Introduction
Redroot pigweed (Amaranthus retroflexus L.) and slender amaranth (Amaranthus viridis L.) are monoecious summer annuals of the Amaranthaceae family. Both species are widely distributed in temperate and warm temperate countries of the world, including countries in Africa, Asia, Europe, and North America (Holm et al. Reference Holm, Doll, Holm, Pancho and Herberger1997; Uva et al. Reference Uva, Neal and DiTomaso1997; Waselkov and Olsen Reference Waselkov and Olsen2014). These species are also emerging problematic weeds on the continent of Australia, where they have infested many cropping systems across New South Wales, South Australia, and Tasmania (Manalil et al. Reference Manalil, Werth, Jackson, Chauhan and Preston2017; Osten et al. Reference Osten, Walker, Storrie, Widderick, Moylan, Robinson and Galea2007; Walker et al. Reference Walker, Taylor, Milne, Osten, Hoque and Farquharson2005).
These weeds are common C4 weed species in cultivated lands, in which their wide distribution, aggressive growth habits, and prolific seed production cause monetary losses to crop production globally (Ward et al. Reference Ward, Webster and Steckel2013). A single A. retroflexus plant can produce up to 300,000 seeds, while A. viridis (which can self-fertilize) can produce up to 7,000 seeds plant−1 (Costea et al. Reference Costea, Weaver and Tardif2004; Holm et al. Reference Holm, Doll, Holm, Pancho and Herberger1997; McLachlan et al. Reference McLachlan, Murphy, Tollenaar, Weise and Swanton1995). The spread of seeds from these species mainly occurs by rainwater, wind, farm equipment, cotton gin trash, livestock, and cover crop seeds (Farmer et al. Reference Farmer, Bradley, Young, Steckel, Johnson, Norsworthy, Davis and Loux2017; Holm et al. Reference Holm, Doll, Holm, Pancho and Herberger1997).
The high competitiveness of A. retroflexus and A. viridis for resources results in a significant yield loss in many crops (Carvalho and Christoffoleti Reference Carvalho and Christoffoleti2008; Rajagopalan et al. Reference Rajagopalan, Devi and Raghavendra1993). For example, the presence of 12 plants m−2 of A. viridis reduced leaf area (25%), leaf biomass (72%), and stem biomass (74%) of red pepper (Capsicum baccatum L.) (Barbasso et al. Reference Barbasso, Orzari, Silva, Braga and Alves2018). Bensch et al. (Reference Bensch, Horak and Peterson2003) reported that soybean [Glycine max (L.) Merr.] yield could be reduced by 38% depending on A. retroflexus density and its emergence date. Overreliance on chemical weed management strategies has led to the evolution of acetolactate synthase– and photosystem II–inhibitor herbicide-resistant biotypes (Heap Reference Heap2020). Although the presence of these species has been reported in Australia, there are no official reports on the level of infestation in summer crops, economic impact, herbicide-resistant biotypes, and other eco-biological weed management information for these species in Australia. Therefore, comprehensive studies on Australian populations of these weeds are required before these weeds become problematic across Australia.
Weed density, weed interference duration, and weed emergence time are major factors causing significant yield losses in crop fields (Estorninos et al. Reference Estorninos, Gealy, Gbur, Talbert and McClelland2005; Hussain et al. Reference Hussain, Khaliq, Matloob, Fahad and Tanveer2015; Lindström and Kokko Reference Lindström and Kokko2002). Emergence time has a significant effect on the growth and fecundity of weeds. Previous research showed that the potential peak growth and seed production of annual turnipweed [Rapistrum rugosum (L.) All.] and African mustard (Brassica tournefortii Gouan) occur when both weeds are grown in optimal environmental conditions (temperature and photoperiod) (Mobli et al. Reference Mobli, Manalil, Khan, Jha and Chauhan2020). It has also been observed that when the emergence of these weeds was delayed from April to July, their biomass and seed production was reduced by more than 75%. It has also been reported that the seed production of Palmer amaranth (Amaranthus palmeri S. Watson) could vary between 100 and 250,000 seeds plant−1 depending on the time of emergence (Keeley et al. Reference Keeley, Carter and Thullen1987; Sellers et al. Reference Sellers, Smeda, Johnson, Kendig and Ellersieck2003). Therefore, a better understanding of the effect of emergence time on weed growth and seed production may contribute to more efficient control of weeds with reduced cost and energy inputs.
