Halosulfuron-methyl is a primidinylsulfonylurea herbicide with PPI, PRE, and POST Cyperus spp. activity, leading to its effective use in many vegetable crops (Anonymous 2016; Culpepper et al. Reference Culpepper, Grey and Webster2009; Grey et al. Reference Grey, Culpepper and Webster2007a, Reference Grey, Culpepper and Webster2007b; Johnson et al. Reference Johnson, Grey and Kissel2010). A weak acid with pKa of 3.5, halosulfuron-methyl demonstrates increasing solubility from 15 mg L−1 at pH 5 to 1,630 mg L−1 at pH 7 (Shaner Reference Shaner2014). This increase in solubility enhances activity but also extends soil longevity (Saha and Kulshrestha Reference Saha and Kulshrestha2002). Overall degradation of halosulfuron begins with chemical hydrolysis of the sulfonylurea bridge resulting in loss of herbicidal activity (Zheng et al. Reference Zheng, Yates and Papiernik2008). Chemical degradation tends to be temperature dependent for halosulfuron, with half-life (DT50) decreasing with increasing temperature (Dermiyati and Yamamoto Reference Dermiyati and Yamamoto1997a; Sarmah and Sabadie Reference Sarmah and Sabadie2002).
Fresh market yellow squash and watermelon production in the southeastern United States often involves planting into low-density polyethylene mulch (LDPE) mulch (Culpepper et al. Reference Culpepper, Grey and Webster2009). The use of mulch production systems for fruiting vegetables and cucurbits has been a standard for decades. These systems enhance fruit maturity and quality while providing soil-borne weed, insect, and disease control (Culpepper et al. Reference Culpepper, Grey and Webster2009; Grey et al. Reference Grey, Culpepper and Webster2007a; Johnson and Mullinix Reference Johnson and Mullinix2005; Webster et al. Reference Webster, Csinos, Johnson, Dowler, Sumner and Fery2001). Additionally, these systems often allow growers to produce two to five crops on a single installation of mulch. Producing multiple crops before removing the mulch minimizes expenses associated with the pesticides, mulch, and drip-tape irrigation by distributing these costs over multiple crops. Although the loss of methyl bromide in plasticulture has been challenging, the production system is still critical to vegetable production.
One new challenge since the loss of methyl bromide has been the need for additional herbicides to be added into the systems (Webster et al. Reference Webster, Culpepper and Johnson2003). Nutsedge (Cyperus spp.) is the greatest weedy challenge in plasticulture because of its ability to not only escape the fumigant system but to also penetrate the mulch (Chase et al. Reference Chase, Sinclair, Chellemi, Olson, Gilreath and Locascio1999). When nutsedge is not controlled prior to planting, yield loss occurs (Dittmar et al. Reference Dittmar, Monks, Schultheis and Jennings2008; MacRae et al. Reference MacRae, Culpepper, Batts and Lewis2008). The logical solution would be to apply halosulfuron over mulch before planting to control nutsedge plants penetrating the mulch, especially in watermelon and yellow squash (Anonymous 2016; Culpepper et al. Reference Culpepper, Grey and Webster2009; Johnson and Mullinix Reference Johnson and Mullinix2002, Reference Johnson and Mullinix2005; Shrefler et al. Reference Shrefler, Brandenberger, Webber, Roberts, Payton and Wells2007; Webster and Grey Reference Webster and Grey2014). Halosulfuron is currently labeled for row middle applications in both watermelon and yellow squash and can be applied pretransplant in watermelon as well as PRE in seeded watermelon (Anonymous 2016; Culpepper et al. Reference Culpepper, Grey and Webster2009; Johnson and Mullinix Reference Johnson and Mullinix2002, Reference Johnson and Mullinix2005; Webster and Grey Reference Webster and Grey2014).
Halosulfuron applied over the top of LDPE mulch has been shown to leave residues on the mulch that may subsequently injure transplanted crops (MacRae et al. Reference MacRae, Culpepper, Batts and Lewis2008). However, this research focused on applications of halosulfuron within a week of planting. Depending on the season, growers in the southeastern United States often have at least a 35-d window to control the previous crop, weeds, and other pests before planting the subsequent crop. The potential for using halosulfuron 7 to 35 d prior to transplanting yellow squash and watermelon into mulch has not been studied. Therefore, field trials were initiated and included an analytical approach and a field bioassay initiated in 2013 and 2014 to determine the potential for applying halosulfuron over mulch up to 35 d prior to transplanting yellow squash or watermelon. Additional laboratory experiments examined the effects of time and temperature on halosulfuron stability in aqueous solutions using a thermogradient device.
