Introduction
African mustard (Brassica tournefortii Gouan) is a winter annual broadleaf weed in the eastern region of Australia. It is deemed to be an emerging problematic weed in this part of Australia, as it is a prolific seed producer (Mahajan et al. Reference Mahajan, Singh and Chauhan2020). In Australia, weeds of the Brassicaceae family are rapidly emerging under conservation agricultural systems, as they can adapt to varying environmental conditions and ongoing crop management practices (Chauhan et al. Reference Chauhan, Gill and Preston2006; Osten et al. Reference Osten, Walker, Storrie, Widderick, Moylan, Robinson and Galea2007; Whish et al. Reference Whish, Sindel, Jessop and Felton2002). Brassica tournefortii costs the Australian grain industry A$10.6 million annually and is ranked at the sixth position in regard to revenue loss, owing to reduced crop yield (Llewellyn et al. Reference Llewellyn, Ronning, Ouzman, Walker, Mayfield and Clarke2016). The life cycle of B. tournefortii is concurrent with important cereals and brassica crops that are cultivated in the eastern grain region of Australia, and it matures along with these crops (Mahajan et al. Reference Mahajan, Mutti, Jha, Walsh and Chauhan2018b, Reference Mahajan, Singh and Chauhan2020). This may result in significant crop contamination of grains and can impact the oil quality of canola (Brassica napus L.) due to the similar appearance and size of B. tournefortii and canola seeds (AOF 2015; CABI 2019). Although a winter-dominant weed, it can also grow in summer with sufficient soil moisture and mild temperatures (GRDC 2019; Mahajan et al. Reference Mahajan, Singh and Chauhan2020).
Seed germination is a critical event that is influenced by environmental factors such as light, temperature, pH, burial depth, and soil moisture (Chachalis and Reddy Reference Chachalis and Reddy2000; Koger et al. Reference Koger, Reddy and Poston2004; Taylorson Reference Taylorson1987). The sensitivity of a weed species to light and dark conditions determines the season of germination and growth. In the wake of climate change, the distribution range of weed species may change by adaptation of weeds to agronomic practices (Mahajan et al. Reference Mahajan, Singh and Chauhan2012). Therefore, it is essential to understand seed germination ecology, as it helps to explain plant evolution and ecological adaption of weeds (Baskin et al. Reference Baskin, Milberg, Andersson and Baskin2004). Our understanding of the seed biology of B. tournefortii has improved recently; however, much is still unknown about the germination ecology of this weed, particularly for populations growing in different habitats (Mahajan et al. Reference Mahajan, Singh and Chauhan2020). Germination ecology of different populations of B. tournefortii may change in response to the maternal environment in which these populations are grown (Mahajan et al. Reference Mahajan, Mutti, Jha, Walsh and Chauhan2018b). To develop weed control programs for B. tournefortii, it would be useful to have a detailed understanding of the seed germination biology of different populations of B. tournefortii in response to varying environmental conditions and management practices.
From an ecological perspective, it is important to evaluate the impact of environmental factors on germination and the emergence of B. tournefortii; however, knowledge of its seed ecology for different populations in relation to the maternal environment is limited. It has been reported that management practices imposed by growers (crop vs. non-crop) can cause a major change in dormancy and population behavior of rigput brome (Bromus diandrus Roth) populations (Kleemann and Gill Reference Kleemann and Gill2013). In South Australia, germination of B. tournefortii was higher when seeds were incubated at day/night temperatures of 20/12 C under alternating light and dark conditions (Chauhan et al. Reference Chauhan, Gill and Preston2006). However, a recent study by Mahajan et al. (Reference Mahajan, Mutti, Jha, Walsh and Chauhan2018b) revealed that populations collected from eastern Australia had higher germination when seeds were incubated under dark conditions at 25/15 C compared with 15/5 and 20/10 C. These results suggest that populations may differ in their germination behavior according to the environments or habitats where they were collected.
Soil salinity has been a major issue in Australia, where it has caused a significant constraint in cropping systems (Rengasamy Reference Rengasamy2010). In Australia, more than 60% of the 20 million ha of cropping soils are sodic, where 80% of these sodic soils have an alkaline pH (>8.5) (Rengasamy Reference Rengasamy2002, Reference Rengasamy2010). Water stress plays an important role in weed invasion by affecting weed physiology and weed ecology (Mahajan et al. Reference Mahajan, Singh and Chauhan2020). The germination behavior of weed populations owing to these factors may influence the invasive potential and pattern of spread of weeds (Chauhan and Johnson Reference Chauhan and Johnson2010; Mahajan et al. Reference Mahajan, Matloob, Walsh and Chauhan2018a).
