Introduction
Water stress is one of the major limiting factors for optimum crop production (Benjamin and Nielsen Reference Benjamin and Nielsen2006; Wu et al. Reference Wu, Qu, Hao and Xiong2013). A plant’s water stress resistance mechanisms, which include drought avoidance, drought tolerance, drought recovery, or drought escape, allow C4 plant species to maintain growth and development more than C3 species under water stress and higher irradiance levels (Lawlor Reference Lawlor2013; McLachlan et al. Reference McLachlan, Swanton, Weise and Tollenaar1993; Steckel et al. Reference Steckel, Sprague, Hager, Simmons and Bollero2003; Stoller and Myers Reference Stoller and Myers1989). Being a C4 species, Palmer amaranth (Amaranthus palmeri S. Watson) can adapt to water stress conditions using drought tolerance as a mechanism (Ehleringer Reference Ehleringer1983).
Amaranthus palmeri is the most troublesome weed species in agronomic crop production systems in the United States (Chahal et al. Reference Chahal, Aulakh, Jugulum and Jhala2015, Reference Chahal, Varanasi, Jugulam and Jhala2017; Kohrt and Sprague Reference Kohrt and Sprague2017), and its interference can cause significant yield losses in crops, including corn (Zea mays L.) and soybean [Glycine max (L.) Merr.] (Klingaman and Oliver Reference Klingaman and Oliver1994; Liphadzi and Dille Reference Liphadzi and Dille2006; Massinga et al. Reference Massinga, Currie, Horak and Boyer2001). Amaranthus palmeri and other C4 species such as spotted mallow (Malvastrum rotundifolium A. Gray) can maintain a high photosynthetic capacity and growth under water stress conditions using osmotic adjustment to increase leaf solute concentration, allowing the stomata to remain open longer during conditions of water stress and maintain CO2 diffusion to chloroplasts (Ehleringer Reference Ehleringer1983, Reference Ehleringer1985; Forseth and Ehleringer Reference Forseth and Ehleringer1982).
Amaranthus palmeri was historically located in the Sonoran Desert and began spreading to the southern and midwestern United States in recent years (Chahal et al. Reference Chahal, Aulakh, Jugulum and Jhala2015; Jhala et al. Reference Jhala, Sandell, Rana, Kruger and Knezevic2014). The spread and dominance of A. palmeri over other weed species could be related to its growth characteristics, ability to survive and reproduce under water stress conditions, or evolution of resistance to distinct site-of-action herbicides. Amaranthus palmeri biotypes have evolved resistance to herbicides that inhibit microtubule function (Group 3), acetolactate synthase (Group 2), photosystem II (Group 5), 5-enol-pyruvylshikimate-3-phosphate synthase (Group 9), 4-hydroxyphenylpyruvate dioxygenase (Group 27), and protoporphyrinogen oxidase (Group 14) in different states in the United States (Heap Reference Heap2017).
Amaranthus palmeri, waterhemp [Amaranthus tuberculatus (Moq.) J. D. Sauer], and redroot pigweed (Amaranthus retroflexus L.) are the most commonly found Amaranthus species in Nebraska agronomic crop fields (Vieira et al. Reference Vieira, Samuelson, Alves, Gaines, Werle and Kruger2018); however, they differ in their growth characteristics (Horak and Loughin Reference Horak and Loughin2000). Amaranthus palmeri has the highest plant dry weight, leaf area, height, growth rate (0.10 to 0.21 cm per growing degree day), and water-use efficiency compared with other pigweed species (Berger et al. Reference Berger, Ferrell, Rowland and Webster2015; Horak and Loughin Reference Horak and Loughin2000; Massinga et al. Reference Massinga, Currie and Trooien2003; Wiese Reference Wiese1968). Amaranthus palmeri roots are finer, longer, and greater in number, allowing it to occupy a much larger soil volume and acquire more soil nutrients compared with most crops, especially during conditions of water stress and low fertility (Wright et al. Reference Wright, Jennette, Coble and Rufty1999a). In addition, A. palmeri is a prolific seed producer, and if left uncontrolled, a single female plant can produce as many as 600,000 seeds (Keeley et al. Reference Keeley, Carter and Thullen1987). With increasing soil water stress, some studies have reported reduced growth and fecundity of different weed species, including A. palmeri (Chauhan Reference Chauhan2013; Chauhan and Johnson Reference Chauhan and Johnson2010; Sarangi et al. Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015; Webster and Grey Reference Webster and Grey2008). In addition, a few studies have reported the growth response of A. palmeri exposed to different durations of levels of water stress for a few weeks and then irrigated back to saturated conditions (Chandi et al. Reference Chandi, Jordan, York, Burton, Milla-Lewis, Spears, Whitaker and Wells2013; Paudel et al. Reference Paudel, Grantz, Vu and Shrestha2016). However, A. palmeri fecundity and seed germination parameters were not studied. Weed seed germination is an important component of weed management strategies and is influenced by environmental factors such as water availability, light, temperature, and photoperiod during seed development (Baskin and Baskin Reference Baskin and Baskin1998; Fenner Reference Fenner1991).