Materials and Methods
Analytical Experiments, Laboratory
Halosulfuron stability in high-performance liquid chromatography (HPLC) water (pH 7.0) as affected by temperature and time was evaluated on a thermal gradient table. An 86.2 µM solution of halosulfuron in HPLC water was prepared with formulated product (Sandea®, 75% ai, Gowan Company, Yuma, AZ) followed by constant stirring with a stir bar and stirrer at 22 C. Formulated halosulfuron totally dissolved in 30 min. This solution was pipetted into incubation flasks (30 ml flask−1), and the flasks were sealed with parafilm and then placed onto the thermal gradient table at 11, 15, 20, 25, 30, 38, and 42 C, with four replications per temperature. Duplicate 1-ml samples from each flask were then removed by pipette (Fisher Scientific International, Hampton, NH) at 0, 1, 6, 12, 24, 48, 96, 168, 240, and 336 h and transferred to HPLC vials (Fisher Scientific International). The experiment was repeated in time. The samples were analyzed with a Waters Alliance 2690 system (Waters Corporation, Milford, MA) using a photodiode array with standard curves generated from technical-grade halosulfuron (Sigma-Aldrich, St Louis, MO). For analysis, the mobile phase was acetonitrile with 10% MeOH, pH 4.5, using an YMC ODS column (YMC America, Allentown, PA) with a 0.2 ml min−1 flow rate. Data were processed using Waters Masslynx software (Waters Corporation).
The thermal gradient tables were constructed from solid aluminum blocks measuring 2.4-m long by 0.9-m wide by 7.6-cm thick with a mass of 470 kg. On each end of the aluminum blocks, a 1-cm hole was drilled across the side section to allow fluid to be pumped into the table. The thermal gradient was generated by installation of a chiller and a heating unit on opposite sides of the table that pumped 10 or 44 C ethylene glycol plus water (1:10 mixture), respectively, at a rate of 3.8 L min−1. Along the length of the thermal gradient, approximately 1.38 C increments occurred every 10 cm, with a constant temperature across the width. Thermocouples made from duplex-insulated PR-T-24 wire (Omega Engineering, Stamford, CT) were mounted to the underside of the table from the hot end to the cold end. The thermocouple was within 5 mm of the upper table surface at 10-cm intervals. Data indicated a continuous temperature gradient ranging from 10 to 44 C along the length of the table. Temperature was monitored continuously for each thermocouple and recorded at 30-min intervals with a Graphtec data logger (MicroDAQ, Contoocook, NH). All temperature data for each thermocouple were downloaded daily and used to ensure consistency during experiments and data analysis.
Analytical Experiments, Field
Field studies were conducted near Ty Ty, GA (31.50911°N, 83.64813°W) to evaluate the dissipation of halosulfuron from black LDPE mulch in summer and autumn of 2013. These sites are typical of commercial vegetable crop production in the southeastern United States. Land was prepared by tandem disking followed by a moldboard plowing. A field cultivator with double rolling baskets and S-tine harrows finalized field preparation. Plot size was a 15-cm-tall by 81-cm-wide by 6-m-long bed prepared using a superbedder plastic layer, followed in the same operation with drip tape laid in the center of the bed 2.5 cm below the bed surface, followed immediately with LDPE mulch (0.032 mm, 1.25 mil). Treatments included halosulfuron at 26 and 53 g ai ha−1with a nonionic surfactant (Chem Nut 80-20, Chem Nut, Albany, GA) at 0.25 % v/v. Treatments were applied with a CO2-pressurized backpack sprayer calibrated to deliver 140 L ha−1 at 150 kPa to the LDPE mulch. This corresponds to 427 and 871 µM for 26 and 53 g ai ha−1 treatments, respectively. Herbicides were applied August 27, 2013, for the first experiment and September 30, 2013, for the second experiment. Maximum and minimum temperatures and total daily solar radiation were recorded (Flitcroft Reference Flitcroft2015), and experiments were conducted for 35 d after treatment (DAT).
Each test used a randomized complete block design with treatments replicated four times. LDPE mulch samples were collected at 1 h after treatment, then at 1, 3, 8, 14, 23, 28, and 35 DAT, 8 in total in time. LDPE mulch sample areas were protected only when there was a threat of rain, with sample areas being covered with a rigid stainless aluminum box measuring 30-cm wide by 122-cm long by 20-cm tall. The aluminum box weighed approximately 10 kg and provided a tight seal to prevent rainfall from entering or seeping under the mulch, but did not cut the surface. This ensured that the sample area did not receive rainfall to wash off halosulfuron. Environmental data were collected for each experiment (Table 1).
