Palmer amaranth is a major problematic agronomic weed in most crops, including soybean [Glycine max (L.) Merr.], cotton (Gossypium hirsutum L.), and corn (Zea mays L.), particularly in the southern United States (Korres et al. Reference Korres, Norsworthy, Bagavathiannan and Mauromoustakos2015a; Riar et al. Reference Riar, Norworthy, Steckel, Stephenson, Eubank and Scott2013; Webster and Nichols Reference Webster and Nichols2012), as even small infestations can cause significant yield reductions (Culpepper et al. Reference Culpepper, Grey, Vencill, Kichler, Webster, Brown, York, Davis and Hanna2006; Massinga et al. Reference Massinga, Currie, Horak and Boyer2001; Morgan et al. Reference Morgan, Bauman and Chandler2001). A substantial amount of research to control this weed using various herbicide combinations, nonchemical methods, or a combination of both has been conducted. Despite the remarkable efforts and progress achieved, the glyphosate-resistant Palmer amaranth–infested acreage in U.S. cotton and soybean crops continues to increase (Culpepper et al. Reference Culpepper, Webster, Sosnoskie and York2010; Duke and Powles Reference Duke and Powles2009). Multiple factors are contributing to infestation increases, including the dioecious nature of the weed that forces outcrossing and genetic diversity (Franssen et al. Reference Franssen, Skinner, Al-Khatib, Horak and Kulakow2001), which enhance the ability of the plant to adapt and spread widely (Bawa Reference Bawa1980; Renner and Ricklefts Reference Renner and Ricklefts1995). Palmer amaranth, a species native to North America, is one of the 10 dioecious Amaranthus subgroup species (Steckel Reference Steckel2007).
Dioecious plants have evolved gender-specific functional differences (Dawson and Bliss Reference Dawson and Bliss1989) that have been mainly attributed to differences in reproductive functions (Obeso Reference Obeso2002; Sanchez-Vilas and Retuerto Reference Sanchez-Vilas and Retuerto2012). Functional differences between genders, which are enhanced under abiotic stressful conditions, have also been reported during the vegetative stage (Montesinos et al. Reference Montesinos, Villar-Salvador, Garcia-Fayos and Verdu2012; Zhang et al. Reference Zhang, Jiang, Peng, Korpelainen and Li2011). The latter are mainly related to physiological functions of the plant and involve its photosynthetic performance (Dawson and Geber Reference Dawson and Geber1999), water use (Rowland and Johnson Reference Rowland and Johnson2001), and phenology (Delph Reference Delph1999). A differential response of male and female Palmer amaranth plants in terms of biomass production and height has been reported (Keeley et al. Reference Keeley, Carter and Thullen1987; Webster and Grey Reference Webster and Grey2015). More particularly, Keeley et al. (Reference Keeley, Carter and Thullen1987) detected differences in plant height between male and female Palmer amaranth plants, with females 11% larger than males. Webster and Grey (Reference Webster and Grey2015) observed a 2-fold increase in biomass production by female Palmer amaranth plants compared with male plants in a cotton crop.
Information on the biology of Palmer amaranth (Norsworthy et al. Reference Norsworthy, Korres, Walsh and Powles2016; Ward et al. Reference Ward, Webster and Steckel2013) and its response to abiotic stress such as shade, drought, or herbicide application is available (Jha et al. Reference Jha, Norsworthy, Riley, Bielenberg and Bridges2008; Moran and Showler Reference Moran and Showler2005). However, little is known about gender-specific influence of abiotic stress on the physiology and biology of Palmer amaranth. Knowledge of Palmer amaranth gender biology and physiology in response to stress could be used to optimize the implementation of weed management strategies. Furthermore, the possibility of increasing the short- and long-term efficacy of future weed management strategies will rely heavily on an improved understanding of the stages and processes that are most critical for regulating population growth (Ghersa et al. Reference Ghersa, Benech-Arnoldb, Satorre and Martinez-Ghersa2000; Puricelli et al. Reference Puricelli, Orioli and Sabbatini2002). This is of particular importance, as plants are often simultaneously exposed to diverse abiotic stresses such as nutrient deficiencies and/or shading and have acquired specific mechanisms to combat these stresses (Ramegowdaa and Senthil-Kumar Reference Ramegowdaa and Senthil-Kumar2015), often resulting in complex outcomes (Korres et al. Reference Korres, Norsworthy, Brye, Skinner, Mauromoustakos and Bagavathiannan2017; Zhua et al. Reference Zhua, Fana, Houa, Wua and Wang2014). Nitrogen (N), phosphorus (P), and potassium (K), among other nutrients, are the most influential for plant growth, development, and establishment. Korres et al. (Reference Korres, Norsworthy, Tehranchian, Gitsopoulos, Loka, Oosterhuis, Moss, Gealy, Burgos, Miller and Palhano2017), for example, reported the importance of total soil nitrogen and extractable soil phosphorus on the presence of Palmer amaranth at field margins in Mississippi River Delta area in eastern Arkansas.