Plant phenology is the study of the timing of plant growth stages in response to environmental factors (Hegazy et al. Reference Hegazy, Fahmy, Ali and Gomaa2005). Weed phenology is an important factor in understanding weed–crop competition (Ghersa and Holt Reference Ghersa and Holt1995). Temperature and photoperiod are the most influential factors impacting the phenological development of weeds (Hodges Reference Hodges and Hodges1991; Patterson Reference Patterson1992). The knowledge of weed phenology is critical to understand weed growth, seed production, biomass production, and the level of potential competition with various crops (Grant Reference Grant1989). Therefore, studies on growth and seed production of weeds under different emergence times may provide primary information for more effective management strategies. The main objectives of this study were to evaluate the growth and seed production of two populations of A. retroflexus and A. viridis in response to different planting dates.
Materials and Methods
Weed Populations and Seed Sources
Two populations of A. retroflexus and A. viridis were each collected in 2016 from Goondiwindi (28.41°S, 150.23°E; altitude 210 m) and Gatton (27.45°S, 152.21°E, altitude 90 m), located in the southeast region of Queensland, Australia. Maternal effects were minimized by growing both populations at Gatton, and mature seeds from these plants were used in the study (Mobli et al. Reference Mobli, Matloob and Chauhan2019a). Seeds of both weeds exhibited sufficient germination, so no dormancy-breaking treatment was necessary.
Experimental Approach
An outdoor pot experiment was conducted at the research facility of the University of Queensland, Gatton, during the late spring to summer seasons (October to March) of 2017 to 2018 to evaluate the effects of planting dates on two populations of A. retroflexus and A. viridis. The mean thermal fluctuation and photoperiod of the research site are provided in Figure 1. Seeds were sown on the third day of every month from October to January (four planting dates) in trays containing Platinum Potting Mix (Centenary Landscaping, Darra, QLD, Australia). The potting mix contained biological organic-based products and had a pH of 5.6 and an electrical conductivity of 1.6 dS m−1. When seedlings reached the 5-leaf stage (4- to 6-cm height), they were transplanted uniformly in free-draining plastic pots (30-cm diameter, 40-cm height). The pots were filled with clay loam soil that had a pH of 6.7, electrical conductivity of 0.14 dS m−1, and organic matter content of 2.8%. No nutritional deficiency symptoms were observed throughout the experiment, therefore no fertilizer was added. All the pots were supplied with adequate water through drip irrigation. The research site was kept weed-free by hand weeding. Plant height and number of leaves per plant were recorded at 14-d intervals until plant maturity. Plant height was measured from the soil surface to the tip of the uppermost reproductive structure. For each planting date, plants were considered mature when seed production had ceased and 10% of leaves had senesced. Shoot and root dry biomass, number of inflorescences per plant, and seed production were recorded at maturity. The shoot and root parts (washed to remove the soil particles) were bagged separately and oven-dried for 4 d at 70 C. All seeds were collected and weighed. The weight of 100 seeds was taken. These values were used to determine the total number of seeds per plant. For each planting date, plant development was recorded by calculating cumulative growing degree days (GDD) (Equation 1) as follows:

Figure 1. The mean thermal fluctuation and daylight hours of research site (Gatton, QLD, Australia) during the growth period in 2017−2019.
The flowering time was defined as the number of days required for 50% of flowering for five plants for each planting date. The experiment was repeated at the same location and experimental procedure in the summer (October to March) of 2018 to 2019.
Data Analysis
The experiment (factorial arrangement of species by population by planting date) was conducted in a randomized complete block design. For each planting date, there were 10 replicates for each population. The Shapiro-Wilk and Breusch-Pagan tests were performed to check the normality and homogeneity of data, and the original data were used for an ANOVA (GenStat 16th edition, VSN International, Hemel Hempstead, UK). Data were pooled over the experimental runs, as no significant differences were observed. Fisher’s protected LSD mean comparison test was used with a probability value of 0.05. A three-parameter sigmoidal model was fit to the plant height and leaf number data (Equation 2) using SigmaPlot v. 14 (Systat Software, San Jose, CA, USA). The equation was of the form:
where F is plant height or number of leaves per plant at GDD X, X max is the maximum plant height or number of leaves per plant, X 50 is the GDD required to reach a 50% plant height or number of leaves per plant, and b is the slope.