Table 1 Environmental data for halosulfuron dissipation from low-density polyethylene mulch in 2013 near Ty Ty, GA.

a Experiment 1 conducted August 27 to September 30, 2013.
b Experiment 2 conducted September 30 to November 4, 2013.
c Average daily maximum and minimum temperature for that week.
d Weekly sum of total solar radiation for the week.
Samples of mulch were collected from each plot using an open-faced wooden square frame with an inside area of 0.1 m2. A box-cutting knife was used to harvest the mulch along the inside edge of the square. Needle-nose pliers were then used to mechanically fold the LDPE mulch inward without contacting the treated side of the mulch to prevent contact of the treated surface with any foreign objects. Samples were then carefully stored in ziplock plastic bags. For all studies, care was taken to prevent contamination between samples and to collect a representative sample from each plot. All samples were immediately frozen upon collection and stored at −10 C until analysis.
Field plot replicate sample integrity was maintained throughout sample collection, preparation, and chemical analysis. For halosulfuron analysis, samples were removed from the freezer and allowed to equilibrate to room temperature prior to being placed into a 250-ml volumetric flask sealed with a rubber stopper (Grey et al. Reference Grey, Vencill, Webster and Culpepper2009). Extractions were initiated with a mixture of 50% methanol with HPLC-grade water (Fisher Scientific International). The extraction volumes were 100 ml. Samples with higher concentrations were diluted to maintain instrumental column and detector load capabilities, and these factors were figured into final quantitative analyses. Samples were placed on a reciprocating shaker for 2 h at 150 rpm, a 1,000-μl sample was then transferred to 1.5-ml microcentrifuge tube (Fisher Scientific International), sealed, and centrifuged at 13,500 rpm for 2 min. Samples were then passed through a 0.45 μM PTFE membrane filter (Fisher Scientific International) that was fitted with a Luer-Lok™ syringe (Fisher Scientific International), and then passed into injection vials (Fisher Scientific International). A Waters Alliance 2690 system (Waters Corporation) was used to analyze the samples as described earlier.
Field Experiments
Bioassay studies were initiated in March of 2013 and 2014 at the Coastal Plain Experiment Station Ponder Farm near Ty Ty, GA. For this site, the soil was Tifton loamy sand (fine-loamy, kaolinitic, thermic Plinthic Kandiudults) with 85% sand, 11 % silt, 4% clay, 0.65% organic matter, and a pH of 6.2 in 2013; and 88% clay, 10 % silt, 2% clay, 0.52% organic matter, and a pH of 6.7 in 2014. Land preparation and plot establishment were identical to the field dissipation experiment setup, except that plot length was increased to 9.1 m. Halosulfuron was applied to the surface of the LDPE mulch at 79 or 158 g ai ha−1 at 28 (2013) or 23 (2014) d prior to transplanting (DPT), or at 79 g ai ha−1 at 17, 9, or 1 (2013) or 14, 7, and 1 (2014) DPT. Nonionic surfactant at 0.25 % v/v was included. Treatments were applied with a CO2-pressurized backpack sprayer calibrated to deliver 140 L ha−1 at 150 kPa.
On the day of transplanting, a single row of holes spaced 60-cm apart were poked through the mulch; each hole had a soil depth of 5 cm. Watermelon (‘Fascination’) or yellow squash (‘Gentry’) with 1 true leaf was hand transplanted into respective experiments on April 25, 2013, and April 11, 2014. Experiments were established as randomized complete block design with three and four replications in 2013 and 2014, respectively. Visual crop injury estimates were taken weekly after transplanting using a scale of 0=no crop injury to 100=crop death. Watermelon and yellow squash plant diameters on 10 consecutive plants plot−1 were measured multiple times during the season. At harvest, U.S. No. 1 and U.S. No. 2 marketable yellow squash fruit (U.S. Department of Agriculture 2015) were recorded 8 times over 3 wk for 2013 and 2014. Watermelons were harvested once in 2013, while whole-plant bioassays that included excavated plant roots and vines were taken twice in 2014.
Average rainfall, solar thermal radiation, and daily maximum and minimum temperature data were collected onsite at a University of Georgia Weather Monitoring Network station. This station was located within 1 km of all experiments (Flitcroft Reference Flitcroft2015).
