Introduction
Cereal rye (Secale cereale L.) is a troublesome weed in winter wheat (Triticum aestivum L.)-producing areas in the United States. Secale cereale infests hundreds of thousands of hectares in the western United States (Stahlman and Peterson Reference Stahlman and Peterson1995; Stump and Westra Reference Stump and Westra1993) and causes millions of dollars in losses to growers annually (Burger et al. Reference Burger, Lee and Ellstrand2006; White et al. Reference White, Lyon, Mallory-Smith, Medlin and Yenish2006) due to lower wheat yields and the cost of S. cereale control (Pester et al. Reference Pester, Westra, Anderson, Lyon, Miller, Stahlman, Northam and Wicks2000; Stump and Westra Reference Stump and Westra2000). Due to the similar life cycles of the two species, options for mechanical methods of weed control are limited, even more so when water and soil conservation practices are used. Consequently, growers must rely on cultural or chemical methods instead.
There are very few selective herbicide options to control S. cereale in wheat (Pester et al. Reference Pester, Nissen and Westra2001). With the introduction of imidazolinone-tolerant wheat cultivars, farmers have used imazamox to control S. cereale in the United States (Geier et al. Reference Geier, Stahlman, White, Miller, Alford and Lyon2004; Peeper et al. Reference Peeper, Roberts, Solie and Stone2008). However, there can be a large variation in tolerance of S. cereale to imazamox (Peeper et al. Reference Peeper, Roberts, Solie and Stone2008). Because S. cereale is an obligate outcrossing species, there is a wide range of phenotypic and genotypic variation within and among populations (Burger et al. Reference Burger, Lee and Ellstrand2006, Reference Burger, Holt and Ellstrand2007; Peeper et al. Reference Peeper, Roberts, Solie and Stone2008), and this variability could contribute to the inconsistency of imazamox efficacy. Other environmental or edaphic factors could also play a role in the variability of response of S. cereale to imazamox, such as temperature or spray application volume.
Low temperatures can negatively affect herbicide efficacy (Geier et al. Reference Geier, Stahlman and Hargett1999; Olson et al. Reference Olson, Al-Khatib, Stahlman, Parish and Moran1999; Olson et al. Reference Olson, Al-Khatib, Stahlman and Isakson2000; Varanasi et al. Reference Varanasi, Prasad and Jugulam2016). Fall application is the optimum time to control winter annual grass species (Blackshaw and Hamman Reference Blackshaw and Hamman1998; Geier et al. Reference Geier, Stahlman, White, Miller, Alford and Lyon2004), but impending colder weather can reduce the effectiveness of herbicide applications. Even when a herbicide application occurs under ideal conditions in the fall, temperatures can drop rapidly after application. These temperature decreases may alter plant growth and reduce herbicide translocation and degradation (Bruce et al. Reference Bruce, Carey, Penner and Kells1996). However, few studies have measured herbicide behavior before, during, and after plants experience cold temperatures. Buman et al. (Reference Buman, Gealy and Ogg1992) and Olson et al. (Reference Olson, Al-Khatib, Stahlman and Isakson2000) reported reduced absorption and translocation of metribuzin and sulfosulfuron, respectively, under cool temperatures by some grass species, but not others (neither paper investigated S. cereale). Pester et al. (Reference Pester, Nissen and Westra2001) specifically looked at absorption, translocation, and metabolism of imazamox in S. cereale, but there is no information regarding the influence of temperature on the fate of imazamox.
Inherent genetic variability in S. cereale, coupled with unfavorable conditions such as low temperature, may lead to inconsistent control with imazamox. Thus, it is important to understand how and when factors such as cold temperature affect the activity of imazamox applied to S. cereale. The objective of this study was to determine the effect of temperature on the absorption, translocation, and metabolism of imazamox in S. cereale, and how these effects relate to the activity of imazamox on S. cereale. Temperature treatment regimes were selected to represent typical conditions during fall and spring imazamox application to winter wheat.
Materials and Methods
Low-Temperature Timing Effect on Imazamox Efficacy
Secale cereale seed was collected from plants growing in a field site near Loveland, CO, in 2008. Seeds were planted into potting soil (Fafard® 2 SV, Conrad Fafard, Agawam, MA 01001) in 8 by 8 by 8 cm inserts and thinned to 2 plants insert−1. Plants were grown in greenhouse conditions set to 22/18 C (day/night) with a 14-h day length and natural lighting supplemented with 400-W sodium-halide lamps until the 3-leaf stage (with no tillers).