Results and Discussion
Effect of Planting Date on Amaranthus retroflexus and Amaranthus viridis Flowering and Growth Period
The planting date had significant (P < 0.001) effects on the required number of days to plant maturity and flowering of both weeds (Table 1). Although both weeds reached maturity at a similar number of GDD, these weeds required a different number of days to complete their life cycles within each planting date. The growth period and flowering times of A. retroflexus, when planted in October, occurred at 121 and 52 d after planting, respectively. Similarly, for the October planting date, A. viridis required 123 and 48 d to complete the life cycle and flowering, respectively. When both weeds were planted in January, the growth period and flowering initiation time were reduced to 27 and 72 d, respectively. Similarly, in both species, the number of GDD required for flowering was reduced to 477 GDD when these weeds were planted in January. No significant differences in GDD and the number of days required for growth period and flowering were observed between planting in December and January.
Table 1. The effect of planting date on Amaranthus retroflexus and Amaranthus viridis flowering and growth period. a

a Data were pooled over the populations and experimental runs (n = 40).
NS, nonsignificant.
The planting date was strongly associated with the growth and fecundity of weeds (Bosnic and Swanton Reference Bosnic and Swanton1997; Spaunhorst et al. Reference Spaunhorst, Devkota, Johnson, Smeda, Meyer and Norsworthy2018; Willenborg et al. Reference Willenborg, May, Gulden, Lafond and Shirtliffe2005). Observations from this study suggest that the growth period and the flowering initiation of both species were reduced when planting was delayed. As a result of the delay, these species experienced lower temperatures and shorter daylight hours (Figure 1), which resulted in shorter growth periods and flowering times in comparison with the October planting date. A similar response to temperature and daylight hours (photoperiod) was also observed in A. palmeri and common waterhemp (Amaranthus rudis Sauer) (Spaunhorst et al. Reference Spaunhorst, Devkota, Johnson, Smeda, Meyer and Norsworthy2018; Wu and Owen Reference Wu and Owen2014). In many weeds, temperature and photoperiod are the most influential factors in phenological stages (Hatfield et al. Reference Hatfield, Boote, Kimball, Ziska, Izaurralde, Ort, Thomson and Wolfe2011; Hatfield and Prueger Reference Hatfield and Prueger2015). In the present study, when these species were planted in January, flowering was induced sooner, as plants experienced shorter daylight hours, and consequently, the growth period was significantly reduced. It has been reported that rapid flowering and the shortening of the growth period are associated with photoperiod, and these species are classified as short-day species (Huang et al. Reference Huang, Shrestha, Tollenaar, Deen, Rahimian and Swanton2000).
Effect of Planting Date on Amaranthus retroflexus and Amaranthus viridis Height
The interaction between population and planting date was significant (P <0.001) for plant height for both species (Figure 2; Table 2). When both species were planted in October, they grew taller in comparison with cohorts from other planting dates. Any delay in planting date resulted in a reduction in plant height of both species. When the Gatton and Goondiwindi populations of A. retroflexus were planted in October, their mean plant height reached 106.7 and 87.4 cm, but the plant height was reduced to 50.0 and 52.4 cm when planted in January, respectively. Similarly, the plant height of Gatton and Goondiwindi populations of A. viridis was reduced from 87.4 to 46.7 cm and 74.3 to 46.5 cm, respectively, when planted in January. A three-parameter sigmoidal model estimated that Gatton and Goondiwindi populations of A. retroflexus required 801 to 990 GDD and 700 to 916 GDD, respectively, to attain 50% of their maximum plant height (X 50 parameter) depending on planting date. Similarly, the Gatton and Goondiwindi populations of A. viridis required 665 to 780 GDD and 684 to 978 GDD, respectively, to reach 50% of their maximum plant height.

Figure 2. The effect of planting date on Amaranthus retroflexus and Amaranthus viridis height for populations collected at Gatton and Goondiwindi, QLD, Australia. Data were pooled over the experimental runs. Vertical bars show standard errors of means. Estimated parameters are presented in Table 1.
Table 2. A three-parameter sigmoidal model fit to plant height of Amaranthus retroflexus and Amaranthus viridis when grown at different planting dates.

a F = X max /{1+ exp[−(X−X 50/b)}, F is plant height at GDD X, X max is the maximum plant height, X 50 is the GDD required to reach a 50% plant height, and b is the slope. Values in parentheses are standard errors of means.
In the current study, later-emerged seedlings of these species (October) experienced warmer days and longer daylight hours than seedlings with other planting dates and consequently grew taller. This observation could be attributed to temperature, daylight hours, and growth period. Spaunhorst et al. (Reference Spaunhorst, Devkota, Johnson, Smeda, Meyer and Norsworthy2018) reported that the plant height of A. palmeri was reduced significantly as a result of late emergence, which lowered its competitive ability.