Data Analysis
ANOVA was applied to the data combined across herbicide and timing of experiment to test for interactions. For the LDPE mulch halosulfuron dissipation field experiments, regression analysis was performed using SAS nonlinear regression (SAS Institute 2012). The intent was to determine whether the response could be described by using the exponential decay equation
where y is herbicide concentration, B 0 is the initial concentration, B 1 is dissipation rate, and x is time in days after treatment or cumulative solar energy (MJ m−2). After data were regressed against time in DAT or megajoules per square meter by day, the output from the analysis included the first-order dissipation-rate constants (k) (Ohmes et al. Reference Ohmes, Mueller and Hayes2000). Data for the exponential decay equations were subjected to ANOVA using the general linear models procedures with mean separation using 95% asymptotic confidence intervals. Dissipation time (DT50) was then determined using the equation (Dermiyati and Yamamoto Reference Dermiyati and Yamamoto1997b; Liu et al. Reference Liu, Clay and Clay2002; Mueller et al. Reference Mueller, Shaw and Witt1999):
Data were then graphed in Sigmaplot 13 (Systat Software, San Jose, CA) (Figure 1).
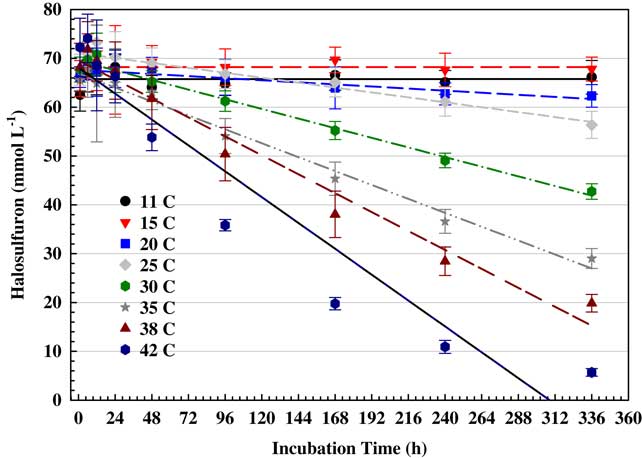
Figure 1 Time course for hydrolysis of halosulfuron in high-performance liquid chromatography water (pH 7) at different temperatures evaluated on a thermal gradient. Error bars represent standard deviations of four replicates per experiment, with experiments conducted twice and combined for presentation.
Differences in halosulfuron stability between incubation temperature regimes were evaluated using regression models that fit halosulfuron concentration (µmol L−1) to incubation time (h) for each temperature regime to establish the first-order rate constant (k). The slope of the line for a given temperature represents the rate of change in halosulfuron concentration. To assess whether slopes differed significantly among incubation temperatures, analysis of covariance was used. Specifically, first-order rate constant (k) for halosulfuron concentration was the response variable, temperature was the independent variable, and time was the covariate. Slope differences were assessed by evaluating the F-test of the interaction term (temperature by time), where rate constants (k) were considered significantly different when P≤0.05. Analyses were conducted using JMP Pro 12 (SAS Institute, Cary, NC). Data were graphed in Sigmaplot 13 (Figure 1).
To accurately characterize temperature effect on halosulfuron stability on the thermal gradient table, the Arrhenius equation, expressed as natural logarithm, was used:
where k is the first-order rate constant, A is the pre-exponential factor, E a is the activation energy expressed (J mol−1), R is the universal gas constant (equal to 8.31 kJ−1 mol−1), and T is absolute temperature expressed in kelvins (Dinelli et al Reference Dinelli, Vicari, Bonetti and Catizone1997). Halosulfuron did not degrade at 11 and 15 C, so only k data for the higher temperatures were used to calculate the activation energy. Data were analyzed and graphed in Sigmaplot 13 (Figure 2).
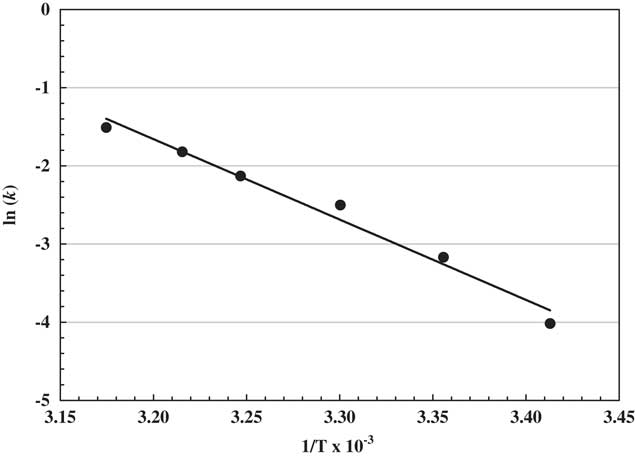
Figure 2 Arrhenius diagram for halosulfuron in high-performance liquid chromatography water (pH 7). The line slope indicates activation energy of 85.55 kJ mol−1. The Arrhenius equation was ln k=−10.29(1/T)+31.26, R2=0.975.