Herbicide Treatment
Plants were treated with 17.5 g ai ha−1 formulated imazamox (Beyond® herbicide, BASF, Research Triangle Park, NC 27709), which included 0.25 % v/v nonionic surfactant (NIS; R-11, Wilbur-Ellis, Fresno, CA 93755) and 3.5 L ha−1 urea ammonium nitrate (UAN; Nortrace®, Loveland Products, Greeley, CO 80632). The imazamox solution was applied with an overhead track sprayer in a spray volume of 187 L ha−1 at 206 kPa with a TT8001 nozzle (TeeJet® Technologies, Wheaton, IL 60139). Plants were placed in 4 C at sequential time points following herbicide application: 0, 1, 2, 3, 4, 5, 6, and 7d, and one at 22/18 C that was never exposed to cold. There were also two nontreated checks subjected to warm or cold temperatures at the 0-d time point. After herbicide application, plants were kept at 4 C for 4 wk (8-h day length). Following the period of cold, plants were exposed to 22/18 C for 1 wk, at which point plants were excised at the soil surface. The plants remained at 22/18 C for an additional week, and regrowth of shoots was evaluated categorically as either present or absent. Plants with no regrowth after 1 wk were considered dead. The experiment was established as a randomized complete block design with six replications and was repeated once in time. Each replicate contained 2 plants, treated as subsamples, per pot. Data were analyzed with Sigma Plot v. 11 (Systat Software, Chicago, IL 60606) and fit to a four-parameter sigmoid model.
Absorption and Translocation of Imazamox in Secale cereale
Secale cereale seeds were planted in 6.5 by 25 cm cones filled with fine silica sand. Plants were grown in a growth chamber set to a 22/18 C day/night cycle (warm conditions) with a 12-h photoperiod and watered daily. Plants receiving cold treatment were placed in a cold room with a constant 4 C temperature and 10-h photoperiod. A slow-release fertilizer (10-10-10 applied at 0.4 g per cone; Osmocote® Flower and Vegetable Smart Release, Scott’s Miracle-Gro, Marysville, OH 43041) was added to the cones after planting, and 5 ml of half-strength Hoagland’s solution (Hoagland’s no. 2 basal salt mixture, Sigma-Aldrich, St Louis, MO 63103) was added weekly to both temperature regimes.
Treatment Groups
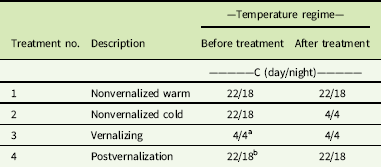
Secale cereale plants were separated into four equal-sized treatment groups. At the 3-leaf growth stage, two groups were treated with 14C-labeled imazamox and subjected to different temperature treatments. Group 1 consisted of plants growing at 22/18 C, while Group 2 was subjected to continuous 4 C. Simultaneously, Groups 3 and 4 were also introduced to continuous 4 C to initiate vernalization. During vernalization, plant growth slowed so that only one additional leaf was present for the subsequent herbicide treatments. After 3 wk of cold temperature, 14C-labeled imazamox was applied to Group 3, but the plants remained at 4 C. Group 4 was vernalized for 6 wk and then placed back in the warm growth chamber at 22/18 C for 1 wk. [14C]Imazamox was then applied to Group 4. These plants were vernalized, as a preliminary study demonstrated that plants would initiate reproduction with only 5 wk of 4 C (unpublished data). The groups represent three growth stages in which imazamox could be applied in field conditions: application to nonvernalized plants under warm or cool conditions (simulated fall/seedling stage; Groups 1 and 2), application during vernalization (nearly dormant; Group 3), and application after vernalization (simulated spring; Group 4). Temperature treatments are further described in Table 1.
Table 1 Temperature treatments for the translocation experiment before and after herbicide application.
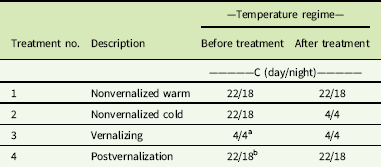
a Plants grown at 4/4 C for 3 wk before imazamox treatment
b Plants grown at 4/4 C for 6 wk followed by 22/18 C for 1 wk before imazamox treatment.