Effect of Planting Date on Amaranthus retroflexus and Amaranthus viridis Number of Leaves
The interaction effect of population and planting date was significant (P < 0.001) for the number of leaves per plant of both species (Figure 3; Table 3). The highest number of leaves of both species was produced in the plants planted in October, and the number of leaves of both species was reduced by delaying the planting date. When Gatton and Goondiwindi populations of A. retroflexus were planted in January, the number of leaves per plant was reduced by 48% and 49%, respectively, in comparison with the number of leaves produced in October. Similarly, the number of leaves of the Gatton and Goondiwindi populations of A. viridis was reduced by 58% and 54%, respectively, as a result of a delay in planting date from October to January. A three-parameter sigmoidal model showed that the Gatton and Goondiwindi populations of A. retroflexus required 598 to 680 GDD and 723 to 1,216 GDD to produce 50% of their leaves (X 50 parameter), respectively. Similarly, the Gatton and Goondiwindi populations of A. viridis required 658 to 1,183 GDD and 801 to 1,120 GDD, respectively, to produce 50% of their maximum leaves per plant.

Figure 3. The effect of planting date on Amaranthus retroflexus and Amaranthus viridis number of leaves for populations collected at Gatton and Goondiwindi, QLD, Australia. Data were pooled over the experimental runs. Vertical bars show standard errors of mean. Estimated parameters are presented in Table 2.
Table 3. A three-parameter sigmoidal model fit to number of leaves of Amaranthus retroflexus and Amaranthus viridis when grown at different planting dates.

a F = X max /{1+ exp[−(X−X 50/b)}, F is number of leaves at GDD X, X max is the maximum number of leaves, X 50 is the GDD required to reach a 50% number of leaves, and b is the slope. Values in parentheses are standard errors of means.
Similar to plant height, the number of leaves per plant was also reduced when the planting date was delayed. Huang et al. (Reference Huang, Shrestha, Tollenaar, Deen, Rahimian and Swanton2000) reported that the number of leaves on the main stem of A. retroflexus was significantly reduced as the amount of daylight hours decreased. In the current study, significant differences were observed between the populations of both species in plant height and number of leaves per plant. Differences in the response of populations of a weed species to environmental conditions can be explained by genetic differences or maternal effects during plant growth and seed production (Bajwa et al. Reference Bajwa, Chauhan and Adkins2018; Mobli et al. Reference Mobli, Mijani, Ghanbari and Rastgoo2019b). In the present study, the effect of maternal conditions was removed by growing both populations under the same conditions in Gatton. Therefore, it could be concluded that observed differences are likely due to genetic differences between populations. Spaunhorst et al. (Reference Spaunhorst, Devkota, Johnson, Smeda, Meyer and Norsworthy2018) reported that A. palmeri populations that originated from different locations exhibited different biological characteristics, and the environmental plasticity of this species contributed to its survival and further distribution into new locations.
Effect of Planting Date on Amaranthus retroflexus and Amaranthus viridis Shoot and Root Biomass
The effect of population was significant (P < 0.001) for A. retroflexus shoot biomass and A. viridis root biomass (Figure 4). Delayed planting time resulted in shoot and root biomass reduction in both species, with the maximum reduction being recorded from the January planting. The shoot biomass from the January-planted Gatton and Goondiwindi populations of A. retroflexus was reduced by 80% and 76%, respectively, when compared with the plants planted in October. Similarly, the shoot biomass of A. viridis was reduced by 73% as a result of a delay in the planting time from October to January. When A. retroflexus was planted in January, the root biomass was reduced by 70% in comparison with planting in October. Although the Gatton and Goondiwindi populations of A. viridis produced different amounts of root biomass in the November planting, the amount of root biomass for both populations was reduced by 65% as a result of delaying the planting from October to January.

Figure 4. The effect of planting date on Amaranthus retroflexus and Amaranthus viridis shoot and root biomass for populations collected at Gatton and Goondiwindi, QLD, Australia. Data were pooled over the experimental runs and populations for shoot biomass of A. viridis and root biomass of A. retroflexus. Vertical bars are LSD values at the 5% level of probability, and letters above bars show grouping differences between means.
In the current study, shoot and root biomass of both species was sharply reduced, followed by a reduction in plant height and the number of leaves as a result of a delay in planting date. Heneghan and Johnson (Reference Heneghan and Johnson2017) reported that when waterhemp [Amaranthus tuberculatus (Moq.) Sauer] emerged in late spring (May), the mean biomass could reach 1,120 g plant−1, but when the planting date was delayed to midsummer (July), the biomass was reduced to 266 g plant−1.