Seed burial depth plays a crucial role in depth-mediated dormancy, which can be induced in several weeds, especially in small-sized weed seeds (Benvenuti et al. Reference Benvenuti, Macchia and Miele2001). All of these factors of the microenvironment, such as moisture, diurnal temperature fluctuation, and light exposure, may have the potential to impact the germination behavior of different populations of B. tournefortii. In addition, it has been reported that with the use of crop residue as a control measure, the emergence of weeds has been reduced (Mobli and Chauhan Reference Mobli and Chauhan2020; Opeña et al. Reference Opeña, Chauhan and Baltazar2014). Therefore, a better understanding is needed to determine the amount of crop residue needed to affect the emergence of B. tournefortii under zero-tillage systems. Such knowledge can generate suitable cultural management tools for this problematic weed.
In this study, our objective was to determine the germination/emergence behavior of four populations of B. tournefortii under different environmental conditions: temperature, light, salt, osmotic stress, burial depth, and crop residue.
Materials and Methods
Seed Description
A series of laboratory experiments were conducted in 2019 at the weed science laboratory of Queensland Alliance for Agriculture and Food Innovation (QAAFI), Gatton, University of Queensland, Australia. Seeds of four populations of B. tournefortii were collected from the Saint George region of Queensland. Populations A and B were collected in October 2016, and Populations C and D were collected in October 2017. The coordinates for population A (a barley [Hordeum vulgare L.] crop), B (barley fence line), C (a chickpea [Cicer arietinum L.] crop), and D (chickpea fence line) were 28.398°S, 147.532°E; 28.119°S, 149.225°E; 28.140°S, 148.613°E; and 27.358°S, 149.156°E, respectively. After collection, seeds were cleaned, dried in a shade house for 3 d, and then stored in plastic containers at room temperature (25 C) until used for production of seeds in May 2018.
For experimental purposes, fresh seeds of each population were collected from mature plants that were grown for production of seeds in May 2018 at Gatton Research Farm, University of Queensland, Australia. All populations were grown in the same environment. Collected seeds then were stored in a lab at room temperature until being used in different experiments. Seed weights of populations A, B, C, and D were 1.08, 1.12, 1.54, and 1.04 mg seed−1 respectively; determined on the basis of 100-seed weight. During production of seeds for each population, plants of each population were surrounded by a 2-m-high plastic fence of 2 m to restrict cross-pollination.
General Germination Test Protocol
Before the germination test, the seeds were treated with sodium hypochlorite (NaOCl 42 g L−1) solution (bleach) for 5 min to break dormancy and thoroughly rinsed thereafter with tap water (Mahajan et al. Reference Mahajan, Mutti, Jha, Walsh and Chauhan2018b). After rinsing, the seeds were placed on blotter paper and left to dry at room temperature for 30 min. The NaOCl-treated seeds were used in all experiments.
Germination of B. tournefortii was evaluated by evenly distributing 25 seeds from each population on two layers of 85-mm Macherey-Nagel filter paper in 9-cm-diameter petri dishes (one dish per population). A pipette was used to moisten the filter papers with 5 ml of tap water. All petri dishes were sealed in resealable ziplock clear plastic bags to avoid evaporation and then placed in incubators according to the respective treatments.
To evaluate the effect of salt stress, osmotic potential, burial depth, and the amount of crop residue on germination/emergence, an incubator was set at 25/15 C day/night temperature. The incubator was set at a 12-h photoperiod to match the thermoperiod cycle. These conditions were ascertained as the optimal germination conditions based on the initial run of the first experiment evaluating the effect of temperature and light on germination of B. tournefortii. The incubator used fluorescent lamps as a light source with a light intensity of 85 mol m−2 s−1.
To test germination in complete darkness, the dishes were wrapped in three layers of aluminum foil. The aluminum foil was only opened once for a germination count after 21 d. Seed germination percentages were calculated for each replicate based on the observation of aggregated germination after 21 d. Seeds were only considered germinated when their radicle was found to be at least 2-mm long. Seeds that were hard or firm were considered viable, and soft seeds that lacked structural integrity were considered nonviable (Mahajan et al. Reference Mahajan, Matloob, Walsh and Chauhan2018a).
Effect of Temperature and Light on Germination
To determine the favorable temperature and light conditions for B. tournefortii populations A, B, C, and D, the seeds were incubated under a wide range of alternating day/night temperatures (15/5, 25/10, 25/15, 30/20, and 35/25 C) in light/dark and dark conditions. These temperature regimes were chosen to simulate fluctuations in temperatures similar to the eastern grain region of Australia during different seasons.
Effect of Salt and Osmotic Stress on Germination
In relation to the salt-stress experiment, sodium chloride (NaCl) solutions of varying concentrations (0, 25, 50, 100, 200, and 300 mM) were used to test the germination of B. tournefortii. Filter papers in each petri dish were moistened with 5 ml NaCl solution according to the respective treatment. The salt concentration range used in this experiment represents soil salinity levels found in many cropping regions of Australia (Rengasamy Reference Rengasamy2002, Reference Rengasamy2010).