Nitrogen is an essential component in proteins, nucleic acids, chlorophylls, and many secondary metabolites in plants (Duque et al. Reference Duque, de Almeida, da Silva, da Silva, Farinha, Santos, Fevereiro and de Sousa Araujo2013; Gan et al. Reference Gan, Jiao, Jia, Wang, Li, Shi, Peng, Polle and Luo2013; Zhua et al. Reference Zhua, Fana, Houa, Wua and Wang2014). Its deficiency affects dry-matter production and partitioning, leaf area development and maintenance, and photosynthetic efficiency (Marschner Reference Marschner1995; Zhua et al. Reference Zhua, Fana, Houa, Wua and Wang2014). Similarly, phosphorus is a component of many plant metabolites such as nucleic acids, phospholipids, adenosine triphosphate (ATP), adenosine diphosphate (ADP), and nicotinamide adenine dinucleotide phosphate (NADP). Phosphorus deficiency can restrict plant growth by reducing shoot growth and leaf area and can inhibit photosynthesis by decreasing the light-saturation point, carboxylation efficiency, ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCo) regeneration, and electron transport rates (De Groot et al. Reference De Groot, Van den Boogaard, Marcelis, Harbinson and Lambers2003; Jacob and Lawlor Reference Jacob and Lawlor1992; Radin and Eidenbock Reference Radin and Eidenbock1984; Yan et al. Reference Yan, Zhang, Xue, Zhang, Wang, Shi and Guo2015). Equally, potassium deficiency results in growth inhibition due to its involvement in a plethora of enzyme activities in addition to its role in maintaining membrane potential and cell turgor (Chinnusamy et al. Reference Chinnusamy, Zhu and Zhu2006; Liu et al. Reference Liu, Fu, Bei and Luan2000), cell elongation, osmoregulation, and promotion of photosynthetic rate (Reddy Reference Reddy2006). Few studies have quantified possible effects of the nutrient regime on Palmer amaranth.
Nevertheless, as mentioned previously, weeds are simultaneously exposed to diverse abiotic stress, for example, nutrient deficiencies and canopy shade. Characterizing the response of weeds to canopy shade (i.e., light stress) is important for understanding the dynamics between the crop and weed (Brainard et al. Reference Brainard, Bellinder and DiTommaso2005). Light stress affects plant weight, number of leaves (Moran and Showler Reference Moran and Showler2005), partitioning of dry weight to stem tissues, stem elongation, specific leaf area (Brainard et al. Reference Brainard, Bellinder and DiTommaso2005; Jha et al. Reference Jha, Norsworthy, Riley, Bielenberg and Bridges2008), and photosynthesis.
Photosynthesis is one of the metabolic processes in which nutrient deficiencies and light stress are involved by altering photosynthetic efficiency significantly. The two primary processes of light in photosynthesis are the driving of electrons from water through the Z-scheme pathway for NADP reduction and the delivery of energy from ATP formation for CO2 reduction and carbohydrate synthesis (Salisbury and Ross Reference Salisbury and Ross1992).