Studies evaluating a plant’s response to water stress are often performed under greenhouse or controlled environmental conditions, with the plants growing in pots to maintain them at a certain water stress level for a limited time or throughout the growing period. The most common approach used to maintain desired water stress level in previous studies has been to weigh the pots to determine water loss from the soil and then add the desired amount of water (Chandi et al. Reference Chandi, Jordan, York, Burton, Milla-Lewis, Spears, Whitaker and Wells2013; Chauhan Reference Chauhan2013; Chauhan and Johnson Reference Chauhan and Johnson2010; Earl Reference Earl2003; Sarangi et al. Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015). Because it is not possible to separately determine the weight of the plant growing in the pot when using this method, plant weight is normally included along with the weight of soil and water, resulting in inaccurate information regarding the actual amount of water present in the pot soil. This could result in an error during rewatering, especially as plants grow larger. Additionally, this method is labor intensive and time-consuming, because all the pots must be weighed at regular intervals until the end of the study. The labor required for weighing and watering the pots can be reduced by using soil moisture sensors such as Decagon 5TE (Decagon Devices, 2365 NE Hopkins Court, Pullman, WA 99163) or Watermark (Irrometer Company, 1425 Palmyrita Avenue, Riverside, CA 92507) sensors. These sensors are very accurate in determining moisture stress in real time. Therefore, moisture sensors would allow researchers to measure soil moisture content more frequently and maintain the water stress level within a narrow, predetermined range (Irmak et al. Reference Irmak, Payero, VanDeWalle, Rees, Zoubek, Martin, Kranz, Eisenhauer and Leininger2016). The Decagon 5TM sensor is a frequency-domain reflectometry sensor that measures soil water content directly as a percent volume and performs well in loam and silt loam soils (Paudel et al. Reference Paudel, Grantz, Vu and Shrestha2016; Zhu Reference Zhu2016). The Watermark granular matrix sensor measures soil matric potential in kilopascals (kPa) or centibars (cb) and converts the matric potential outputs to soil water content through developed soil-specific soil–water retention curves (Saxton and Rawls Reference Saxton and Rawls2006). No information is available regarding the growth and fecundity of A. palmeri exposed to different water stress levels throughout its growing period. The objective of this study was to determine the effect of degree of water stress on growth, fecundity, and seed germination of A. palmeri biotypes from Nebraska using soil moisture sensors.
Materials and Methods
Plant Materials
Amaranthus palmeri seeds were collected from two different production fields located in Thayer County and Fillmore County, NE, and were stored in separate paper bags in a refrigerator at 4 C in the dark for 5 to 6 mo until used in this study. Seeds from both biotypes were planted in germination trays containing potting mix (Berger BM1 All-Purpose Mix, Berger Peat Moss, Saint-Modeste, QC, Canada), and seedlings were later transplanted in 72-cell germination trays and kept under greenhouse conditions. Seedlings of 6 to 8 cm in height were transplanted into round, free-draining black plastic pots (20-cm diameter and 30-cm height) containing 10 kg of finely ground loam texture soil with 1 plant pot−1. Each pot was filled with dry soil, and the soil was lightly tapped from the top using a wooden block to ensure homogeneous soil bulk density within and between the pots. Pots were watered lightly every other day to avoid transplant shock until the initiation of water stress treatments at 7 d after transplanting (DAT). The study was conducted in a greenhouse at the University of Nebraska–Lincoln that was maintained at a 28/24 C day/night temperature, and plants were supplied with 24–8–16 commercial plant fertilizer (Miracle-Gro® Water Soluble All Purpose Plant Food, Scotts Miracle-Gro Products, 14111 Scottslawn Road, Marysville, OH 43041) in irrigation water every 14 d throughout the study period. Overhead metal-halide lamps with 600 mmol photon m−2 s−1 light intensity were used to provide supplemental light in the greenhouse to maintain a 16-h photoperiod. Treatments were arranged in a randomized complete block design with four replications, and the experiment was repeated in time under the same greenhouse conditions as described above.
Soil Water Content
The soil used in this study was collected from a field near Lincoln, NE, with no known history of herbicide usage for the last 10 yr. The soil was a Crete silt loam texture (fine, smectitic, mesic Pachic Udertic Argiustolls) with a pH of 7.7 and particle size distribution of 37% sand, 19% clay, 44% silt, and 1.9% organic matter content. The permanent wilting point and saturation point values of the soil were 12.8% and 44.6% volumetric, respectively. The soil had a bulk density of 1.2 g cm−3 and a field capacity (FC) of 33.5% by volume based on soil test reports, and an FC of 28% by weight calculated using the following equation (Hillel Reference Hillel1998):
where θg is the percent gravimetric soil water content, θv is the percent volumetric soil water content, and ρb is the soil bulk density in grams per cubic centimeter.