To examine the effects of osmotic stress, water solution with osmotic potentials of −0.1, −0.2, −0.4, −0.8, and −1.6 MPa were prepared by dissolving 94.9, 134.3, 189.9, 268.5, and 379.6 g of polyethylene glycol (PEG) 8000 in 1 L of distilled water, respectively (Michel and Radcliffe Reference Michel and Radcliffe1995). Filter papers in each petri dish were moistened with 5 ml PEG solution according to the respective treatment.
Effect of Wheat Residue Amount on Seedling Emergence
Twenty-five seeds of each population of B. tournefortii were placed on the surface of soil in 10-cm-diameter experimental plastic pots. The soil used in the experiment was collected from the Gatton farm of the University of Queensland and had a clay loam texture with 2.7% total organic matter. Wheat (Triticum aestivum L. ‘Spitfire’) straw (leaves and stem) was finely chopped (2 to 3 cm) and spread evenly on the surface of the soil. Crop residue equivalent to 0, 1,000, 2,000, 4,000, and 6,000 kg ha−1 was spread on the soil surface. All pots were placed in plastic trays and moved to the incubator set at 25/15 C under light/dark conditions, and water was applied daily to keep the soil moist. Emergence was recorded at 1-wk intervals for a period of 4 wk. The criteria for emergence was the appearance of the radicle.
Effect of Seed Burial Depth on Seedling Emergence
To analyze the effect of burial depth on seedling emergence, 25 seeds of each population of B. tournefortii were planted at soil depths of 0, 1, 2, 3, 4, and 8 cm in 10-cm-diameter plastic pots filled with soil. The soil used in this experiment was the same as described for the crop residue experiment. Emergence was recorded at 1-wk intervals for a period of 4 wk.
Statistical Analyses
A complete randomized design with six replications (combined over two runs) was used in all the experiments. Each experiment was repeated after the termination of the first experiment. ANOVA was used to identify any significant treatment and interaction effects (P < 0.05). Data were subjected to ANOVA using the software Elementary Designs Application 1.0 Beta (AgriStudy.com: www.agristudy.com; verified with GEN STAT 16th ed., VSN International, Hemel Hempstead, UK). The data represent the average of two runs, as there was no time by treatment interaction, as determined by ANOVA. Before ANOVA, data were also validated for meeting the assumptions of normality. Where treatments were significant (P < 0.05), means were separated using Fisher’s protected LSD test.
Nonlinear regression analysis was used to determine relationships between germination and salt concentrations or osmotic potential or burial depth. These data were described with a functional three-parameter sigmoid model using SigmaPlot v. 14.0 Notebook (Systat Software, San Jose, CA, USA):
where G is the total germination or emergence (%) at salt concentration or osmotic potential or burial depth x, G max is the maximum germination or emergence (%), x 50 is the NaCl concentration or osmotic potential or burial depth for 50% inhibition of the maximum germination/emergence, and G rate indicates the slope.
Results and Discussion
Effects of Light and Temperature
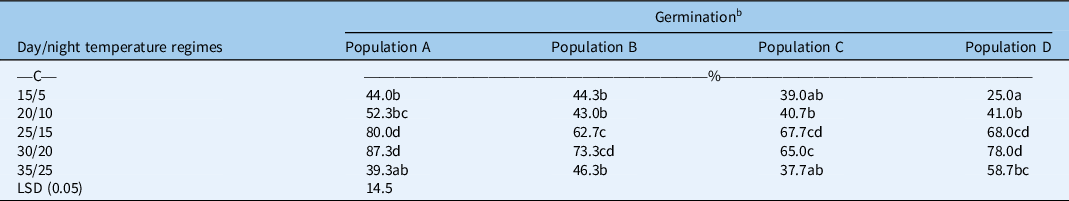
A significant interaction between different temperature regimes and populations was observed for the germination of B. tournefortii (Table 1). Averaged over different light conditions, all tested populations had higher germination at 25/15 and 30/20 C compared with other temperature regimes. At 20/10 C, populations did not differ for seed germination. However, at 25/15 C, population A (collected from a barley field) had higher germination than population B (barley fence line); and at 30/20 C, population A had higher germination than population C (collected from a chickpea field). At the highest temperature regime (35/25 C), population D (collected from a chickpea fence line) had higher germination than population A. A previous study on B. diandrus revealed that management practices imposed by growers (crop vs. non-crop) may influence the dormancy and germination behavior of seeds (Kleemann and Gill Reference Kleemann and Gill2013). Similarly, we also found the populations of B. tournefortii collected from different habitats varied in their germination behavior.