[14C]Imazamox Application and Harvest
Plants were oversprayed with 17.5 g ha−1 formulated imazamox with 0.25 % v/v NIS and 3.5 L ha−1 UAN. The imazamox solution was applied with an overhead track sprayer at a target rate of 187 L ha−1 at 206 kPa. An aliquot was taken from this spray solution before application and mixed with technical grade [14C]imazamox. This solution was used to treat the newest fully expanded leaf with a total of 333 Bq imazamox (specific activity 1,850 kBq mg−1) applied in five 0.5-µl droplets within 10 min of overspraying. Plants were harvested at 0, 0.5, 1, 3, and 7 d after treatment (DAT). The [14C]imazamox-treated leaf of each plant was excised and washed with 5 ml of leaf wash solution (methanol and NIS, 10% and 0.25% by volume, respectively, in water) for 20 min in a reciprocating shaker. Treated leaves were removed, and 14C was measured in the remaining leaf wash solution via liquid scintillation spectroscopy (LSS; Packard 2500R, PerkinElmer, Waltham, MA 02451) by adding 10 ml of scintillation cocktail (Ultima Gold LLT, PerkinElmer). The remaining plant tissue was divided into shoot, crown, and root samples. Crown tissue was considered all belowground growth, excluding roots. All tissues, including the washed treated leaf, were dried at 60 C for 96 h, and dry biomass weight was recorded. Dried tissue was combusted with a biological oxidizer (OX500, R. J. Harvey Instrument, Tappan, NY 10983) where 14C was captured in a trapping cocktail (OX-161, R.J. Harvey Instrument) and quantified using LSS. Root exudation of 14C was measured by adding 50 ml of water to the rooting medium. The samples were shaken and then allowed to settle, and a 5-ml aliquot was added to 10 ml of scintillation cocktail.
Data Analysis
The uptake and translocation experiment was designed as a randomized complete block with a split-plot arrangement and three replications and repeated once in time. Time of herbicide application was the main plot effect, and harvest time points were the subplot effect. Imazamox absorption was calculated as the total quantity of 14C applied minus that recovered in the leaf wash. Homogeneity of variance was determined for both experiments using SAS v. 9.2 (SAS Institute, Cary, NC 27513), where PROC GLM was used to analyze means, standard errors, and mean separation. Data were subjected to Levene’s test for homogeneity of variance to determine whether data sets could be pooled. A weighted estimate of t statistic was calculated for comparisons (Fisher’s protected LSD) between all treatment combinations for uptake and translocation means. Sigma Plot v. 11 (Systat Software) was used to apply an exponential rise to maximum model (f(y)=66.55e−3.69t ; where t=days), as per Kniss et al. (Reference Kniss, Vassios, Nissen and Ritz2011), to absorption data.
Imazamox Metabolism in Secale cereale
Secale cereale seeds were pre-germinated and transplanted into 6.5 by 25 cm cone pots filled with washed silica sand. Plants were grown in the greenhouse under ambient and supplemental light (400-W sodium-halide lamps) until they reached the 3-leaf stage. Plants were watered daily with 7.5 ml Hoagland’s solution for the duration of the experiment.
[14C]Imazamox Application and Harvest
Technical-grade [14C]imazamox was added to a 35 g ai ha−1 imazamox spray solution containing NIS (0.25% v/v) and UAN (2.5% v/v) to create the radiolabeled application solution. The application was quality-control tested for accurate dosing of 1,666 Bq plant−1 by placing 10 µl of the solution plus 10 ml of scintillation cocktail into each of three scintillation vials. 14C radioactivity was measured using LSS. Each leaf of each plant was treated such that each plant received 10 µl of application solution.
Three replications of plants were randomly selected for each temperature treatment for harvest at one of five time points (0, 1, 2, 4, and 6 DAT). All treated plants, except those to be evaluated at 0 DAT, were placed either in a cold room (4C) or a growth chamber (22/18 C).