The study included five soil water stress treatments: 100%, 75%, 50%, 25%, and 12.5% of the soil FC corresponding to no, light, moderate, high, and severe water stress levels, respectively (Sarangi et al. Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015). Soil water content in the pots was measured using Decagon 5TM and Watermark soil moisture sensors (Figure 1A and B). To convert the Watermark sensor–measured matric potential values to volumetric water content, a retention curve was developed for this specific experimental soil using the Soil-Water Characteristics Software (v. 6.02.74) developed by Saxton and Rawls (Reference Saxton and Rawls2006), which calculates soil hydraulic properties from soil textural properties (Irmak et al. Reference Irmak, Payero, VanDeWalle, Rees, Zoubek, Martin, Kranz, Eisenhauer and Leininger2016; Rawls Reference Rawls1983; Rawls et al. Reference Rawls, Gimenez and Grossman1998; Saxton et al. Reference Saxton, Rawls, Romberger and Papendick1986). The retention curve equation is:
where SWC is the percent volumetric soil water content and SMP is the soil matric potential measured by the Watermark sensor in kilopascals.
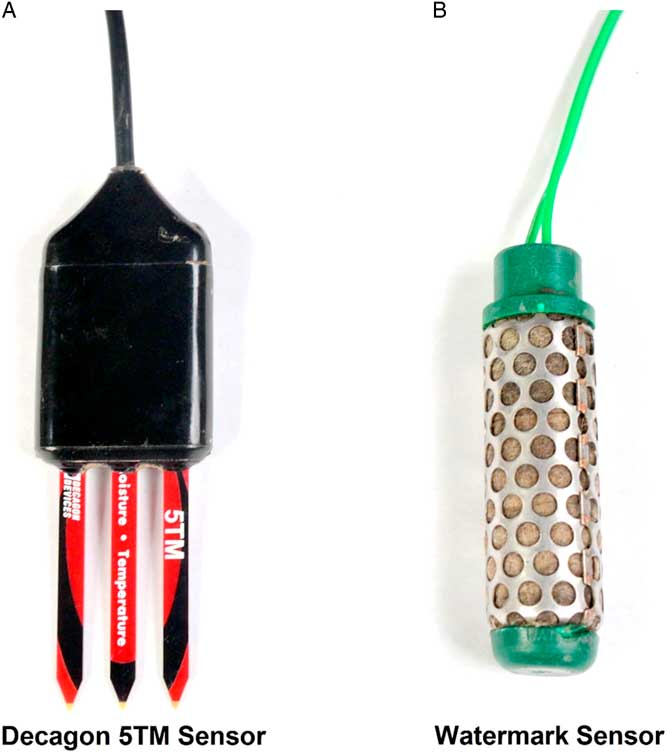
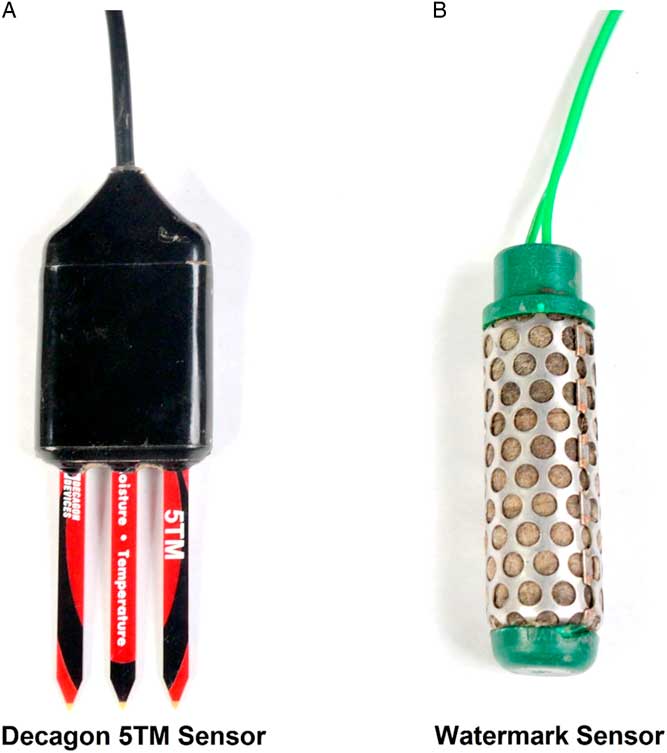
Figure 1 Soil moisture content in pots was measured using (A) Decagon 5TM and (B) Watermark soil moisture sensors to determine degree of water stress on growth and fecundity of Amaranthus palmeri biotypes in a greenhouse study conducted for 77 d after transplanting at the University of Nebraska–Lincoln.