Table 1. Effect of alternating day/night temperatures (15/5 to 35/25 C) on the germination of four populations (A, B, C, and D) of Brassica tournefortii averaged over light/dark (12-h photoperiod) and complete dark (24-h photoperiod). a
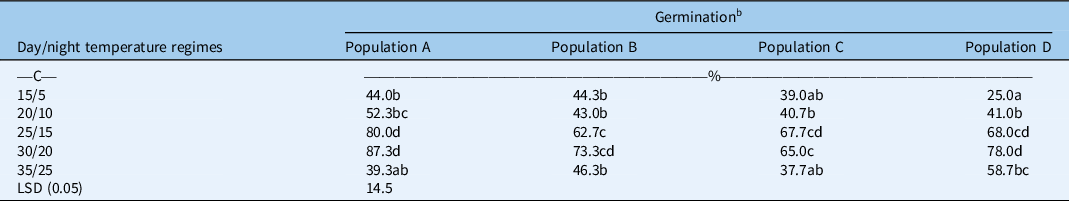
a Seeds were incubated for 21 d.
b Means with the same letter are not significantly different from each other.
Our results further suggest that the populations of B. tournefortii differ for seed germination, especially at relatively higher temperatures. This study also demonstrates that the germination of selected populations was significantly reduced when seeds were exposed to a very high (35/25 C) or low-temperature regime (20/10 or 15/5 C). The previous study conducted in South Australia reported reduced germination of B. tournefortii at low temperature (15/9 C) compared with high temperature (20/12 C) (Chauhan et al. Reference Chauhan, Gill and Preston2006). Likewise, we also found reduced germination of B. tournefortii at 15/5 C; however, in addition to that, our results revealed that populations of B. tournefortii behaved differently for germination at low temperatures. Differential germination responses of Brassicaceae weeds to a range of temperatures have been observed by many researchers (Karimmojeny et al. Reference Karimmojeny, Rezvani, Zaefarian and Nikneshan2014; Kleemann et al. Reference Kleemann, Chauhan and Gill2007; Mahajan et al. Reference Mahajan, Matloob, Walsh and Chauhan2018a). For example, the germination of Oriental mustard (Sisymbrium orientale L.) was reduced at 15/5 C or 20/10 C compared with 25/15 C (Karimmojeny et al. Reference Karimmojeny, Rezvani, Zaefarian and Nikneshan2014). Similarly, the germination of perennial wall rocket [Diplotaxis tenuifolia (L.) DC.] was reduced at lower temperatures (10 to 20 C) compared with high temperature (30 C) (Kleemann et al. Reference Kleemann, Chauhan and Gill2007). In contrast to this, the germination of African turnip weed (Sisymbrium thellungii O. E. Schulz) was not inhibited at 15/5 C (Mahajan et al. Reference Mahajan, Matloob, Walsh and Chauhan2018a).
Brassica tournefortii is a winter weed; therefore, reduced germination is expected at high temperatures. Relatively high germination of B. tournefortii at 25/15 and 30/20 C in the present study suggests that this winter-season weed is more adapted to the spring season in eastern Australia and may increase its invasion in the wake of climate change. It has been suggested that winter-season annual weeds may germinate any time of the year if they get optimum moisture and temperature (Baskin and Baskin Reference Baskin and Baskin1998). Previous studies also suggested that B. tournefortii had optimum germination in the temperature range of 16 to 32 C (Bangle et al. Reference Bangle, Walker and Powell2008; Thanos et al. Reference Thanos, Georghiou, Douma and Marangaki1991). However, these studies each examined just one population. Our study suggests that populations of B. tournefortii differed for seed germination under a range of temperatures, and the differences in germination could be due to genetic variants or adaptive traits among populations collected from various geographic or agroecological conditions. Gene erosion among populations is a slow process; therefore, adaptive traits due to the maternal environment cannot be removed by growing these populations under the same environment in one season (Van de Wouw et al. Reference Van de Wouw, Kik, Van Hintum, Van Treuren and Visser2010).
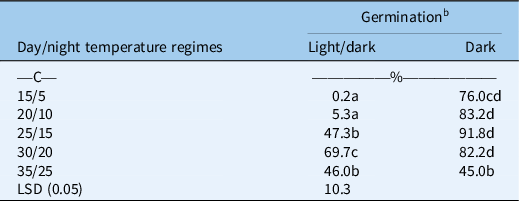
A significant interaction for different light conditions and temperature regimes was also observed for the germination of B. tournefortii (Table 2). Averaged over populations, B. tournefortii had higher germination in the dark compared with alternating light/dark conditions at different temperature regimes, except at 35/25 C. In alternating light/dark conditions, germination of B. tournefortii was highest at 30/20 C and lowest at 15/5 C. However, in dark conditions, the highest germination was observed at 25/15 C, which was similar to that at temperature regimes of 20/10 and 30/20 C. At 35/25 C, light conditions did not influence the germination of B. tournefortii. Previous studies on closely related species revealed that in canola, seed germination is stimulated by light. However, in wild radish (Raphanus raphanistrum L.), seed germination is inhibited by light (Cheam and Code Reference Cheam and Code1995; Pekrun et al. Reference Pekrun, Lutman and Baeumer1997). These results suggest that B. tournefortii germination had a distinct preference for dark conditions, particularly within a certain range of temperatures (15/5 to 30/20 C).
