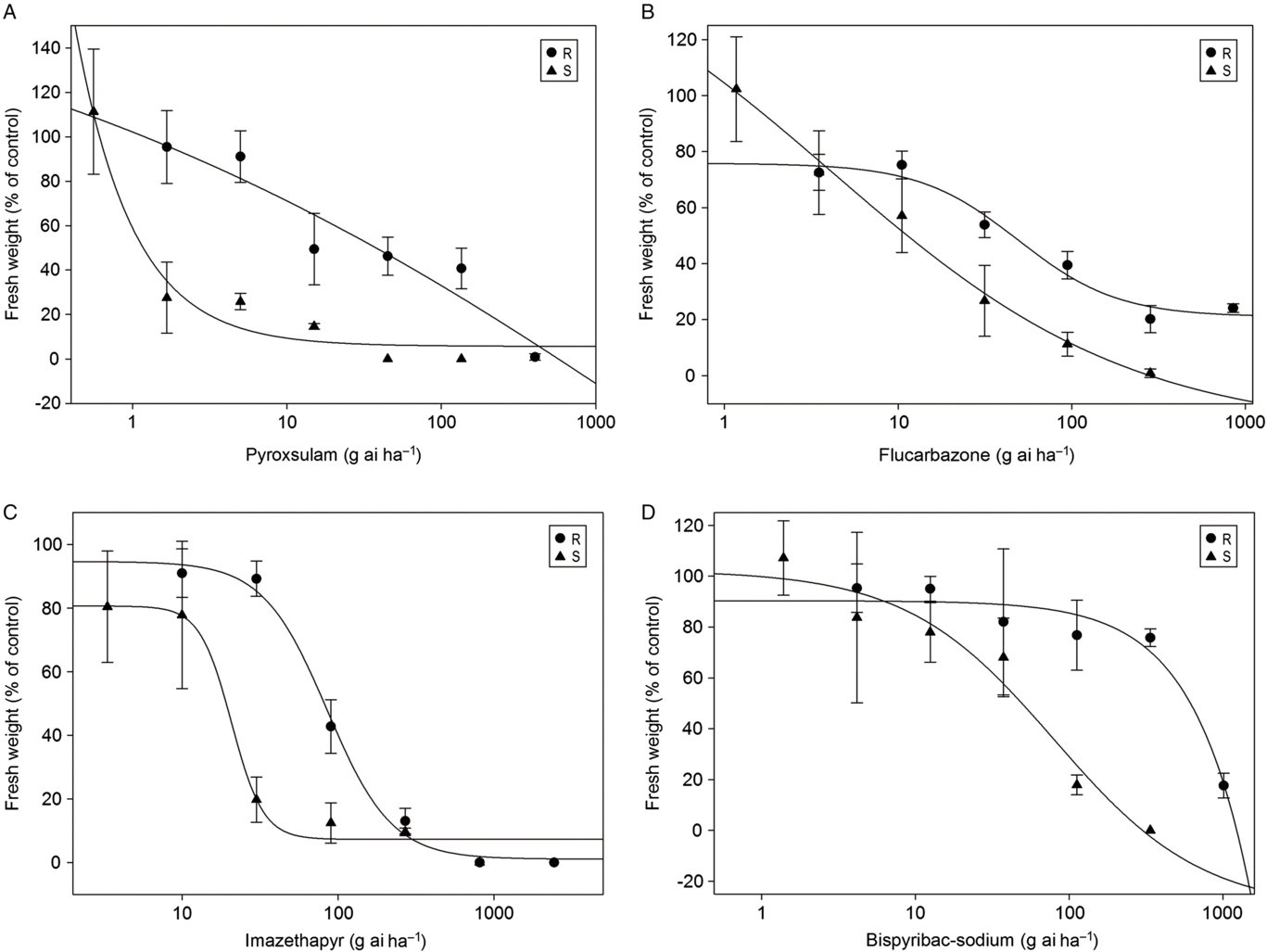
A series of herbicide experiments were conducted to determine the resistance level to mesosulfuron-methyl and the cross-resistance pattern to other ALS-inhibiting herbicides in an R population of Beckmannia syzigachne. At 10 d after treatment (DAT), the S population was more injured than the R population for all herbicides tested at the recommend dosage. The results indicated that the R population had 4.1-fold resistance to mesosulfuron-methyl (Figure 1). The R population had different degrees of resistance to four other ALS inhibitors tested. Among them, the resistance level to pyroxsulam was the highest (RI = 600); the resistance to imazethapyr was the lowest (RI = 4.4); and the RI was 12 for both flucarbazone and bispyribac-sodium (Figure 2).