The aim of this research was to investigate the morphological and physiological responses of Palmer amaranth gender to nutrient deficiency and light stress by combining both mechanistic and phenomenological approaches. The objectives of this study were 1) to quantify the growth response of Palmer amaranth male and female plants under nitrogen-, phosphorus-, and potassium-deficient conditions and/or various light-intensity gradients; 2) to examine the effects of these stresses on Palmer amaranth gender phenology; 3) to examine the effects of these stresses on Palmer amaranth gender photochemistry through the application of chlorophyll fluorescence technique; and 4) to examine whether chlorophyll content is altered under abiotic stresses.
Materials and Methods
Plant Material and Cultured Cuttings
Palmer amaranth seeds were collected from the field at the University of Arkansas–Agricultural Research and Extension Center, Fayetteville, AR. Seed samples were stored in sealed vials at 5 C until planting. Seeds of Palmer amaranth were planted in trays (52.5 by 25.5 by 5.5 cm) containing a commercial potting mix (Sunshine LC1 potting mix, SunGro Horticulture, Agawam, MA). Trays were placed in a greenhouse with a 35/23 C day/night temperature and a 14-h photoperiod with light intensity between 1,000 to 1,200 μmol m−2 s−1 of photosynthetically active radiation (PAR) at the top of the trays as measured at midday with a sunfleck ceptometer (AccuPAR, model LP-80, Decagon Devices, Pullman, WA).
One hundred vigorous Palmer amaranth 1-leaf seedlings without any sign of injury or damage were selected, transplanted into Jiffy pots (Jiffy-7® peat pellets, HummertTM International, Earth City, MO), and placed in 72-plug plastic trays until the 6- to 8-leaf stage. Each individual plant was then moved to a polyvinylchloride pot 37-cm height by 30-cm diameter containing commercial potting mixture and left to grow under greenhouse conditions similar to those described previously, until reproductive development. These plants were supplemented with water-soluble all-purpose plant food (Scotts Miracle-Gro Products, Marysville, OH) containing 24%, 8%, and 16% N, P, and K, respectively, every 10 to 14 d.
When plant gender, based on the inflorescence characteristics, was distinguishable, the most vigorous mature plants from each gender were chosen, and Palmer amaranth cuttings were obtained as described by Teaster and Hoagland (Reference Teaster and Hoagland2014), with some modifications. More particularly, the excised petioles (∼10- to 20-mm long) were planted soon after their imbibition into rooting powder containing 0.1% indole-3-butyric acid (Hormex, Brooker Chemical, Chatsworth, CA), in Jiffy pots that were kept in a Conviron growth chamber (CMP 6050, Conviron, Winnipeg, Canada) at a constant temperature of 25 C and continuous low-light intensity (i.e., 150 μmol m−2 s−1) for 10 d to allow root initiation and growth. The rooted cuttings, after this period, were moved into individual 37 by 30 cm pots containing a 3:1:0.5:0.5 substrate mixture of washed sand (Oldcastle® Lawn & Garden, Hope, AR), vermiculite, perlite, and Sunshine® Canadian sphagnum peat moss (SunGro Horticulture, Agawam, MA) and placed into three Conviron growth chambers, each of which delivered a different light intensity, under a 14-h photoperiod and 35/25 C day/night temperature.
Experimental Design and Treatments
A three by three by two factorial experiment, arranged as a randomized complete block design, with light intensity (chamber) as main plot treatment and gender with nutrient deficiency as blocked treatments, was conducted. Each treatment consisted of 10 replications for each combination of nutrient deficiency and Palmer amaranth gender. Each growth chamber was programmed to deliver a low, medium, and high light intensity equal to 150, 450, and 1,300 μmol m−2 s−1, mimicking light interception by a dense, a moderately dense, and a relatively open soybean canopy at the early crop-growth stages, respectively, as had previously been measured under clear-sky field conditions at midday between 1100 and 1300 hours between the end of June and middle of July in Fayetteville, AR. Light intensity with three different light gradients as treatment levels comprised the first factor of the experiment. This coincides with the findings made by Sage and Pearcy (Reference Sage and Pearcy1987), who indicated that growth room light intensities of 600 μmol m−2 s−1, although well below the maximum that occurs in the field, produce plants including redroot pigweed (Amaranthus retroflexus L.) and common lambsquarters (Chenopodium album L.) with similar characteristics as field-grown plants.