Table 2. Effect of alternating day/night temperatures (15/5 to 35/25 C) on the germination of Brassica tournefortii under light/dark (12-h photoperiod) and complete dark (24-h photoperiod) averaged over populations. a
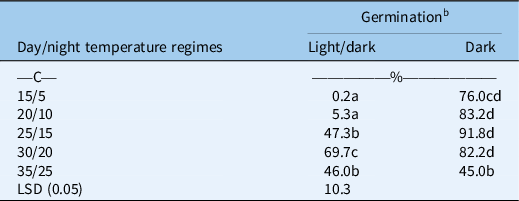
a Seeds were incubated for 21 d.
b Means with the same letter are not significantly different from each other.
Like photoblastic seeds, exposure of seeds to light at a low temperature may convert phytochrome far-red (Pfr) into inactive phytochrome red (Pr) and could inhibit germination of B. tournefortii (Rollin Reference Rollin, Mitrakos and Shropshire1972). These results further suggest that shallow tillage may help in greater germination of B. tournefortii by burying seeds at shallow depths. A study carried out in New South Wales, Australia, revealed that light inhibited the germination of turnip weed [Rapistrum rugosum (L.) All.] at 10 C compared with 15 C (Cousens et al. Reference Cousens, Armas and Baweja1994). Our results also revealed that light inhibits germination of B. tournefortii at low temperatures, but did not influence germination at 35/25 C. However, the previous study of Chauhan et al. (Reference Chauhan, Gill and Preston2006) carried out in South Australia revealed that different light conditions did not influence the germination of B. tournefortii even at 20/12 and 25/15 C.
Overall, our results imply that in eastern Australia, distinct population behaviors under varying temperatures, high germination ability under dark environment, and high dormancy of freshly harvested seeds make B. tournefortii adaptable to diversified environments and may further spread its invasion.
Effect of Salt Stress
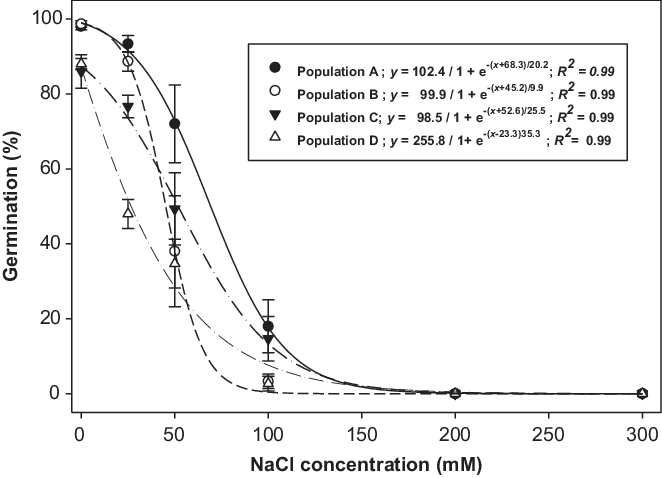
Brassica tournefortii seed germination was influenced by the interaction between population and salt concentration (Figure 1). In the control treatment, germination remained similar for all tested populations. At 50 mM NaCl, germination of populations B, C, and D was lower than for population A. Germination of population D was inhibited even at 25 mM NaCl. Population A had 72% germination at 50 mM NaCl; however, populations B, C, and D had less than 50% germination at 50 mM. Soils are categorized as salt-affected if the total salt concentration in the soil exceeds 20 mM (Abrol et al. Reference Abrol, Yadav and Massoud1988). A sigmoid response was observed in the germination of B. tournefortii seeds at various salt concentrations (Figure 1). NaCl concentration required to inhibit germination of 50% of the populations A, B, C, and D was 68, 45, 53, and 45 mM, respectively. These results suggest that populations behaved differently with varying concentrations of NaCl. Germination of all populations was completely inhibited at 100 mM NaCl. In contrast to this, a study conducted in South Australia revealed that germination of B tournefortii was unaffected at 80 mM NaCl, and it was completely inhibited at 320 mM NaCl (Chauhan et al. Reference Chauhan, Gill and Preston2006). However, high germination of population A at 50 mM NaCl, compared with other populations in this study, suggests that under saline soil conditions of Australia, populations such as A could thrive and pose serious competition to crops.

Figure 1. Effect of sodium chloride (NaCl) concentration on germination of four populations (A, B, C, and D) of Brassica tournefortii at alternating day/night temperatures of 25/15 C under 12-h photoperiod. Seeds were incubated for 21 d. Vertical bar represents the ± standard error of the mean (n = 6).