Watermelon and yellow squash data were subjected to arcsine square-root transformations. Interpretations were not different from nontransformed data; therefore, nontransformed data are presented. Data for injury, plant diameter, and marketable fruit yields were subjected to ANOVA using the PROC GLIMMIX in SAS 9.2 (SAS Institute 2012). As data were different for the biological measures for each experiment, data were analyzed separately by year. Means were separated using the appropriate Fisher’s protected LSD test at P≤0.05.
Results and Discussion
Halosulfuron Dissipation and Degradation
ANOVA indicated there were no herbicide by experiment interactions for either the field dissipation studies or the stability laboratory studies. Therefore, data within each experiment were combined for presentation.
Halosulfuron Stability in Aqueous Solution on Thermal Gradient Table
Hydrolysis of halosulfuron followed first-order kinetics for each temperature (Table 2), with a stepwise progression of degradation with increasing temperature (Figure 1). Half-lives could not be determined at 11 and 15 C, indicating that halosulfuron was stable at lower temperatures. Half-lives were 38.5 d and fewer when temperatures exceeded 20 C. Zheng et al. (Reference Zheng, Yates and Papiernik2008) reported similar results for halosulfuron, with <30% dissipation after 2.5 d at 15 C and nondetectable after 1.5 d at 45 C. They noted that in acid and neutral aqueous solutions with pH≤7, a cleavage occurs for the halosulfuron sulfonylurea bridge, resulting in carbon dioxide and other inactive metabolites. Sulfosulfuron degradation has been reported to rapidly degrade at high temperatures with 50% reduction within 6 d at 50 C (Saha and Kulshrestha Reference Saha and Kulshrestha2002).
Table 2 Degradation constants (k±standard error) and half-lives (t 1/2) in days for halosulfuron in aqueous solutions (pH 7) at different temperatures when evaluated on a thermal gradient table.Footnote a

a Each value is the average of four replicates per experiment, with experiments conducted twice and combined for presentation. Values for each rate constants within a column followed by the same letter are not significantly different at P<0.001 probability level.
b Degradation rate constants were calculated by linear regression of the herbicide quantity with respect to time (0 to 336 h).
c CND, cannot determined.
d Arrhenius equation ln k=−10.29(1/T) + 31.26, r2=0.975, T=absolute temperature (in kelvins). P<0.0001. Only k from 20, 25, 30, 35, 38, and 42 C were used to model the activation energy.
The magnitude of the activation energy according to the Arrhenius equation corresponds to the effect of temperature on hydrolysis rate constants for sulfonylurea herbicides (Dinelli et al. Reference Dinelli, Vicari, Bonetti and Catizone1997; Zheng et al. Reference Zheng, Yates and Papiernik2008). Data indicated the activation energy was 85.55 kJ mol−1 for temperatures of 20 C and greater (Table 2) when comparing the degradation constant to the inverse effect of temperature (Figure 2). Zheng et al. (Reference Zheng, Yates and Papiernik2008) reported similar activation energies of 80.7 and 84.4 kJ mol−1 for halosulfuron at pH solutions of 3 and 9, respectively, for temperatures ranging from 15 to 45 C. Dinelli et al. (Reference Dinelli, Vicari, Bonetti and Catizone1997) noted the hydrolytic degradation of four sulfonylurea herbicides, indicating that prosulfuron and rimsulfuron were similar to halosulfuron with 86.08 and 92.28 kJ mol−1, respectively, but primisulfuron (58.02 kJ mol−1) and thifensulfuron (34.44 kJ mol−1) were much lower. Activation energy relates to dissipation, in that energy was required to dissipate halosulfuron and was comparable to other sulfonylurea herbicides. The greater the activation energy, the longer the half-life, as indicated with prosulfuron (86.08 kJ mol−1) at 2.64 d compared with primisulfuron (58.02 kJ mol−1) at 1.06 d at 25 C (Dinelli et al. Reference Dinelli, Vicari, Bonetti and Catizone1997).
Halosulfuron Dissipation from LDPE
Differences in radiation and temperature occurred over the course of each experiment (Table 1). Cumulative rainfall of 5.9 and 3.8 cm was measured for the first and second run of the experiment, respectively. However, harvested mulch was protected by the previously described metal covers.