Figure 1. Dose–response curves for aboveground fresh weights of resistant (R) and susceptible (S) Beckmannia syzigachne populations treated with mesosulfuron-methyl. Vertical bars represent mean ± SE.

Figure 2. Dose–response curves for aboveground fresh weights of the resistant (R) and susceptible (S) Beckmannia syzigachne populations treated with a range of (A) pyroxsulam, (B) flucarbazone, (C) imazethapyr, and (D) bispyribac-sodium rates. Vertical bars represent mean ± SE.
Recently, Wang et al. (Reference Wang, Li, Li, Han, Li, Yu and Cui2018) reported that a B. syzigachne population (R1) had low resistance to mesosulfuron-methyl (3.3-fold) and imazapic (2.8-fold), moderate resistance to pyribenzoxim (7.4-fold), and high resistance to flucarbazone (11-fold) but was susceptible to pyroxsulam (1.6-fold) due to the increased metabolic capacity of CYTP450s (Wang et al. Reference Wang, Li, Li, Han, Li, Yu and Cui2018). Our results were similar to those of Wang et al. (Reference Wang, Li, Li, Han, Li, Yu and Cui2018) for resistance to flucarbazone, but different for pyroxsulam, to which our R plants were 600-fold more resistant.
ALS activity assay in vitro
To clarify the resistance mechanism to mesosulfuron-methyl in the R population, the in vitro activity of the ALS enzyme was determined (Figure 3). The results indicated that the I50 of the R population was 0.52 ± 0.045 μM, which was not different from that of the S population (0.69 ± 0.029 μM). Therefore, whole-plant resistance in the R population is not due to target-site ALS enzyme insensitivity. That insensitive ALS enzyme mechanisms usually result in high levels of resistance has been well documented and reviewed as an important resistance mechanism in weeds (Powles and Yu Reference Powles and Yu2010; Yu and Powles Reference Yu and Powles2014). Resistance in a plant with a sensitive ALS enzyme will be due to an NTSR mechanism (Guo et al. Reference Guo, Riggins, Hausman, Hager, Riechers, Davis and Tranel2017; Liu et al. Reference Liu, Bai, Zhao, Jia, Li, Zhang and Wang2018; Owen et al. Reference Owen, Goggin and Powles2011; Yu et al. Reference Yu, Abdallah, Han, Owen and Powles2009; Zhao et al. Reference Zhao, Fu, Yu, Huang, Yan, Li, Shafi, Zhu, Wei and Ji2017). Recently, an insensitive ALS enzyme was identified in an B. syzigachne population (R1) that had low resistance to mesosulfuron-methyl (Wang et al. Reference Wang, Li, Li, Han, Li, Yu and Cui2018).

Figure 3. Dose–response curves of acetolactate synthase isolated from resistant (R) and susceptible (S) Beckmannia syzigachne populations treated with mesosulfuron-methyl. Vertical bars represent mean ± SE.
ALS gene sequencing
Analyses of the ALS genes in many sulfonylurea-resistant weed biotypes proved that the resistance was caused by mutations at a single site or two sites in the conserved region of the ALS gene (Yu and Powles Reference Yu and Powles2014). In our study, no known resistant mutations were identified in either individuals from the R population who survived the mesosulfuron-methyl treatment or individuals from both populations who were killed by mesosulfuron-methyl treatment. This result is in accordance with our enzyme-sensitivity experiment (Table 2). Therefore, resistance to mesosulfuron-methyl in the R population is not caused by amino acid mutations in the target enzyme.
Table 2. Amino acids at sites associated with ALS resistance in resistant (R) and susceptible (S) populations.

Determination of relative expression level of ALS gene in the R population
The fluorescent dye in relative fluorescence quantification can monitor the real-time production of PCR products in each PCR cycle. This method was employed to calculate the ALS gene expression level in B. syzigachne in this study. Our experiments indicated that the ALS gene expression level of the R population was not different from that of the S population (Figure 4). Therefore, the mesosulfuron-methyl resistance in the R population was not caused by an overexpressed ALS gene.