The Watermark sensor operates at a range of 0 to 239 kPa (Irmak and Haman Reference Irmak and Haman2001; Irmak et al. Reference Irmak, Payero, VanDeWalle, Rees, Zoubek, Martin, Kranz, Eisenhauer and Leininger2016), which is an effective range of soil-water status for most agricultural soils and corresponds to saturated to 58% of the volumetric FC conditions in this study. Therefore, this sensor was used to measure soil water content for the 100% and 75% FC treatments, and Decagon 5TM sensors were used for the 50%, 25%, and 12.5% FC treatments. A Watermark sensor was installed vertically 10- to 12-cm deep in each pot for the 100% and 75% FC treatments, and a Decagon 5TM sensor was installed vertically at the same depth in each pot for the 50%, 25%, and 12.5% FC treatments. Because the soil has a gravimetric FC of 28%, 2.8 L of water (28% of 10 kg soil) was added per pot at 7 DAT to maintain 100% gravimetric FC. Similarly, 2.1 (75% of 2.8 L), 1.4 (50% of 2.8), 0.7 (25% of 2.8), and 0.35 (12.5% of 2.8) L of water was added to maintain 75%, 50%, 25%, and 12.5% soil FC, respectively, with a range of ±2% set for all water stress treatments. Soil moisture data from Watermark and Decagon data loggers were recorded twice a day, and the required amount of water was added evenly on top of the soil to maintain soil FC.
A preliminary study was conducted under the same greenhouse conditions described earlier to compare the efficacy of the Decagon 5TM and Watermark sensors in the pots. Both sensors were installed 10- to 12-cm deep in the same pot filled with 10 kg soil with a total of 6 pots and 2.8 L of water added to each pot to maintain 100% soil FC. Soil moisture data were recorded from the data loggers 1 d after watering, and the results suggested that volumetric FC values recorded from Decagon and Watermark loggers corresponded very closely with the volumetric FC of soil provided by the soil test report (unpublished data).
Data Collection
Amaranthus palmeri height, number of leaves per plant, and growth index were determined at 7-d intervals starting from 21 DAT until plants were harvested upon maturity at 77 DAT. Growth index can be defined as a quantitative indicator of plant growth rate used to compare plants grown under different soil water conditions and was calculated using the following equation (Irmak et al. Reference Irmak, Haman, Irmak, Jones, Campbell and Crisman2004; Sarangi et al. Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015):
where w is the width of the plant calculated as an average of two widths, one measured at the widest point and another at 90° to the first; and h is the plant height measured from the soil surface to the last stem node at the top.
Upon maturity, all leaves were removed from each stem to measure the total leaf area for each plant using a leaf area meter (LI-3100C Area Meter, Li-Cor, Lincoln, NE 68504). Seed heads were collected from female plants, and seeds were cleaned with a seed cleaner. Plant stems were cut from the soil surface and roots were removed from the pots; stems and roots were washed with water in a container and air-dried for 24 h. The leaves, shoots, and roots from each plant were stored separately in paper bags and oven-dried at 65 C for 7 d. The aboveground and root biomass were weighed. The number of seeds produced per female plant from each water stress treatment were calculated by dividing total seed weight per plant by an average weight of four samples of 200 seeds plant−1. Seeds were stored in the refrigerator at 4 C in the dark for 4 to 5 mo. To determine the germination percentage of seeds, 200 seeds from each female plant were placed on a piece of moist Whatman No. 4 filter paper (GE Healthcare UK, Amersham Place, Little Chalfont, Buckinghamshire HP7 9NA, UK) in a petri dish. All petri dishes were stored for 21 d in a growth chamber maintained at a 35/28 C day/night temperature with a 16-h photoperiod, and an appropriate amount of water was added every 2 d to keep the filter paper wet. Fluorescent lamps were used to produce a light intensity of 85 mmol m−2 s−1. The total number of seeds germinated was counted and converted to percent germination compared with the number of seeds placed in each petri dish.
Statistical Analysis
Amaranthus palmeri height (cm), number of leaves per plant, growth index (cm3), aboveground and root biomass per plant (g), total leaf area per plant (cm2), and number of seeds per female plant from both A. palmeri biotypes were subjected to ANOVA using the PROC GLIMMIX procedure in SAS v. 9.3 (SAS Institute, Cary, NC 27513). Amaranthus palmeri biotypes, water stress treatments, and experimental runs were considered fixed effects in the model, and blocks were considered random effects. No significant experimental run by treatment or biotype by treatment interaction was observed; therefore, data were combined over two experimental runs and A. palmeri biotypes. Before analysis, data were tested for normality and homogeneity of variance using a Shapiro-Wilk goodness-of-fit test and Levene’s test in SAS. The normality and homogeneity of variance assumptions were met; therefore, no data transformation was required. Where the ANOVA indicated treatment effects were significant, means were separated at P≤0.05 with Tukey-Kramer’s pairwise comparison test to reduce type I error for series of comparisons.