Halosulfuron at 53 and 26 g ha−1 are equivalent to 5.3 and 2.6 mg m−2 applied to LDPE mulch (Figure 3A and B). The exponential decay equation (Equation 1) accurately described dissipation of halosulfuron at 53 and 26 g ha−1 with first-order rate constants (k) of 0.249 and 0.270 and half-lives (DT50) of 2.8 and 2.6 d, respectively (Table 3). With regard to cumulative energy from solar radiation, k was 0.0125 and 0.0136 with half-lives of 55 and 51 MJ m−2 for halosulfuron at 53 and 26 g ha−1, respectively (Table 3). Pesticide dissipation is multifaceted and, depending on abiotic processes affecting it, quantifying dissipation in terms of time after application in days may not provide an accurate model when halosulfuron is used in LDPE mulch vegetable production. Cumulative energy may be a more logical method of estimating the dissipation of halosulfuron half-life (Figure 3B) from mulch instead of time in days (Figure 3A), as it can be quantified over time from weather station data. The reason is that dissipation of halosulfuron was rapid, with greater than 80% diminished by 7 DAT. However, a more accurate halosulfuron dissipation measure could be achieved when megajoules per square meter is applied to Equations 1 and 2. The predicted k was 0.0125 and 0.0136 mg m−2 with DT50 of 55 and 51 MJ m−2 for halosulfuron applied at 53 and 26 g ha−1 (Table 2), respectively, when solar radiation data from the local weather station (Flitcroft Reference Flitcroft2015) were used instead of DAT. Ham et al. (Reference Ham, Kluitenberg and Lamont1993) noted that temperatures within the top 5 cm of black LDPE mulch surfaces were 3 to 5 C greater than ambient air temperature at 1.5 m heights during daylight hours. They also reported that temperatures remained greater overnight for the air above black LDPE versus bare soil. This indicates the heat-sink properties of LDPE mulches and has been reported on by other researchers (Chase et al. Reference Chase, Sinclair, Chellemi, Olson, Gilreath and Locascio1999; Standifer et al. Reference Standifer, Wilson and Porche-Sorbet1984). Utilizing solar energy as a means of estimating herbicide dissipation has been previously used for paraquat on LDPE mulch (Gilreath et al. Reference Gilreath, Santos and Duranceau2006) and sethoxydim in water (Sevilla-Morán et al. Reference Sevilla-Morán, López-Goti, Alonso-Prados and Sandin-Espańa2014). Halosulfuron is a weak acid with negligible photodegradation losses (Shaner Reference Shaner2014), similar to other SU herbicides that exhibit little to no photodegradation, including pyrazosulfuron ethyl (Zheng et al. Reference Zheng, Yates and Papiernik2008) and imazosulfuron (Morrica et al. Reference Morrica, Fidente and Seccia2004). Halosulfuron does dissipate with irrigation or rainfall. In experiments that quantified halosulfuron removal from LDPE mulch that was either washed daily or left dry for 7 d, differences in dissipation were evident (Grey et al. Reference Grey, Vencill, Webster and Culpepper2009). Half-lives (DT50) for halosulfuron were 18 h for dry versus 3 h for wash-off experiments.

Figure 3 Halosulfuron dissipation under dry conditions by rate for low-density polyethylene mulch in Georgia using the exponential decay equation. Nonlinear regression was applied for days after treatment (A) and for cumulative solar radiation (B). The lines represent the first-order regression equation. Data points are the means of replications with bars indicating the standard error of the mean:
Days after treatment
 $$\eqalignno{&\matrix{ {{\rm \quad Halosulfuron}\,53\,{\rm g}\,{\rm ha}^{{{\minus}1}} \,\colon\,} \hfill & {\gamma {\equals}3.03e^{{\left( {{\minus}0.249x} \right)}} } \hfill & {{\rm P}\,\lt\,0.0001} \hfill \cr & {{\rm R}^{2} {\equals}0.901} \hfill \cr {{\rm \quad Halosulfuron}\,26\,{\rm g}\,{\rm ha}^{{{\minus}1}} \,\colon\,} \hfill & {\gamma {\equals}1.27e^{{\left( {{\minus}0.27x} \right)}} } \hfill & {{\rm P}\,\lt\,0.0001} \hfill \cr & {{\rm R}^{2} {\equals}0.944} \hfill \cr } $$