The other factors consisted of two levels of Palmer amaranth gender (i.e., male and female plants) and three levels of nutrient deficiency, each containing only 10% N, P, or K of the standard N, P, and K stock solutions (i.e., 91.4 g NH4NO3 L−1 solution, 40.3 g NaH2PO4×2H2O L−1 solution, or 71.4 g K2SO4 L−1 solution), as described in Yoshida et al. (Reference Yoshida, Forno, Cock and Gomez1976) and recommended by the Soil Analytics Lab at the University of Arkansas, Altheimer Lab, Fayetteville, AR (TL Roberts, personal communication). Das and Sen (Reference Das and Sen1981) used a similar percentage reduction level of nitrogen, phosphorus, and potassium to induce deficiency in chickpea (Cicer arietinum L.). Hence, for each light-intensity gradient mentioned earlier, each of the 20 Palmer amaranth cuttings (10 cuttings from male parents and 10 cuttings from female parents) were supplied with 300 ml of a nitrogen-, potassium-, or phosphorus-deficient solution, every 2 d. The solution was modified to contain all essential macro- and micronutrients, but only 10% N or 10% P or 10% K of the standard N, P, and K stock solution at each time of fertilization. Plants were regularly rotated within each growth chamber to avoid possible shading effects. In addition, 10 plants from each Palmer amaranth gender receiving full nutrition (i.e., 91.4 g NH4NO3 L−1 solution, 40.3 g NaH2PO4×2H2O L−1 solution, and 71.4 g K2SO4 L−1 solution) (Yoshida et al. Reference Yoshida, Forno, Cock and Gomez1976) and left to grow under greenhouse conditions (i.e., 12- to 14-h photoperiod with 32/25 C day/night temperature and 700 to 1000 μmol m−2 s−1 light intensity) were used as controls. A control group, common to all treatments, was allowed due to 1) space restrictions and 2) the complexity of the study i.e., it is actually impossible to implement a group that simultaneously has several levels of treatment factors and receives no treatment at all. In summary, the experiment consisted of 3 light-intensity gradients by 2 Palmer amaranth genders by 3 nutrient deficiency regimes by 10 replications per gender plus 10 controls per gender, totaling 200 experimental units (pots). The same set of experimental treatments was repeated. Experimental runs were conducted during 2015 and 2016.
Growth Analysis Measurements
Growth rate (GR) for each Palmer amaranth gender, averaged across nutrient and light regimes, was estimated by height measurements taken every 2-d interval period until the final harvest (Equation 1) (Korres Reference Korres2005).
where
![]() ${\rm height}_{{t_{n} }}$
=height at time n, and
${\rm height}_{{t_{n} }}$
=height at time n, and
![]() ${\rm height}_{{t_{n-1} }} $
=height at time n−1.
${\rm height}_{{t_{n-1} }} $
=height at time n−1.
At final harvest, when Palmer amaranth plants reached maturity 46 d after transplanting (DAT), measurements were made of leaf area per plant, using a LI-3100C leaf area meter (LI-COR® Bioscience, Lincoln, NE); number of leaves per plant; total dry weight; leaf dry weight; stem dry weight (plants were dried in a forced-draft oven at 70 C for 48 h); number of inflorescences; and the length of the main and lateral inflorescences for the entire Palmer amaranth population. Specific leaf area (SLA) was estimated based on leaf area and leaf dry weight (Equation 2) (Korres Reference Korres2005).
Chlorophyll Fluorescence Measurements
Efficacy of the photosystem II complex was estimated via the electron transport rate (ETR) efficiency, using light-adapted chlorophyll fluorescence techniques as described by Flexas et al. (Reference Flexas, Escalona and Medrano1999) and Motohashi and Myouga (Reference Motohashi and Myouga2015), for light stress and nutrient deficiency using an OS5p modulated fluorometer (Opti-Science, Tyngsboro, MA). Five light-exposed, fully expanded young leaves from the upper half of the main stem of five randomly selected plants from each treatment were chosen for fluorescence measurements. Nondestructive fluorescence measurements were obtained on a weekly basis for both experimental runs. The change in irradiance (ΔF) was measured under actinic light conditions. The adaxial surface of the leaf was illuminated stepwise with increasing intensity (2850, 5700, and 8550 µmol m−2 s−1) for approximately 0.95 s, which provided the estimate of F′M (maximum fluorescence) when all reaction centers have been fully occupied (Maxwell and Johnson Reference Maxwell and Johnson2000). An estimate of ETR was calculated based on Equation 3 (Motohashi and Myouga Reference Motohashi and Myouga2015).