A four-parameter log-logistic function was fit to number of leaves per plant, plant height, and growth index data using the ‘drc’ package c (drc 2.3, Christian Ritz and Jens Strebig; R 3.1.0, Kurt Hornik, online) in R statistical software (R Foundation for Statistical Computing, Vienna, Austria) (Knezevic et al. Reference Knezevic, Streibig and Ritz2007):
where Y is the number of leaves per plant, plant height, or growth index; x is the days after transplanting; c is the estimated minimum number of leaves per plant, plant height, or growth index; d is the estimated maximum leaves per plant, plant height, or growth index; e is the time taken to achieve 50% of leaves per plant, plant height, or growth index; and b represents the relative slope around the parameter e. A t-test was used to determine whether the water stress treatments significantly affected minimum and maximum plant height, number of leaves per plant, or growth index; rate of change of plant height, number of leaves per plant, or growth index with time; and time taken to achieve 50% of maximum plant height, number of leaves per plant, or growth index.
Results and Discussion
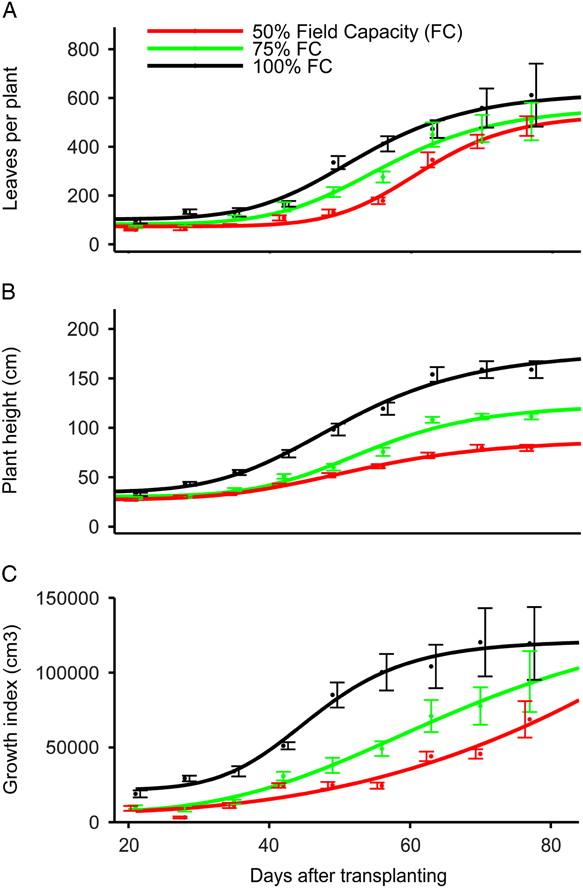
Water stress treatments at 100%, 75%, and 50% FC did not affect the number of leaves produced (534 to 626 leaves plant−1) as estimated by the four-parameter log-logistic model (Table 1; Figure 2A). In contrast, Sarangi et al. (Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015) reported a greater number of A. tuberculatus leaves produced at 100% FC (231 leaves plant−1) compared with 75% FC (185 leaves plant−1) and 50% FC (161 leaves plant−1). Chauhan and Abugho (Reference Chauhan and Abugho2013) reported 134 to 147 leaves plant−1 for spiny amaranth (Amaranthus spinosus L.) at 100% and 75% FC compared with 104 leaves at 50% FC. Amaranthus palmeri plants at 75% and 100% FC took 53 to 56 d to produce 50% of the maximum number of leaves compared with 60 d at 50% FC. Similarly, A. spinosus at 75% and 100% FC took 30 to 32 d to produce 50% of the maximum number of leaves compared with 26 d at 50% FC (Chauhan and Abugho Reference Chauhan and Abugho2013). Amaranthus palmeri maintained at 25% and 12.5% FC did survive only up to 35 DAT, and the four-parameter log-logistic model did not provide a good fit for the number of leaves per plant, plant height, or growth index; therefore, data are not presented.