Effect of Osmotic Stress
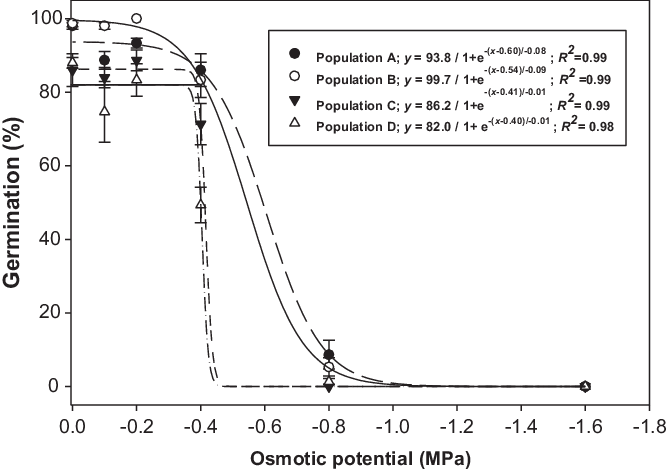
Brassica tournefortii seed germination was influenced by the interaction between population and osmotic potential (Figure 2). The germination of tested populations was not affected by the decrease in osmotic potential from 0 to −0.2 MPa. However, at the osmotic potential of −0.4 MPa, seed germination of populations C and D decreased by 20% and 41%, respectively, when compared with the osmotic potential of −0.2 MPa. Populations A and B had >80% seed germination at −0.4 MPa. None of the populations germinated at −1.6 MPa. Osmotic potentials that could cause a 50% reduction in germination based on the regression models were −0.60, −0.54, −0.41, and −0.40 MPa, for populations A, B, C, and D, respectively (Figure 2). The osmotic potential study suggests that populations of B. tournefortii have adaptability to water-stressed environments. Populations A and B can tolerate high water stress (−0.4 MPa). These results are in agreement with the observations of earlier studies, which reported the prevalence of this weed in dry and desert areas (USDA 2005). In the eastern region of Australia, the surface layer of vertosol soil dries rapidly after rainfall (Dalal et al. Reference Dalal, Weston, Strong, Probert, Lehane and Cooper2004). In such situations, populations such as A and B could thrive and might produce seeds for reinfestation.
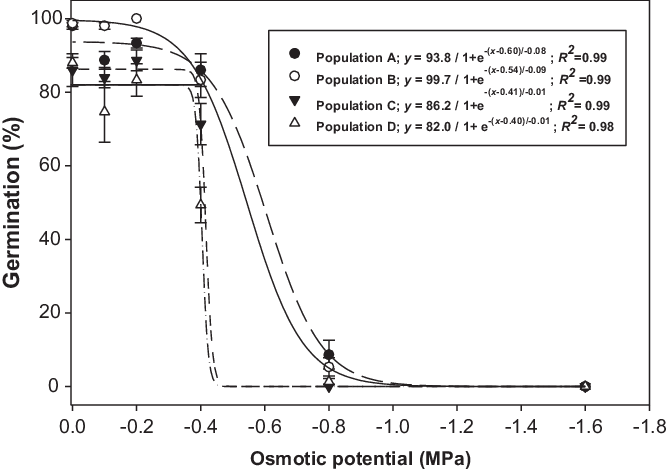
Figure 2. Effect of osmotic potential on germination of four populations (A, B, C, and D) of Brassica tournefortii at alternating day/night temperatures of 25/15 C under 12-h photoperiod. Seeds were incubated for 21 d. Vertical bar represents the ± standard error of the mean (n = 6).
Effect of Wheat Residue
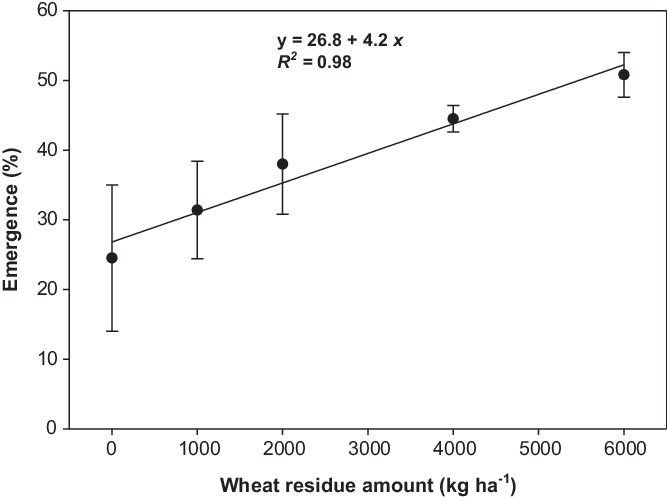
Seedling emergence in response to wheat residue amount was not affected by population. Averaged over populations, emergence was increased by 55%, 82%, and 107% with residue amounts of 2,000, 4,000, and 6,000 kg ha−1, respectively, compared with the no-residue treatment (Figure 3). The addition of crop residues at 6,000 kg ha−1 decreased the emergence of many weeds (Facelli and Pickett Reference Facelli and Pickett1991; Mahajan et al. Reference Mahajan, Matloob, Walsh and Chauhan2018a; Opeña et al. Reference Opeña, Chauhan and Baltazar2014). However, in our study, emergence increased with the addition of wheat crop residue upto 6,000 kg ha−1. Recent study by Mobli and Chauhan (Reference Mobli and Chauhan2020) revealed that sorghum [Sorghum bicolor (L.) Moench ssp. bicolor] residue cover did not increase the emergence of B. tournefortii, and it might be due to the allelopathic potential of sorghum residue. Sorghum residue releases allelochemicals like caffeic acid, m-coumaric acid, and chlorogenic acid, which may have an inhibitory effect on weed emergence (Cheema et al. Reference Cheema, Mushtaq, Farooq, Hussain and Islamud-Din2009). Wheat residues were used in the current study, and these residues may or may not have an allelopathic effect on weeds, depending on the phytochemistry of wheat cultivars (Wu et al. Reference Wu, Pratley, Lemerle and Haig2001).