Weed competition is strongly associated with weed biomass and phenological characteristics (Huang et al. Reference Huang, Shrestha, Tollenaar, Deen, Rajcan, Rahimian and Swanton2001). The prediction of the phenological stages and potential competitiveness of weeds in different conditions is an integral part of integrated weed management strategies (Deen et al. Reference Deen, Hunt and Swanton1998; Ghersa and Holt Reference Ghersa and Holt1995). In this study, the competitiveness of A. retroflexus and A. viridis was not evaluated, but late spring–emerged seedlings of these species grew vigorously and produced a higher amount of biomass in comparison with other planting dates. It seems that any early weed management practice for these species could be beneficial to minimize the subsequent cost and energy inputs toward their control. Although the effect of planting date on the biomass of these weeds was evaluated in the current outdoor pot study, field studies should be conducted to confirm the results under natural conditions. Weed phenology also contributes to developing better weed management through accurate prediction of the beginning of the weed interference (Hegazy et al. Reference Hegazy, Fahmy, Ali and Gomaa2005). Therefore, the identification of the most sensitive phenological stage of these weeds to weed management strategies and their competitiveness under different emergence times should be evaluated in future studies.
Effect of Planting Date on Amaranthus retroflexus and Amaranthus viridis Inflorescence Number and Seed Production
The interaction effect of population and planting time had a significant (P < 0.001) effect on the number of inflorescences of both species (Figure 5). The number of inflorescences and seed production of both species were reduced by a delay in planting time, and the reduction was highest in the plants planted in January. When Gatton and Goondiwindi populations of A. retroflexus were planted in January, the number of inflorescences was reduced by 75% and 62% compared with the October-planted plants, respectively. Similarly, the number of inflorescences of the Gatton and Goondiwindi populations of A. viridis was also reduced by 66% and 68%, respectively, in comparison with the October planting date. In the plants planted in October, A. retroflexus and A. viridis produced 11,350 and 5,780 seeds plant−1, but this number dropped to 770 and 365 seeds plant−1 in the plants planted in January, respectively.
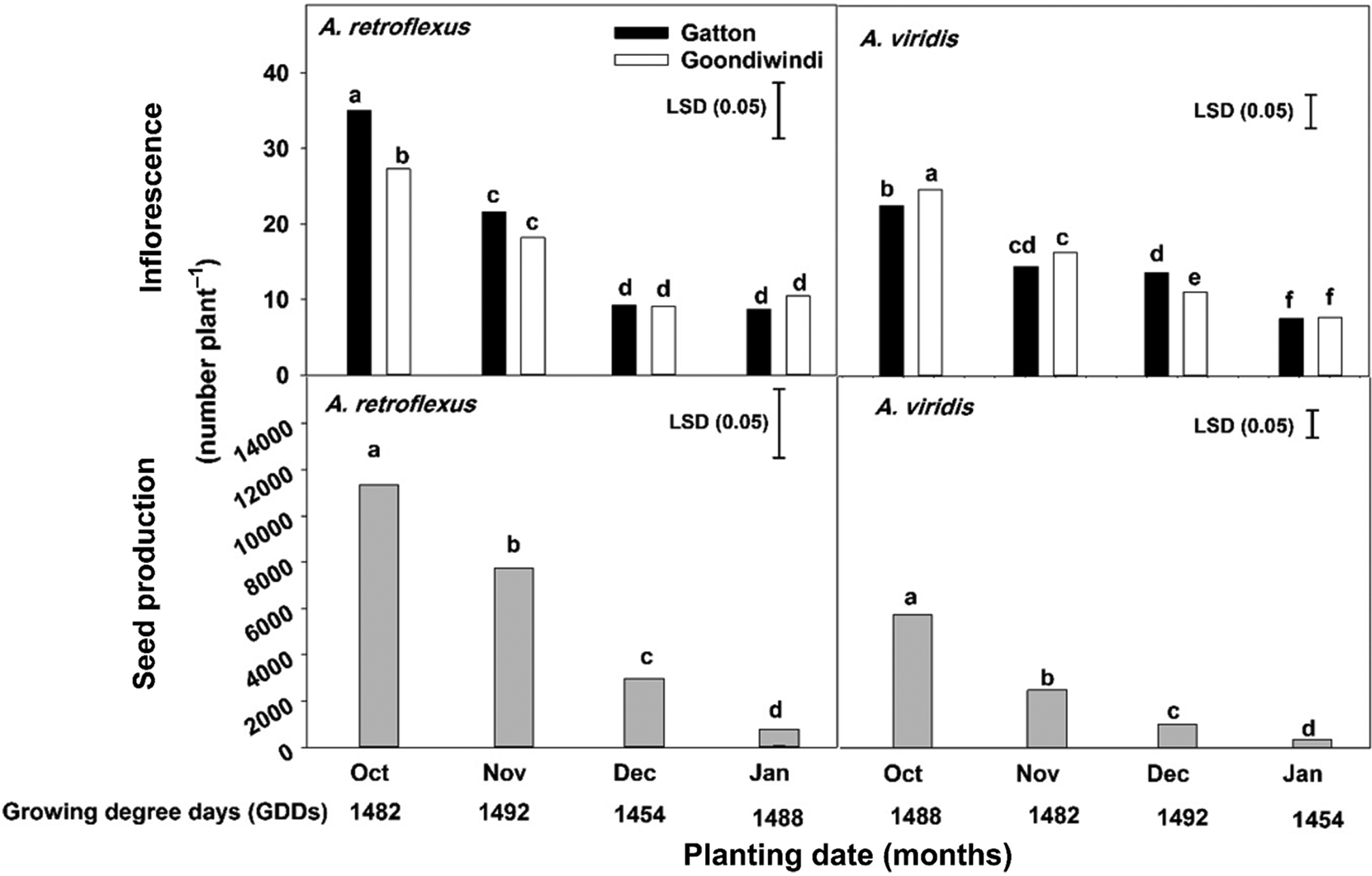
Figure 5. The effect of planting date on Amaranthus retroflexus and Amaranthus viridis number of inflorescences and seed production for populations collected at Gatton and Goondiwindi, QLD, Australia. Data were pooled over the experimental runs and populations (for root biomass). Vertical bars are LSD values at the 5% level of probability, and letters above bars show grouping differences between means.