where PAR=photosynthetically active radiation at the leaf’s surface, F′M=maximal fluorescence level from leaves in light, and F′V=variable fluorescence level of leaves in light (F′v=F′M−F′O, with F′O representing the minimal fluorescence level of leaves in light prior to irradiance change ΔF). The constant 0.5 corresponds to the excitation energy being divided among both photosystems I and II, and 0.85 is the leaf absorbance coefficient for C4 plants (Oberhuber et al. Reference Oberhuber, Dai and Edwards1993).
Chlorophyll Content Measurements
A nondestructive optical method, as described by Richardson et al. (Reference Richardson, Duigan and Berlyn2002), was used to compare Palmer amaranth plants on the basis of their chlorophyll content. Five measurements were obtained from each of five fully expanded, light-exposed leaves from the upper half of the main stem (the same leaves were used for chlorophyll fluorescence measurements) of five randomly selected plants per treatment, using a handheld SPAD-502 (Minolta Camera, Osaka, Japan) meter. This device has a 0.06 cm2 measurement area and calculates an index in “SPAD units” based on absorbance at 650 and 940 nm at ±1.0 SPAD units accuracy level. Measurements were taken on a weekly basis for both experimental runs. The estimation of total chlorophyll was based on a calibration equation (Equation 4) developed by Richardson et al. (Reference Richardson, Duigan and Berlyn2002).
where total chlorophyll=chlorophyll α + chlorophyll b and SPAD=SPAD units.
Data Analysis
One-way ANOVA was performed to clarify whether male and female controls were statistically different. Adjustments by expressing data from treated plants as control percentages were employed if statistical differences between gender controls were observed, otherwise data from both male and female controls were pooled and percentages were performed accordingly. Electron transport rate and chlorophyll content were pooled, as no statistical differences were detected between Palmer amaranth gender controls. Growth rate estimations and height were excluded from this adjustment. ANOVA was employed for final data analysis. Log-transformations based on natural logarithms were performed where necessary to secure normality assumptions. JMP Pro v. 12.0.1, (SAS Institute, Cary, NC), SigmaPlot v. 13.0 (Systat Software, San Jose, CA), and Genstat 10 (VSN International, Hemel Hempstead, UK) were used for statistical analyses and curve-fitting regressions.
Results and Discussion
Palmer Amaranth Gender Biology in Response to Abiotic Stress
The biological characteristics of Palmer amaranth male and female plants were measured at plant maturity for the evaluation of Palmer amaranth gender response to abiotic stress. Height, stem dry weight, and total dry weight were the growth characteristics for which the response of female Palmer amaranth plants was significantly higher than that of male plants to light-intensity levels, nutrient deficiency, or the combination of nutrient deficiency by light intensity (Table 1). Female plants were significantly taller (α=0.001) compared with male plants under any combination of the experimental factors (Table 1). Nitrogen was the most influential nutrient, as its deficiency negatively influenced all Palmer amaranth growth characteristics independent of gender. This is particularly noticeable at high light intensity, where the height of both nitrogen-deficient genders was significantly reduced (α=0.05) compared with other nutrient-deficient plants (Table 1). Nevertheless, stem elongation in female Palmer amaranth plants was statistically higher (α=0.05) compared with male plants when nitrogen was not deficient, particularly at low light intensities (Table 1). Under shade conditions, plants exhibit several well-known “shade-avoidance” responses, including accelerated stem extension growth (Morgan and Smith Reference Morgan and Smith1976). In addition, female plants invested in stem dry weight, which most probably contributed to the statistically significant higher (α=0.001) total dry weight production by female compared with male Palmer amaranth plants (Table 1). These results demonstrate a differential response of the female Palmer amaranth plants to abiotic stress in favor of size through increases in height and dry matter partitioning.