The shoot tissue of each treated plant was analyzed for [14C]imazamox at each time point using a Beta-RAM radio high-performance liquid-chromatography detector (RHPLC; Ram Radioactivity Detector Model 2B LabLogic Systems, Inc., Tampa, FL 33619). Plants were excised at the root–shoot interface. The shoots were placed in 15-ml plastic tubes and washed for 20 min with a shaker on the high setting. Wash solution consisted of 10 ml of 90% HPLC-grade water and 10% HPLC-grade methanol solution to remove any unabsorbed material. Five milliliters of scintillation cocktail was added to the solution that remained after the leaf wash, and radioactivity was measured using LSS. Analysis of the leaf wash allowed indirect correction of RHPLC disintegrations per minute (dpm) data for percent imazamox absorption. Shoots and roots were triple rinsed with 100 ml of HPLC grade water and allowed to dry, and shoots were cut into smaller tissue segments. Roots were placed in coin envelopes and dried for 24 h at 60C. Radioactivity in root samples was measured using a biological oxidizer as described earlier. Negligible amounts of 14C radioactivity were detected in the roots during the course of the experiment (unpublished data).
[14C]Imazamox was extracted by grinding shoot tissue from each sample with 10 ml of 90% HPLC-grade acetone and 10% HPLC-grade water solution and centrifuging for 30 min. Glass tubes for each sample were weighed before and after the addition of the acetone supernatant to volume-corrected dpm measured by RHPLC. Remaining water in the supernatant was removed using a vacuum evaporation system (RapidVap, Labconco, Kansas City, MO 64132). Samples were transferred to filter tubes and centrifuged on high for 10 min, and supernatants for each sample were transferred to HPLC vials.
The HPLC column had two mobile phases. Mobile phase A contained 10% HPLC-grade acetonitrile, 0.05% phosphoric acid, and 89.95% HPLC-grade water. Mobile phase B contained 30% HPLC-grade acetonitrile, 0.05% phosphoric acid, and 69.95% HPLC-grade water. Each sample was processed by running helium through both mobile phases for 5.8 min. [14C]Imazamox standard solutions were used at the beginning and end of each HPLC run. The standard solution was 10 µl [14C]imazamox+20 µl normal imazamox+2 ml mobile phase A.
The experiment was repeated once in time, and data from both experiments were combined and compared by fitting two-parameter exponential decay models where f(y)=14C remaining as [14C]imazamox (% of [14C]imazamox absorbed), k=rate of decay, and t=time (d). Temperature treatment was considered a categorical variable with two levels (i.e., cold or warm), and all data were fit to models simultaneously. Statistical analyses were performed on radioactive material remaining as [14C]imazamox measured as a percent of absorbed [14C]imazamox at each time point using SAS v. 9.2 and SigmaPlot v. 11.
Results and Discussion
Low-Temperature Timing Effect on Imazamox Efficacy
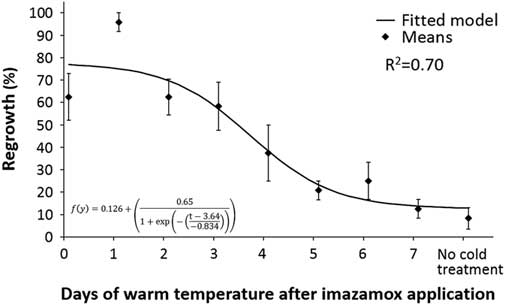
Imazamox efficacy on S. cereale decreased as plants were exposed to 4 C shortly after herbicide application. Plants not exposed to cold temperature had 90% mortality (Figure 1). When cold-temperature treatment was applied between 0 and 3 DAT, 60% or more of the plants had tissue regrowth. Secale cereale that had at least 5 d of 22/18 C performed similar to plants in the treatment with no cold-temperature exposure (GLM pairwise comparison).
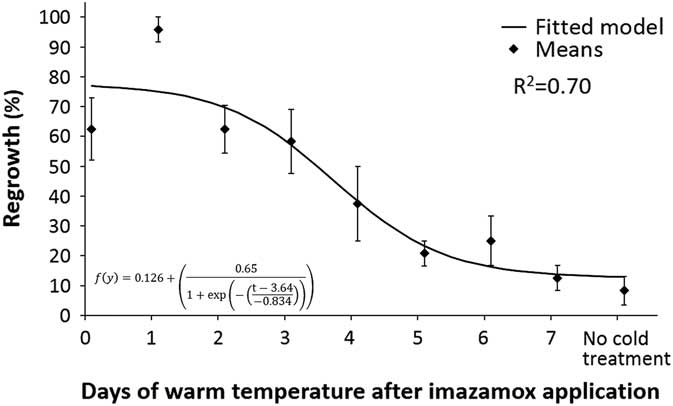
Figure 1 Regrowth of Secale cereale plants following imazamox application and exposure to different lengths of warm temperature (22/18 C) followed by continuous cold (4/4 C).