In the current study, it was observed that both species could germinate and complete their life cycles throughout the summer growing season (October to March). Although the growth and fecundity of these species was dependent on planting time, these weeds could emerge throughout the summer growing season under irrigation or sufficient rainfall and produce a significant number of seeds. Similar results were observed for A. palmeri, in which seed production was reduced as a result of late emergence (Spaunhorst et al. Reference Spaunhorst, Devkota, Johnson, Smeda, Meyer and Norsworthy2018). Heneghan and Johnson (Reference Heneghan and Johnson2017) reported that when the emergence date of A. tuberculatus was delayed from late spring to midsummer (May to July), seed production was reduced by 70%, but late-emerging plants of this weed could produce more than 276,000 seeds plant−1. It could be concluded that the seed control of these weeds should play an important role in their management, as late cohorts of these species could produce a significant number of seeds and enrich the soil seedbank. Although it has been reported that weed seed harvest control strategies are effective methods for managing Amaranthus species (Norsworthy et al. Reference Norsworthy, Korres, Walsh and Powles2016; Schwartz et al. Reference Schwartz, Norsworthy, Young, Bradley, Kruger, Davis, Steckel and Walsh2016), late-emerging plants of these species are shorter (below crop harvest height) and may escape this control practice. Therefore, more studies and consideration should be dedicated to the control of late cohorts of these species.
Amaranthus retroflexus and A. viridis germinated throughout the study period (October to January), and germination was not inhibited during the course of experiment; therefore, both species may predominate weeds in summer-season crops. It was observed that the growth and fecundity of both species were strongly associated with planting date (temperature and photoperiod). Plants had a tendency to flower earlier with decreasing daylight hours and temperature, which led to a shorter growth period and vegetative phase. When these weeds were planted in October, they grew vigorously and produced more seeds in comparison with other planting dates. The growth and seed production of both species were reduced as a result of the reduction in the growth period. Although the effect of planting date on crop competition of these species was not evaluated in the current study, it seems that a delay in planting date may result in a less competitive weed, as weed competition is highly associated with weed biomass and phenological characteristics. It could be concluded that any early weed management practice for these species could be beneficial to minimize the subsequent cost and energy inputs toward their control. However, preventing early-emergence cohorts of these weeds alone would not guarantee success in the management of these species, as a significant amount of seed was produced by late-emerged cohorts.
Acknowledgments
This work was supported by a grant from Cotton Research and Development Corporation (CRDC) under Project UQ1703.
No conflicts of interest have been declared.