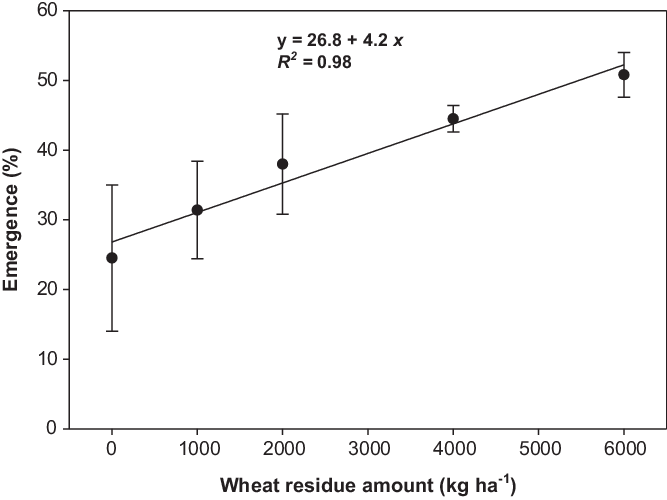
Figure 3. Effect of wheat residue on seedling emergence of Brassica tournefortii after 21 d of incubation at 25/15 C day/night temperature (averaged over populations). The line represents a linear model fit to the data. Vertical bar represents the ± standard error of the mean (n = 6).
Increased emergence with the increase in the residue amount suggests seeds prefer darkness for emergence. The addition of crop residue may have a shading effect on seeds. The light/dark experiment also confirmed higher germination of B. tournefortii in completely dark conditions when compared with light/dark conditions. Increased emergence under high residue cover may also be due to high moisture conservation that supported increased germination and emergence. The position and uniformity of crop residue could also increase the shading, as in the present study, as the loose and chopped residue was spread evenly on the soil surface. However, under field conditions, residue cover is usually not uniform and loose, and these conditions may affect results. Therefore, the results of this study require confirmation under field conditions. Our results suggest that no-till systems with high residue cover in the field could promote greater emergence of B. tournefortii. This implies that no-till and conservation agriculture systems in Australia are likely to favor B. tournefortii invasion.
Effect of Seed Burial Depth on Emergence
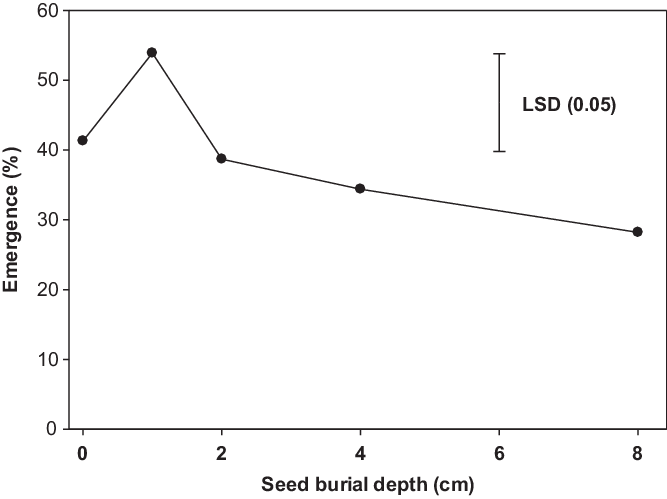
Populations of B. tournefortii did not differ for emergence when sown at different seed burial depths. Averaged over populations, emergence decreased with increased burial depth after 1 cm (Figure 4). Emergence was similar when seeds were sown on the surface and at 1-cm depth (41% to 54%). It was interesting that 28% of seedlings of B. tournefortii emerged from the 8-cm depth. Emergence in pots was lower than seed germination in petri dishes due to the increased contact of seeds with water in the petri dishes. Our results are in contrast with the earlier study by Chauhan et al. (Reference Chauhan, Gill and Preston2006) conducted in South Australia, which revealed no emergence of B. tournefortii from 5-cm or greater soil depths. The results of the burial study revealed that shallow burial could increase the emergence of B. tournefortii. This could be due to better soil moisture–seed contact and preference for darkness. Decreased germination with increased soil depth has been reported for many small-seeded weeds (Mahajan et al. Reference Mahajan, Matloob, Walsh and Chauhan2018a; Manalil et al. Reference Manalil, Ali and Chauhan2018), as carbohydrate reserves do not support the emergence of the seedlings of small-seeded species from greater depths (Teasdale et al. Reference Teasdale, Beste and Potts1991). Our results indicate that B. tournefortii could be a problematic weed in no-till or under shallow-tillage situations. Deep tillage (>8 cm) could reduce its emergence; therefore, inversion tillage and preventing seed production of new plants could be a weed management strategy under high infestation conditions.