Figure 4. Ct value of ALS gene in resistant (R) and susceptible (S) Beckmannia syzigachne populations. Vertical bars represent mean ± SE.
Increased expression of target enzyme genes is a common target resistance mechanism in glyphosate-resistant weeds (Chatham et al. Reference Chatham, Wu, Riggins, Hager, Young, Roskamp and Tranel2015; Chen et al. Reference Chen, Huang, Zhang, Wei, Huang, Chen and Wang2015; Gaines et al. Reference Gaines, Shaner, Ward, Leach, Preston and Westra2011; Koo et al. Reference Koo, Jugulam, Putta, Cuvaca, Peterson, Currie, Friebe and Gill2018). Recently, this mechanism was identified in large crabgrass [Digitaria sanguinalis (L.) Scop.] resistant to acetyl-CoA carboxylase inhibitor (Laforest et al. Reference Laforest, Soufiane, Simard, Obeid, Page and Nurse2017). Overexpression of the ALS gene has been reported in resistant shortawn foxtail (Alopecurus aequalis Sobol.) from Japan and China (Iwakami et al. Reference Iwakami, Shimono, Manabe, Endo, Shibaike, Uchino and Tominaga2017; Zhao et al. Reference Zhao, Yan, Wang, Bai, Wang, Liu and Wang2018).
Effect of malathion pretreatment on resistance to mesosulfuron-methyl in R plants
Plant responses to mesosulfuron-methyl with or without malathion pretreatment were determined (Figure 5). The GR50 values for mesosulfuron-methyl treatments of R plants with and without malathion were 27 ± 7 g ai ha−1 and 110 ± 9 g ai ha−1, respectively, while the GR50 for mesosulfuron-methyl treatment of S plants was 20 ± 4 g ai ha−1. These results prove that malathion inhibited metabolism of mesosulfuron-methyl.

Figure 5. Comparison of whole-plant responses resistant (R) and susceptible (S) Beckmannia syzigachne populations to mesosulfuron-methyl without (−) or with (+) malathion. Mean responses were measured using fresh aboveground weight. Vertical bars represent mean ± SE.
Malathion pretreatment has been used as a method to identify whether CYTP450 was involved in herbicide resistance (Christopher et al., Reference Christopher, Preston and Powles1994; Feng et al. Reference Feng, Gao, Zhang and Dong2016; Guo et al. Reference Guo, Riggins, Hausman, Hager, Riechers, Davis and Tranel2017; Zhao et al. Reference Zhao, Fu, Yu, Huang, Yan, Li, Shafi, Zhu, Wei and Ji2017). Malathion obviously reversed the resistance to mesosulfuron-methyl in the R population in our experiment, so it is likely that CYTP450 was involved in mesosulfuron-methyl resistance in the R population.
Difference in the metabolic rate to mesosulfuron-methyl
The UPLC-MS-MS experiment measured the concentration dynamic of mesosulfuron-methyl in B. syzigachne (Figure 6). The concentration of the herbicide in both the R and S populations peaked at 4 h after treatment and decreased slowly over the next 24 h. At 5 DAT, the concentration of mesosulfuron-methyl in the R population decreased sharply and was lower than in the S population. Similar results were also measured at 10 and 17 DAT. These results indicate that R plants metabolized mesosulfuron-methyl more rapidly than S plants.

Figure 6. Concentration of mesosulfuron-methyl in resistant (R) and susceptible (S) populations of Beckmannia syzigachne at 4 h, 1 d, 5 d, 10 d, and 15 d after treatment. Vertical bars represent mean ± SE.
Enhanced metabolism is a mechanism for herbicide resistance. Water starwort [Myosoton aquaticum (L.) Moench] and flixweed [Descurainia sophia (L.) Webb ex Prantl] from China are resistant to tribenuron-methyl via enhanced herbicide metabolism (Bai et al. Reference Bai, Liu, Wang, Zhao, Jia, Zou, Guo and Wang2018; Yang et al. Reference Yang, Li, Shen, Xu, Liu, Deng, Li and Zheng2018). A resistant blackgrass (Alopecurus myosuroides Huds.) population from Germany can degrade fenoxaprop-p-ethyl and mesosulfuron-methyl more rapidly than a susceptible population (Kaiser and Gerhards Reference Kaiser and Gerhards2015). Nicosulfuron was shown to metabolize faster in resistant D. sanguinalis (Mei et al. Reference Mei, Si, Liu, Qiu and Zheng2017).
In conclusion, our R B. syzigachne population is resistant to mesosulfuron-methyl and cross-resistant to pyroxsulam, flucarbazone, imazethapyr, and bispyribac-sodium because of enhanced metabolism of mesosulfuron-methyl.
Author ORCIDs
Lingxu Li, https://orcid.org/0000-0003-1436-0069
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (no. 31301680) and Advanced Talents Foundation of Qingdao Agricultural University (6631115023). The authors thank all the workers for their assistance in conducting this research. No conflicts of interest have been declared.