Table 1 Growth analysis on the logarithmically transformed growth characteristics of Palmer amaranth gender at harvest (46 DAT) and expressed as control percentagesFootnote a prior to data transformation.

a Height analysis was based on raw data for both genders.
b Standard error of the mean, in the far right column of the table, stands for the interactions of N×L, N×G, L×G, and N×L×G on height, no. of leaves plant−1, stem dry weight, and total dry weight, respectively
c Standard error of the mean within the row of each dependent variable stands for the main effects of Palmer amaranth; specifically, G, N, and L on height, no. of leaves plant−1, stem dry weight, and total dry weight, respectively
*Significant at α=0.05; ***significant at α=0.001; ns=nonsignificant.
Palmer amaranth male plants were mostly invested in leaf area and leaf dry weight production (Table 2). Moreover, leaf area of male Palmer amaranth plants was higher in most treatment combinations, although statistically significant increases were mostly recorded under low and medium light-intensity regimes and phosphorus deficiency (Table 2). This relationship was inversed under high light intensity, where female plants exhibited a statistically significant higher leaf area under phosphorus deficiency (Table 2). Differences in leaf area investments by male plants under high light intensity and nitrogen deficiency were significantly greater compared with those in female plants, with the former exhibiting a leaf area increase of 60%. Nevertheless, leaf area under nitrogen deficiency was significantly reduced in both Palmer amaranth genders compared with other nutrient deficiencies under any light regime (Table 2). Radin and Eidenbock (Reference Radin and Eidenbock1984) and Zhao et al. (Reference Zhao, Reddy, Kakani and Reddy2005) reported that leaf expansion in nitrogen-deficient cotton and sorghum [Sorghum bicolor (L.) Moench] plants was limited by hydraulic conductance with direct consequences in leaf area.
Table 2 Effect of nutrient deficiency and light-intensity gradients on Palmer amaranth gender leaf area and leaf dry weight.Footnote a

a Data were measured at harvest, expressed as control percentages, and logarithmically transformed prior to data analysis.
b Standard error of the mean, at the far right column of the table, stands for the interactions of N×L; N×G; L×G and N×L×G on height, no. of leaves plant−1, stem dry weight, and total dry weight, respectively.
c Standard error of the mean within the row of each dependent variable stands for the main effects of Palmer amaranth; specifically, G, N, and L on height, no. of leaves plant−1, stem dry weight, and total dry weight, respectively.
*Significant at α=0.05; ***significant at α=0.001; ns=nonsignificant.
Leaf dry weight in male plants was less affected by potassium deficiency under medium and high light intensity (Table 2). This might be an indication of differentiation in regulating mechanisms between Palmer amaranth genders under potassium deficiency. The assimilation of nitrogen in female plants, for example, under medium and high light intensities could have been affected by potassium deficiency as compared with male plants. Qu et al. (Reference Qu, Liu, Ze, Gong, Hong, Wang and Hong2011) reported that nitrogen assimilation was impaired under potassium-deficiency stress in corn, which according to Zhua et al. (Reference Zhua, Fana, Houa, Wua and Wang2014) and Marschner (Reference Marschner1995) affects the allocation of resources and dry matter partitioning.
Fitness Differentiation of Palmer Amaranth Gender
Geber (Reference Geber1990) along with Cheplick (Reference Cheplick1995) and Solbrig (Reference Solbrig1981) stated that variation in size and biomass allocation contributes to variation in fitness. Significant differences between Palmer amaranth genders, in favor of female plants, were observed in size (i.e., tallness, greater stem and total dry weights) along with differential biomass allocation. It is therefore reasonable to accept the existence of variation in fitness between the two Palmer amaranth genders, which may express an adaptive plasticity, a “gain of fitness,” possibly due to reproductive effort (Obeso Reference Obeso2002; Sanchez-Vilas and Retuerto Reference Sanchez-Vilas and Retuerto2012). Nevertheless, possible gain of female fitness as a function of reproductive effort declines progressively at 30 to 42 DAT (Figure 1) when flowering reaches its peak. After that period the growth rate of female and male plants, averaged across nutrient deficiency and light-intensity treatments, becomes comparable, making it easier for the males to be established and eventually dominate.