Based on the number of warm days required for maximum efficacy, I50 and I80 values (the points at which 50% and 80% mortality are reached, respectively) can be calculated. The I50 was 3.3 DAT, and I80 was 5.5 DAT. The minimum growing temperature for domesticated S. cereale is 4 C (Kantar and Porter Reference Kantar and Porter2014). Therefore, the I80 value identified in this research represents the longest period of time that S. cereale would be likely to survive. Temperatures below 4 C cause S. cereale dormancy, and warmer temperatures would reduce the amount of time needed to ensure S. cereale mortality. Thus, if S. cereale does not experience cold temperatures until after 5 DAT, mortality should be high, unless plant dormancy is induced by other adverse environmental conditions.
Absorption and Translocation of Imazamox in Secale cereale
Imazamox Absorption
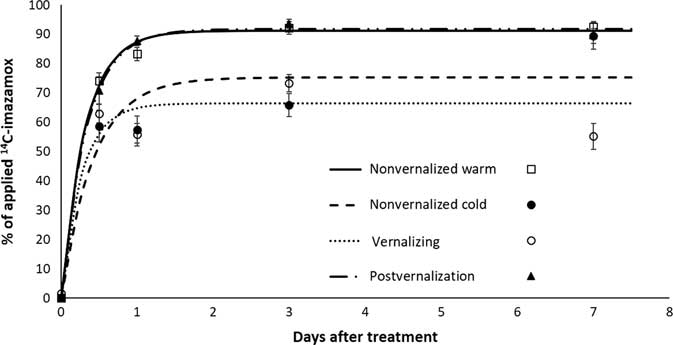
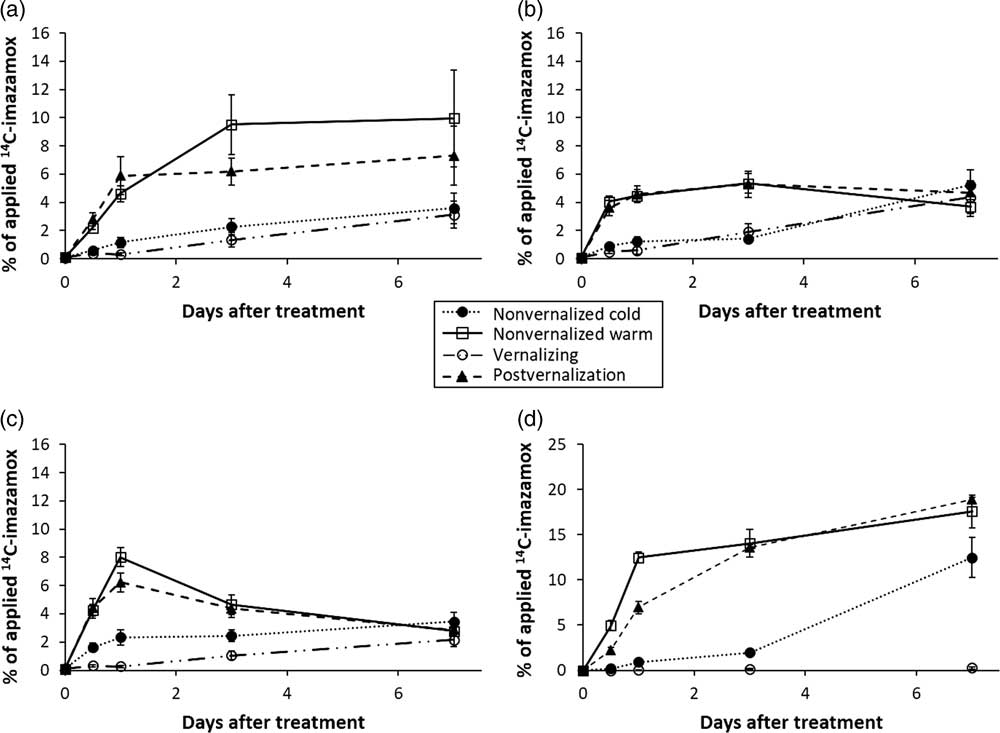
Absorption of imazamox was greatest in warm-temperature treatments and lowest in cold-temperature treatments (Figure 2). Beginning at 1 DAT, temperature treatments affected absorption by approximately 20% of applied imazamox. Growth stage (pre- vs. postvernalization) of S. cereale had little effect on uptake of imazamox. In warm-temperature treatments, absorption was always greater than 80%, similar to other reports (Pester et al. Reference Pester, Nissen and Westra2001). Maximum absorption was reached at 3 DAT, except for plants in the nonvernalized cold treatment, which did not reach maximum absorption until 7 DAT. At that time, final absorption was 89%, similar to results for the warm-temperature treatments. Parameters for absorption models are listed in Table 2.