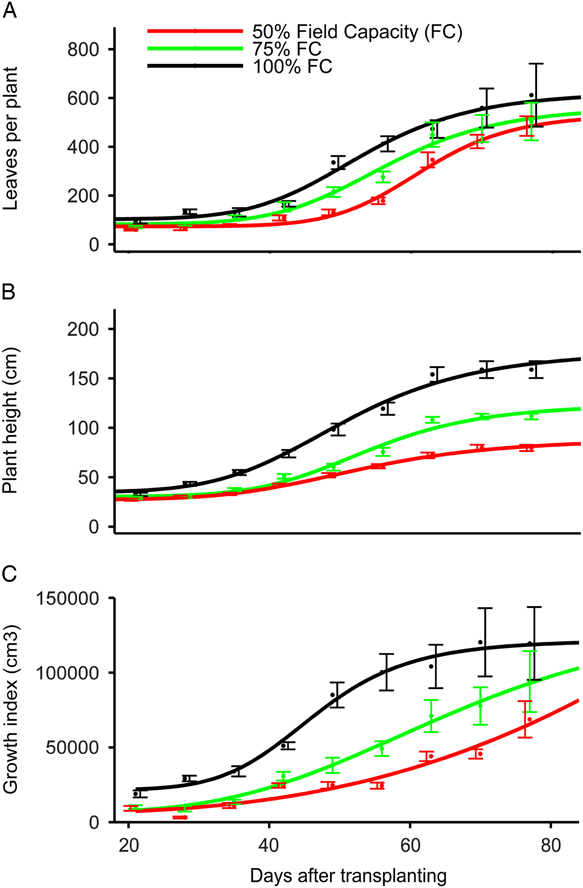
Figure 2 Effect of degree of water stress on (A) leaves per plant, (B) plant height, and (C) growth index of Amaranthus palmeri in a greenhouse study conducted for 77 d after transplanting at the University of Nebraska–Lincoln. The 100%, 75%, 50%, 25%, and 12.5% field capacity (FC) treatments were considered as no, light, moderate, high, and severe water stress level, respectively. Amaranthus palmeri maintained at 25% and 12.5% soil FC did not survive more than 35 d after transplanting, and the four-parameter log-logistic model did not provide a good fit for number of leaves per plant, plant height, or growth index; therefore, curves are not presented for 25% and 12.5% FC.
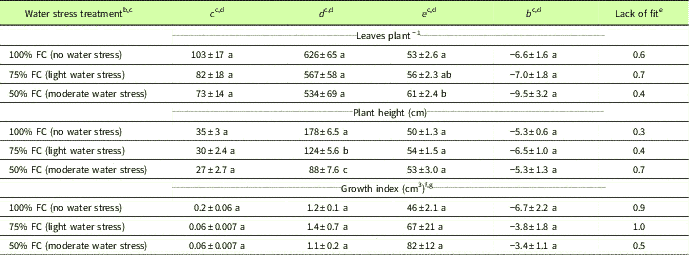
Table 1 Parameter estimates and test of lack of fit at 95% level for the four-parameter log-logistic functionFootnote a fit to Amaranthus palmeri leaves per plant, plant height, and growth index under different degrees of water stress in a greenhouse experiment conducted for 77 d after transplanting at the University of Nebraska–Lincoln.
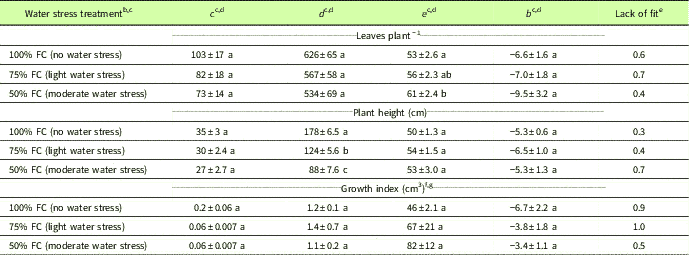
a Y = c + {d – c/1 + exp[b (log x – log e)]}, where Y is the number of leaves per plant, plant height, or growth index; x is the days after transplanting; c is the estimated minimum number of leaves per plant, plant height, or growth index; d is the estimated maximum number of leaves per plant, plant height, or growth index; e is the time taken to achieve 50% of leaves per plant, plant height, or growth index; and b represents the relative slope around the parameter e.
b Abbreviation: FC, field capacity.
c Amaranthus palmeri maintained at 25% and 12.5% soil FC did not survive more than 35 DAT, and the four-parameter log-logistic model did not provide a good fit for number of leaves per plant, plant height, or growth index; therefore, data are not presented for 25% and 12.5% FC.
d Means within columns with no common letter(s) are significantly different at P ≤ 0.05.
e A test of lack of fit at the 95% level was not significant for any of the curves tested for the water stress treatments, indicating that the fitted model was correct.
f Growth index=π×(w/2)2×h, where w is the width of the plant calculated as an average of two widths, one measured at the widest point and another at 90° to the first; and h is the plant height measured from the soil surface to the last stem node at the top.
g Values presented for estimated minimum (c) and maximum growth index (d) are divided by 105.