Figure 1 Growth rate of Palmer amaranth gender throughout the experimental period. Data at the lower right side of the figure indicate the comparability of growth rate between female and male Palmer amaranth plants as both genders approach the peak of their reproductive stage. Vertical bars represent the standard error of the mean.
The increase of female leaf dry weight compared with male plants under high light-intensity regime and nitrogen deficiency resulted in significantly (α=0.05) lower female SLA (i.e., 0.76; standard error of the mean=0.2) compared with male counterparts (i.e., 3.0; standard error of the mean=0.2) under nitrogen-deficient conditions. According to Wilson et al. (Reference Wilson, Thompson and Hodgson1999), SLA reflects the expected return on previously captured resources, and high-SLA leaves are short-lived. These authors suggested that high-SLA leaves are more suitable in resource-rich environments, while low-SLA leaves perform better in resource-poor environments, where retention of captured resources is a higher priority. Jha et al. (Reference Jha, Norsworthy, Riley, Bielenberg and Bridges2008) reported that Palmer amaranth acclimation to shade can be achieved, other than by height, with increases in SLA. The results presented in this work are in agreement with this statement when comparisons are made between low and high light-intensity regimes, particularly with nitrogen-deficient plants. However, further research is needed to better understand Palmer amaranth acclimation potential to shading under various nutrient conditions.
Effects of Gender and Abiotic Stress on Palmer Amaranth Flowering
Reports in the literature on nonphotoperiodic flowering or stress-induced flowering (i.e., the tendency to flower under unsuitable growth conditions) indicate that factors responsible for stress-induced flowering include, among others, nutrient deficiencies and light stress (i.e., low or high light intensity) (Takeno Reference Takeno2012). As mentioned previously, the most typical phenotype of the shade-avoidance response is rapid stem elongation or increased SLA, but recent reports suggest that acceleration of flowering is another strategy plants adopt as a shade-adaptive mechanism (Adams et al. Reference Adams, Allen and Whitelam2009). Accelerating flowering under unfavorable environments increases the probability of the survival of the individual and therefore of the species (Takeno Reference Takeno2012).
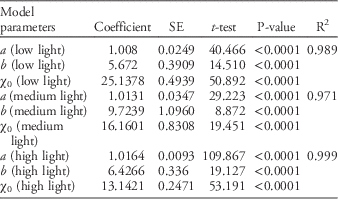
A sigmoidal model, the parameters of which are shown in Table 3, was used to examine the relationship of flowering (i.e., floral initiation and flowering) to light-intensity gradients. The results, as presented in Figure 2, demonstrate the influence of low light intensity in extending flowering induction compared with medium and high light regimes (i.e., the inflection point at low light intensity equals 25 DAT compared with 16 and 13 DAT for medium and high light intensity, respectively). In addition, the rate of flowering induction is greater at lower light intensity compared with medium and high light, as indicated by each slope (Table 3). High and to a certain extent medium light intensities promote flowering, as indicated by the higher saturation point values in the corresponding curves (Figure 2; Table 3). Takeno (Reference Takeno2012) reported similar results with respect to the effects of light intensity on flowering.

Figure 2 Cumulative number of Palmer amaranth flowering plants throughout the experimental period, averaged across nutrient deficiency regimes and Palmer amaranth genders; ns=nonsignificant.
Table 3 Coefficients and accumulate analysis of variance of the three-parameter sigmoidal model fit onto the cumulative number of Palmer amaranth flowering plants for each light-intensity regime throughout the experimental period.