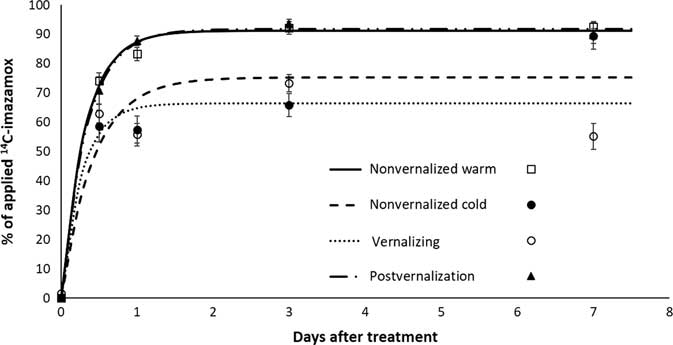
Figure 2 Absorption of imazamox measured as total applied [14C]imazamox in Secale cereale plants.
Table 2 Estimated parameters of imazamox absorption in Secale cereale plants at various growth stages.

Imazamox Translocation
[14C]Imazamox recovery ranged from 70% to 100%, largely dependent on temperature and growth stage (unpublished data). Lowest recovery was realized during the postvernalization time point. This could be due to loss of exuded imazamox during daily watering from the bottom of the container during the long growth period of these S. cereale plants (see Figure 4D).
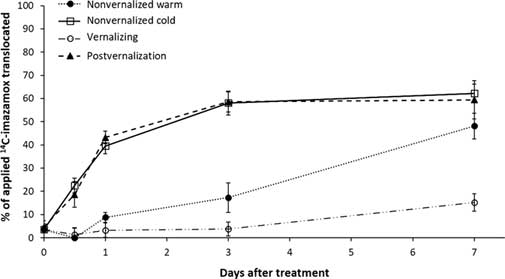
The two warm-temperature treatments had a nearly identical response of 14C translocation from the treated leaf (Figure 3). Translocation of imazamox was lowest at continuous 4 C, with a maximum of 15% at 7 DAT, whereas almost 60% of the applied 14C translocated out of the treated leaf in warm temperatures. In the nonvernalized cold treatment, minimal translocation was observed before 1 DAT, but herbicide translocation continued in a near linear fashion until reaching nearly 50% of the applied total by the end of the experiment. This value was approaching the amount translocated by plants in the warm-temperature treatments (~60%).
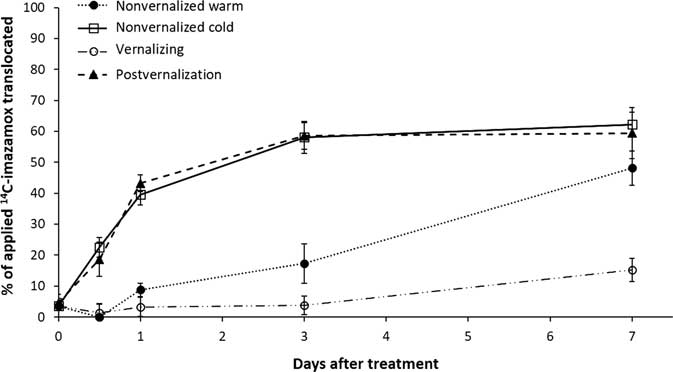
Figure 3 Translocation from the treated leaf as measured by absorbed [14C]imazamox minus [14C]imazamox remaining in the treated leaf of Secale cereale plants.