Amaranthus palmeri height was reduced with increasing levels of water stress as estimated by the log-logistic model. Amaranthus palmeri maintained at 100% FC achieved a maximum height of 178 cm compared with 124 and 88 cm at 75% and 50% FC, respectively (Table 1; Figure 2B). Similarly, Chandi et al. (Reference Chandi, Jordan, York, Burton, Milla-Lewis, Spears, Whitaker and Wells2013) reported 5% to 15% reduction in the height of 15 A. palmeri biotypes under water stress conditions employed for 3 to 9 d compared with no water stress. Sarangi et al. (Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015) also reported an A. tuberculatus height of 163 cm at 100% FC compared with 146 and 115 cm at 75% and 50% FC, respectively, in a greenhouse study. However, A. spinosus produced the greatest height of 128 to 137 cm at 100% and 75% FC compared with 73 cm at 50% FC (Chauhan and Abugho Reference Chauhan and Abugho2013). The model estimated that A. palmeri grown at 100%, 75%, or 50% FC took a similar number of days (50 to 54 DAT) to achieve 50% of maximum height. Amaranthus palmeri at 100% FC had greater growth index compared with 75% and 50% FC at 21 to 70 DAT based on the fitted model curve (Figure 2C); however, the maximum growth index did not vary (1.1×105 to 1.4×105 cm3 plant−1) among 100%, 75%, and 50% FC treatments (Table 1; Figure 2C). In contrast, Sarangi et al. (Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015) reported the highest growth index of A. tuberculatus (4.4 × 105 cm3 plant−1) at 100% FC compared with 75% (3.1 × 105 cm3 plant−1) and 50% FC (1.5 × 105 cm3 plant−1), possibly because of greater width of A. tuberculatus plants compared with A. palmeri.
Amaranthus palmeri maintained at 25% and 12.5% FC died without producing seeds; therefore, only root, leaf, and stem biomass data collected at 35 DAT are presented (Table 2). The permanent wilting point of soil used in this study was 12.8% by volume, corresponding to 46% FC; therefore, the soil water available at 25% and 12.5% FC was below the permanent wilting point and could have resulted in plant death. Plants maintained at 100% FC were the tallest; therefore, they produced the greatest dry stem biomass of 22.3 g plant−1 compared with 13.3 to 15 g plant−1 at 75% and 50% FC and 3.4 to 3.8 g plant−1 at 25% and 12.5% FC. The total leaf area per plant was not significantly different (571 to 693 cm2 plant−1) at 100%, 75%, and 50% FC. In contrast, A. tuberculatus produced the greatest leaf area at 100% FC compared with 75% and 50% FC (Sarangi et al. Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015). The leaf area of plants exposed to 25% and 12.5% FC is not presented, because plants did not survive, and leaves were dry and rolled up. Similarly, dry leaf biomass was similar (5.4 to 6.4 g plant−1) among 100%, 75%, and 50% FC at harvest; however, 25% and 12.5% FC plants had only 1.2 to 1.4 g leaf biomass plant−1. Amaranthus palmeri at 100% FC produced the greatest total aboveground biomass of 38.3 g plant−1 at 100% FC compared with 25 to 27.6 g plant−1 at 75% and 50% FC and 4.8 to 5.0 g plant−1 at 25% and 12.5% FC (Table 2). Water stress treatments, except for 25% and 12.5% FC (0.6 to 0.7 g plant−1), did not affect the root biomass production (2.3 to 3 g plant−1). In addition, A. palmeri root length at 100%, 75%, and 50% FC was similar (unpublished data). This demonstrates that A. palmeri under light to moderate water stress would be able to efficiently acquire water from soil and quickly exhaust the soil water available to crops (Wright et al. Reference Wright, Coble, Raper and Rufty1999a, Reference Wright, Jennette, Coble and Rufty1999b).
Table 2 Effect of degree of water stress on Amaranthus palmeri biomass, seed production, and seed germination in a greenhouse experiment conducted for 77 d after transplanting at the University of Nebraska–Lincoln.

a Abbreviation: FC, field capacity.
b Means within columns with no common letter(s) are significantly different at P≤0.05.
c Plants maintained at 25% and 12.5% FC did not survive more than 35 d after transplanting, and leaves were dried and rolled up; therefore, total leaf area is not presented.
d Plants maintained at 25% and 12.5% FC died without producing seeds; therefore, the number of seeds per plant and seed germination data are not presented.
Amaranthus palmeri at 100% FC produced the highest number of seeds (41,696 plant−1) compared with 75% and 50% FC (14,835 to 18,796 plant−1). Similarly, A. tuberculatus produced the highest number of seeds at 100% FC (34,450 seeds plant−1) compared with 27,775 seeds plant−1 at 75% and 10,194 seeds plant−1 at 50% FC (Sarangi et al. Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015). In contrast, Chauhan (Reference Chauhan2013) reported greater seed production of itchgrass [Rottboellia cochinchinensis (Lour.) Clayton], a C4 grass species, at 75% compared with 100% FC. Amaranthus palmeri seed germination was similar (38% to 46%) at 50% to 100% FC. Similarly, seed germination of A. tuberculatus (Sarangi et al. Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015) and junglerice [Echinochloa colona (L.) Link] (Chauhan and Johnson Reference Chauhan and Johnson2010) did not differ when mother plants were maintained at 25% to 100% FC under greenhouse conditions. In contrast, previous studies have reported increased seed germination or reduced dormancy of different weed species, including A. retroflexus, when the mother plant was grown under water stress conditions compared with no water stress (Benech-Arnold et al. Reference Benech-Arnold, Fenner and Edwards1992; Karimmojeni et al. Reference Karimmojeni, Bazrafshan, Majidi, Torabian and Rashidi2014; Peters Reference Peters1982).