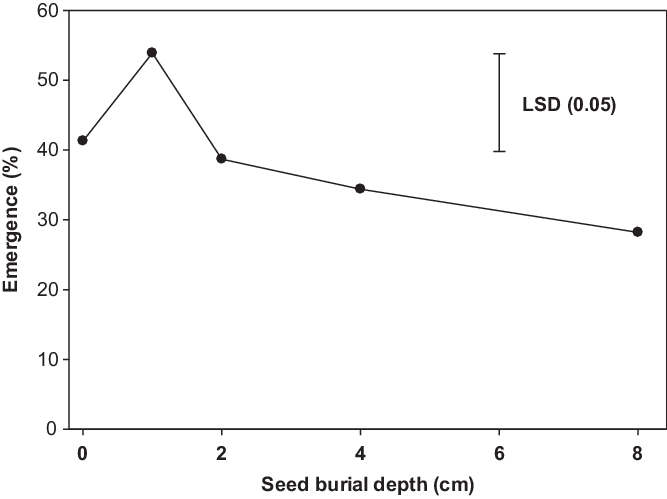
Figure 4. Effect of seed burial depth on seedling emergence of Brassica tournefortii after 21 d of incubation at 25/15 C day/night temperature (averaged over populations). LSD is the least significant difference at a 5% level of significance.
In summary, Brassica tournefortii is a winter weed; however, high germination within the temperature range of 25/15 to 30/20 C under dark conditions suggests that it could also be a problematic weed in the autumn and spring seasons in Queensland and New South Wales, requiring an extra level of weed management after seedling emergence. A second observation was that germination was stimulated under dark conditions; however, dark was not an absolute requirement. Greater germination/emergence of B. tournefortii on the surface layer (0 to 1 cm) suggests that no-till farming systems could favor this weed. Control of this weed in the no-till farming system for 3 yr may deplete its seedbank if new seed production is restricted, as its seed persistence in the soil is short (Mahajan et al. Reference Mahajan, Singh and Chauhan2020). Low emergence of B. tournefortii from greater (>4 cm) soil depths suggests that the practice of inversion tillage may reduce its emergence, and strategic tillage could reduce its seedbank. Retention of crop residues on the surface may promote its germination by increasing moisture conservation and providing a dark environment.
Overall, our results demonstrated that B. tournefortii could germinate over a wide range of environmental conditions. Seed germination of B. tournefortii was affected by osmotic stress, suggesting that retaining crop residue on the soil surface or conservation tillage may stimulate emergence of this weed by conserving soil moisture. As B. tournefortii is tolerant to salt and water stress, it may compete with crops in problematic soils and under water-stress situations. Therefore, chemical control integrated with cultural management (e.g., manipulation of crop sowing time, use of narrow-row spacing, and use of quick-growing crop cultivars) may suppress this weed through early canopy closure, reduce the seed production of this weed, and prevent the fields from further reinfestation. A recent study reported that B. tournefortii seeds decayed fast on the soil surface (Mahajan et al. Reference Mahajan, Singh and Chauhan2020); therefore, the seedbank of B. tournefortii could be depleted in a no-till system if new seed production is prevented through integrated weed management practices. This suggests that a strategy of diligent control of seedlings during a 6- to 12-mo fallow could exhaust the seedbank, resulting in a reduced weed problem in the following crop. Populations of B. tournefortii collected from different habitats differed in germination behavior in response to temperature, salt, and water stress, and this variation in germination response of seeds collected from different habitats suggests that further studies are required to understand the effect of different crop husbandry practices on the evolutionary mechanism of B. tournefortii seeds. Information gathered from this study provides a better understanding of the factors that favor germination and emergence of B. tournefortii, which will aid in developing management strategies in countries like Australia, Africa, and the United States, where it is emerging as a problematic weed in winter crops.
Acknowledgments
The authors would like to thank the Grains Research and Development Corporation (GRDC) for investing in this research. No conflicts of interest have been declared.