$$\eqalignno{&\matrix{ {{\rm \quad Halosulfuron}\,53\,{\rm g}\,{\rm ha}^{{{\minus}1}} \,\colon\,} \hfill & {\gamma {\equals}3.03e^{{\left( {{\minus}0.249x} \right)}} } \hfill & {{\rm P}\,\lt\,0.0001} \hfill \cr & {{\rm R}^{2} {\equals}0.901} \hfill \cr {{\rm \quad Halosulfuron}\,26\,{\rm g}\,{\rm ha}^{{{\minus}1}} \,\colon\,} \hfill & {\gamma {\equals}1.27e^{{\left( {{\minus}0.27x} \right)}} } \hfill & {{\rm P}\,\lt\,0.0001} \hfill \cr & {{\rm R}^{2} {\equals}0.944} \hfill \cr } $$
Cumulative solar radiation
 $$\matrix{ {{\rm \quad Halosulfuron}\,53\,{\rm g}\,{\rm ha}^{{{\minus}1}} \,\colon\,} \hfill & {\gamma {\equals}3.10e^{{\left( {{\minus}0.0125x} \right)}} } \hfill & {{\rm P}\,\lt\,0.0001} \hfill \cr & {{\rm R}^{2} {\equals}0.920} \hfill \cr {{\rm \quad Halosulfuron}\,26\,{\rm g}\,{\rm ha}^{{{\minus}1}} \,\colon\,} \hfill & {\gamma {\equals}1.19e^{{\left( {{\minus}0.0136x} \right)}} } \hfill & {{\rm P}\,\lt\,0.0001} \hfill \cr & {{\rm R}^{2} {\equals}0.934} \hfill \cr } $$
$$\matrix{ {{\rm \quad Halosulfuron}\,53\,{\rm g}\,{\rm ha}^{{{\minus}1}} \,\colon\,} \hfill & {\gamma {\equals}3.10e^{{\left( {{\minus}0.0125x} \right)}} } \hfill & {{\rm P}\,\lt\,0.0001} \hfill \cr & {{\rm R}^{2} {\equals}0.920} \hfill \cr {{\rm \quad Halosulfuron}\,26\,{\rm g}\,{\rm ha}^{{{\minus}1}} \,\colon\,} \hfill & {\gamma {\equals}1.19e^{{\left( {{\minus}0.0136x} \right)}} } \hfill & {{\rm P}\,\lt\,0.0001} \hfill \cr & {{\rm R}^{2} {\equals}0.934} \hfill \cr } $$
Table 3 First-order dissipation rate constants (k) and half-lives (DT50) of halosulfuron from low-density polyethylene mulch for dry scenarios from experiments conducted in August and September of 2013 by time and cumulative solar radiation.

a First-order dissipation rate constants were calculated by nonlinear regression of the herbicide quantity with respect to time (1 h to 35 d) and solar radiation (MJ m−2). Values for each rate for first-order rate constants within a column followed by the same letter are not significantly different at the P<0.05 probability level. General linear model procedures were used with mean separation using 95% asymptotic confidence intervals.
Bioassay Experiments
There was variation in weather conditions for rainfall and solar radiation between years (Table 4), resulting in differences in bioassay data. Therefore, data for yellow squash and watermelon are presented by year. Analysis of data indicated no difference between rates of halosulfuron at 79 and 158 g ha−1 applied at 28 or 23 DPT in 2013 and 23 or 14 DPT in 2014, respectively (Tables 5 to 7). Data for these two halosulfuron applications were combined for presentation by DPT for their respective years.
Table 4 Environmental data for halosulfuron dissipation from low-density polyethylene mulch bioassay at Ty Ty, GA.

a DPT, days prior to transplanting.
b Average daily maximum and minimum after application and prior to transplanting.
c Total rainfall after application and prior to transplanting.
d Sum of total solar radiation after that treatment and prior to transplanting.
Table 5 Yellow squash response to halosulfuron applied over mulch prior to transplanting in Ty Ty, GA, in 2013.

a Abbreviations: DAT, days after transplanting; DPT, days prior to transplanting.
b Means followed by same letter in a column do not differ significantly (P≤0.05).
c Halosulfuron was applied at 79 g ha−1 or 158 g ha−1; however, ANOVA indicated there was no significant difference between these two treatments for 28 DPT, and they are therefore combined for presentation.
d Halosulfuron applied at 79 g ha−1.
*Significant at P=0.05 to 0.01.
**Significant at P=0.01 to 0.001
***Significant at P<0.001.
Table 6 Yellow squash response to halosulfuron applied over mulch prior to transplanting in Ty Ty, GA, in 2014.

a Abbreviations: DAT, days after transplanting; DPT, days prior to transplanting.
b Means followed by same letter in a column do not differ significantly (P≤0.05).
c Halosulfuron was applied at 79 g ha−1 or 158 g ha−1; however, ANOVA indicated there was no significant difference between these two treatments for 28 DPT, and they are therefore combined for presentation.
d Halosulfuron applied at 79 g ha−1.
Table 7 Watermelon response to halosulfuron applied over mulch prior to transplanting in Ty Ty, GA, in 2013 and 2014.