Amaranthus palmeri at 100%, 75%, and 50% FC took a statistically similar number of days (45 to 56 DAT) to reach flowering during the study (unpublished data). Early flowering might limit the production of photosynthates in plants, resulting in reduced growth and seed production (Shitaka and Hirose Reference Shitaka and Hirose1998), and A. palmeri plants at 75% and 50% FC did not initiate flowering early in the season and produced the same number of leaves per plant, leaf area, and leaf biomass as plants under no water stress. Plant growth parameters, including leaf area, leaf cuticle thickness, or number of leaves per plant, might play an important role under varying soil water stress levels in determining herbicide efficacy by affecting herbicide interception and absorption in plants (Zhou et al. Reference Zhou, Tao, Messersmith and Nalewaja2007).
Practical Implication
This is the first study, to our knowledge, evaluating response of A. palmeri to the degree of water stress by using soil moisture sensors to measure soil water content in the pots more frequently to maintain precise levels of water stress throughout the growing period. Amaranthus palmeri height and stem biomass were more sensitive to water stress than leaf number, leaf biomass, or total leaf area per plant. Amaranthus palmeri under light (75% FC) to moderate (50% FC) water stress allocated fewer photosynthates to the stem and produced the same leaf number, total leaf area, and root biomass as nonstressed plants. Water stress might also reduce or increase the critical weed-free period in various crops infested with different weed species compared with saturated conditions (Coble et al. Reference Coble, Williams and Ritter1981; Jackson et al. Reference Jackson, Kapusta and Schutte Mason1985). The growth characteristics of A. palmeri at different water stress levels could indicate its competitive ability under water stress conditions (Bond and Oliver Reference Bond and Oliver2006; Horak and Loughin Reference Horak and Loughin2000; Radosevich et al. Reference Radosevich, Holt and Ghersa1997), and this information may be useful for evaluating weed–crop interaction using competition models (Knezevic et al. Reference Knezevic, Horak and Vanderlip1999; Sarangi et al. Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015).
Amaranthus palmeri biotypes used in the study were collected from growers’ fields under continuous corn production in Thayer County, NE, and corn–soybean rotation in Fillmore County, NE, and the growth characteristics of A. palmeri observed in this study could vary if A. palmeri biotypes were collected from different cropping systems or rotations: for instance, Bravo et al. (Reference Bravo, Leon, Ferrell, Mulvaney and Wood2017) reported greater height and biomass of A. palmeri biotypes collected from tall-statured crops such as corn compared with shorter-canopy crops such as vegetables. The results observed in this study could also vary under field conditions, because A. palmeri plants were not able to grow to their full potential due to limited pot size under greenhouse conditions. In addition, a single A. palmeri plant was grown in each pot without any inter- or intraspecific competition; however, plants growing with crops might produce flowers earlier or later in the season depending on the competitive nature of the crops (Bolmgren and Cowan Reference Bolmgren and Cowan2008; Franks et al. Reference Franks, Sim and Weis2007). Additionally, water stress treatments were imposed throughout the growing season in this study, and timing of water stress can also play an important role in determining A. palmeri’s growth response (Chandi et al. Reference Chandi, Jordan, York, Burton, Milla-Lewis, Spears, Whitaker and Wells2013). Therefore, A. palmeri grown under field condition will have better chance of survival and higher seed production due to limited periods of water stress compared with continuous water stress conditions in this study. Previous research has shown reduced control of different weed species, including A. tuberculatus, with POST herbicides when grown under high soil water stress conditions, due to reduced herbicide retention, absorption, or translocation (Lubbers et al. Reference Lubbers, Stahlman and Al-Khatib2007; Morrison et al. Reference Morrison, Lownds and Sterling1995; Ruiter and Meinen Reference Ruiter and Meinen1998; Skelton et al. Reference Skelton, Ma and Riechers2016; Zhou et al. Reference Zhou, Tao, Messersmith and Nalewaja2007). Therefore, more research needs to be conducted regarding herbicide efficacy on A. palmeri plants under varying water stress levels.
Author ORCIDs
Mithila Jugulam, http://orcid.org/0000-0003-2065-9067; Amit J. Jhala, http://orcid.org/0000-0001-8599-4996.
Acknowledgments
The authors gratefully acknowledge Ian Rogers, Adam Leise, and Murtaza Nalwala for their assistance in this project. We acknowledge the Nebraska Corn Board for partial funding of this study. No conflicts of interest have been declared.