The translocated portion of [14C]Imazamox was divided into shoot, crown, and root tissue and soil exudation to determine its ultimate fate. Cold-treated plants translocated imazamox to plant shoots in a similar manner (Figure 4A) and at a lower rate than plants in either warm-temperature treatment. Warm-temperature treatments followed different trends. Variances in warm-temperature data make it difficult to distinguish between the warm-temperature treatments.
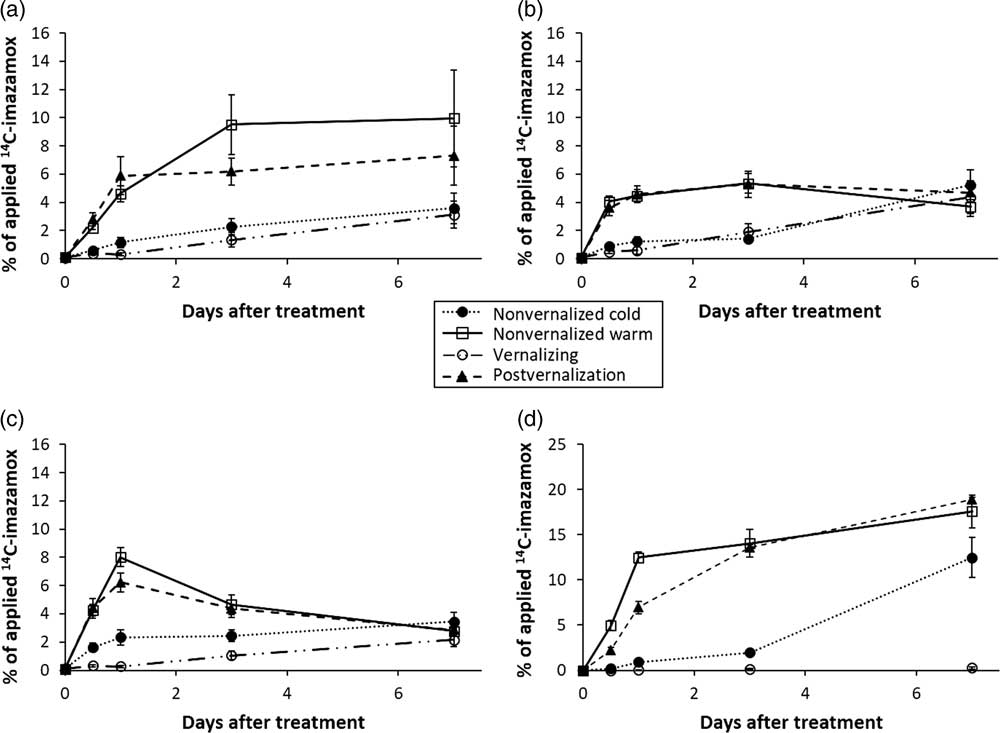
Figure 4 Imazamox translocation in (A) nontreated shoot tissue, (B) crown tissue, (C) roots of Secale cereale plants, and (D) amount of [14C]imazamox recovered from the soil.
Crown tissue accumulated a maximum of nearly 6% of total applied [14C]imazamox regardless of temperature treatment (Figure 4B). Cold temperature–treated plants accumulated imazamox more slowly than warm temperature–treated plants; however, all plants accumulated similar amounts of imazamox in the crown by 7 DAT regardless of growing conditions. Differences of 4% to 5% applied imazamox existed between the two temperature treatments for all sampled dates before 7 DAT.
Root tissue reached a maximum accumulation of applied [14C]imazamox (8%) in warm-temperature treatments at 1 DAT and then decreased (Figure 4C). Both warm- and cold-temperature treatments resulted in approximately 4% of applied imazamox in root tissue by 7 DAT, lower than the amount detected in crown tissue. In warm temperatures, it appears that imazamox was more readily exuded from root tissue into the soil. In cold temperatures, the concentration of imazamox in root tissue continued to increase.