a Abbreviations: DAT, days after transplanting; DPT, days prior to transplanting.
b Means followed by same letter in a column do not differ significantly (P≤0.05).
c Halosulfuron was applied at 79 g ha−1 or 156 g ha−1; however, ANOVA indicated there was no significant difference between these two treatments for 28 DPT, and they are therefore combined for presentation.
d Halosulfuron applied at 79 g ha−1.
Yellow Squash Results
Yellow squash visual injury was 22% and 16% for halosulfuron at 79 g ha−1 applied at 1 and 9 DPT in 2013, respectively (unpublished data). In 2014 visual injury was <16% and was observed only for the 1 DPT treatment (unpublished data). Yellow squash plant diameters were significantly reduced by halosulfuron applied 9 and 1 DPT compared with the nontreated control for the 13 and 20 DAT measures in 2013 (Table 5). This same trend was observed in 2014 for the 7 and 1 DPT plant diameters at 25 DAT (Table 6). Rainfall for 2013 after halosulfuron application was 8.4, 6.2, and 2.7 cm for the 28, 17, and 9 DPT. In 2014 rainfall measured 13.5, 13.5, and 9.6 cm for the 23, 14, and 7 DPT. Grey et al. (Reference Grey, Vencill, Webster and Culpepper2009) reported halosulfuron half-life of 0.75 d when halosulfuron-treated LDPE mulch remained under dry conditions, and 0.13 d after irrigation or rain-off events. The bioassay data in this experiment for yellow squash indicate that a combination of rainfall plus heat from solar radiation would effectively dissipate halosulfuron from the mulch.
Across the first four harvests, yellow squash biomass yield and fruit per hectare were reduced in 2013 and 2014 for the 9 and 1 DPT, and 1 DPT treatments, respectively (Tables 5 and 6). This indicates that halosulfuron residues significantly injured yellow squash as described by plant diameter reductions (Tables 5 and 6), leading to yield loss. Overall, there was little effect on the size of yellow squash fruit for timing of halosulfuron applications for the first four or eight total harvests. Culpepper et al. (Reference Culpepper, Grey and Webster2009) noted that when halosulfuron at 51 g ha−1 was applied to LDPE mulch 1 d prior to transplanting without the mulch being washed with rainfall or irrigation, yellow squash injury was 75% at 3 wk. When irrigation occurred after halosulfuron application but prior to transplanting, injury was 46%. Growers would be advised to only apply halosulfuron to the surface of LDPE mulch if 14 d and 9.6 cm or 17 d and 2.7 cm of rainfall occur.
Watermelon Results
Watermelon visual injury was 10% or less when applied at 1 or 9 DPT in 2014 and 1 DPT in 2014 (unpublished data). Watermelon is more tolerant to halosulfuron than yellow squash (Anonymous 2016). Watermelon vine length reductions were reflective of the early-season visual injury; overall growth was reduced by the 1 DPT treatment in 2013 and 2014 when evaluated at 20 and 32 DAT, respectively (Table 7). Overall, watermelon grew rapidly, and halosulfuron injury was transient. Yield in 2013 was affected only by the 1 DPT application, but not significantly. However, plant biomass was significantly reduced for the 1 DPT treatment at 28 DAT in 2014 (Table 7). These data indicate that watermelon has more tolerance to halosulfuron than yellow squash, and growers could apply halosulfuron 7 DPT as long as 9.6 cm of rainfall occurs or 9 DPT if 2.7 cm of rainfall occurs. As previously described by Dittmar et al. (Reference Dittmar, Monks, Schultheis and Jennings2008), watermelon recovered from injury and had no yield differences compared with nontreated controls when halosulfuron was POST and directed to the lower stem and could be used for weed control given this level of tolerance. These same researchers noted that watermelon was not tolerant to POST over the top applications of halosulfuron.
Relating the halosulfuron stability laboratory experiments to the field dissipation studies indicates that as temperatures increased in both studies, halosulfuron dissipation was rapid with input of thermal energy. These analytical data indicate the importance of quantifying halosulfuron for comparison to bioassay research. For the bioassay research, data indicated the greatest potential for halosulfuron dissipation occurs as a result of hydrolysis from rainfall and irrigation (Dinelli et al. Reference Dinelli, Vicari, Bonetti and Catizone1997; Sarmah and Sabadie Reference Sarmah and Sabadie2002; Zheng et al. Reference Zheng, Yates and Papiernik2008). However, even under dry conditions, halosulfuron degrades due to thermal energy from sunlight (Grey et al. Reference Grey, Vencill, Webster and Culpepper2009).
Acknowledgments
We acknowledge the technical contributions of Jenna Smith, Sidney Cromer, Ashley Williams, Jody Thompson, and Hayden Davis.