The amount of 14C in the soil was determined after root removal. Less than 0.3% of applied imazamox was detected under continuous 4 C treatment (Treatment 3) across all time points (Figure 4D). All other treatments exuded 12% to 18% of total applied imazamox by 7 DAT regardless of the growing temperature (Figure 4D). The amount of 14C in the soil of warm temperature–treated plants approached near-maximum levels by 3 DAT, though accumulation continued through 7 DAT. However, the nonvernalized cold temperature–treated plants showed a much different pattern of 14C exudation. By 3 DAT, less than 5% of the applied 14C was detected in the soil, but by 7 DAT, the amount had increased to approximately 12% of the applied herbicide (Figure 4D). It is possible more herbicide could be exuded from this treatment if plants were left in cold temperatures for a longer time (this was beyond the scope of this study), simultaneously with a steadily increasing amount of imazamox being translocated from the treated leaf. The amount of translocated imazamox exuded ranged from 26% to 30% of the translocated total for all but the continuous-cold treatment. Other studies have shown that roots of other grassy species exude imidazolinones under normal growing conditions (Little and Shaner Reference Little and Shaner1991; Shaner Reference Shaner1988). The difference between cold-temperature treatments was that imazamox was applied to the nonvernalized treatment under warm conditions, where normal plant function is expected. Plants were then subjected to cold temperatures, causing slow growth and development. Plants treated during the vernalization process would already be acclimated to the cooler growing conditions. Thus, the nonvernalized cold treatment tends to have an intermediate response compared with the vernalizing treatment and either warm treatment.
Metabolism of Imazamox
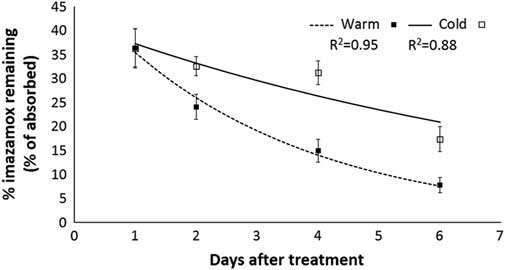
The ability of S. cereale to metabolize imazamox under different temperature regimes was measured. Results indicate that S. cereale was capable of metabolizing imazamox while growing under either warm or cold temperatures (Figure 5). Metabolism by warm-treated plants was similar to what was reported by Pester et al. (Reference Pester, Nissen and Westra2001) in assessing the ability of S. cereale to metabolize imazamox. Metabolism still occurred in cold-treated plants, albeit at a much slower rate, creating a larger window for detoxification before, and thus averting, mortality. With a longer duration of the experiment, the intact imazamox concentration may reach a point where both temperature treatments are similar.

Figure 5 Imazamox metabolism in shoot tissue of warm- and cold-treated Secale cereale plants.
The temperature treatments differed by only 10% of intact imazamox at the conclusion of the experiment, leaving a final imazamox concentration of approximately 10% and 20% for warm- and cold-treated plants, respectively (Figure 5). From Figure 1, it was estimated (I80=5.5 d), there would be 8.8% intact imazamox remaining. Introducing cold temperatures before 5.5 d could reduce growth enough to increase the likelihood of S. cereale survival. Imazamox translocation steadily increases in cold-treated plants (Figure 3), particularly between 3 and 7 DAT after imazamox application, with 29.5% and 18.6% remaining active, respectively. This makes the amount of active imazamox much greater in cold-treated plants compared with warm-treated plants, especially at later time points. Yet it is the warm-treated plants that are likely to die.
When temperatures decrease, imazamox efficacy on S. cereale decreases due to the effect of temperature on absorption, translocation, and metabolism. Imazamox absorption decreases approximately 50% at 4 C compared with 22/18 C. There is also a concomitant decrease in the amount of imazamox translocated out of the treated leaf to the rest of the plant at continuous 4 C compared with 22/18 C. However, S. cereale continues to metabolize imazamox, although at a reduced rate at continuous 4 C. The reduction in absorption and translocation of imazamox at low temperature coupled with continued metabolism of the herbicide results in the loss of efficacy. Even though plants were not developing appreciably in cool temperatures, biological activity within the plant remained high enough to effectively detoxify a large percent of the herbicide at a time when the plant is not likely to be producing high levels of branched-chain amino acids.
The practical application of these results is that a grower should not apply imazamox to imidazolinone-tolerant wheat to control S. cereale if cold temperatures are predicted within several days of application. If temperatures drop and remain low for 3 d after application, these results suggest that treatment may not elicit a lethal response. Five or more days of warmer temperatures may be required to effectively control S. cereale with imazamox.
Acknowledgments
The Colorado Wheat Research Foundation provided partial support for this project. No conflict of interest has been declared.