Fitted curves for low, medium, and high light intensity were pairwise compared to evaluate the “parallelism” of these curves between each light-intensity regime. The outcome of the analysis highlights the statistically significant differences between each pair of the fitted curves, except those between medium and high light intensity (Figure 2). Brainard et al. (Reference Brainard, Bellinder and DiTommaso2005) found no effect on the number of days to flowering in Powell amaranth (Amaranthus powellii S. Wats.) exposed to various regimes of shading under field conditions. In contrast, McLachlan et al. (Reference McLachlan, Swanton, Weise and Tollenaar1993) reported flowering delays of redroot pigweed due to reduced irradiance conditions under a corn canopy shaded environment compared with nonshaded controls. Weiner (Reference Weiner1988) stated that reduced irradiance and changes of red:far-red ratio under canopy shade could delay flowering. Korres et al. (Reference Korres, Norsworthy, Green, Godwin, Martin and Lancaster2016a) reported that flowering in Palmer amaranth was induced earlier at high weed population densities present in wide-row soybean, hence increasing light interception by both soybean and Palmer amaranth canopy. This suggests a pleiotropic trade-off between reproduction and survival of the species. To the best of our knowledge, no studies have been conducted to investigate the response of Palmer amaranth gender flowering under light-stress gradients. Further research is required to better understand the physiological mechanisms and ecological consequences of Palmer amaranth flowering time in response to light stress.
A three-parameter sigmoidal model was also used to examine the relationship between Palmer amaranth gender and flowering. Despite the slightly better curve fit of the female data set (R2=0.992) compared with male plants (R2=0.989) and the higher t-test values for the female data set, the curves were not statistically different. This indicates a similar flowering trend between male and female Palmer amaranth plants. Nevertheless, flowering in female Palmer amaranth plants, expressed as a percentage of the control and averaged across nutrient deficiencies, light-intensity levels, and time, was significantly higher (α=0.05) compared with male plants (i.e., 0.73 for female vs. 0.68 for male plants; standard error of the mean=0.008).
A significant difference (α=0.05) in the number of flowering plants averaged across light-intensity regimes was revealed for Palmer amaranth gender by nutrient deficiency by time (Figure 3). Flowering occurrence of female Palmer amaranth phosphorus-deficient plants was higher compared with other nutrient-deficient female and male plants up to 24 DAT. After that period, flowering occurrence of female nitrogen-deficient plants along with male nitrogen- and potassium-deficient plants and to lesser extent female potassium-deficient plants was not different compared with phosphorus-deficient female plants. As stated by Wada and Taneko (2010), flowering in most cases is enhanced under nutrient deficiencies, although flowering in nitrogen-deficient plants of lesser duckweed (Lemna aequinoctialis Welw.) was induced (Tanaka et al. Reference Tanaka, Nakayama, Emori, Takeba, Sato and Sugino1997). Perhaps this may offer an explanation as to why female Palmer amaranth nitrogen-deficient plants responded later than those under phosphorus deficiency.

Figure 3 Effects of nutrient deficiency by Palmer amaranth gender on the cumulative percentage of flowering plants at harvesting, averaged across light-intensity regimes. Vertical bars represent standard error of the mean.
Nitrogen limitation has been previously reported to accelerate flowering in perilla mint [Perilla frutescens (L.) Britt. var. crispa (Benth.)], even under continuous light conditions (Shinozaki and Takimoto Reference Shinozaki and Takimoto1982; Wada et al. Reference Wada, Kondo and Takeno2010). In Japanese morningglory (Ipomoea nil (L.) Roth, formerly Pharbitis nil), nutrient starvation and high light intensity induced flowering (Hirai et al. Reference Hirai, Kojima, Koshimizu, Shinozaki and Takimoto1993; Shinozaki Reference Shinozaki1985; Shinozaki and Takimoto Reference Shinozaki and Takimoto1982; Tanaka et al. Reference Tanaka, Nakayama, Emori, Takeba, Sato and Sugino1997; Wada et al. Reference Wada, Kondo and Takeno2010). Flowering induction under nutrient-deficient conditions is often accompanied by an increase in phenylalanine ammonia lyase (PAL) activity (Hatayama and Takeno Reference Hatayama and Takeno2003; Wada and Takeno Reference Wada and Takeno2010). PAL is a critically regulated enzyme that controls the diversion of phenylalanine from protein biosynthesis to phenylpropanoid biosynthesis (Hopkins and Hüner Reference Hopkins and Hüner2009). Inhibition of PAL could manipulate Palmer amaranth flowering initiation and, possibly, regulate population structure.
It is also notable that female plants completed initiation of flowering 6 to 8 d earlier than male plants (Figure 3), exhibiting a type of “compactness” in their flowering pattern, particularly under phosphorous and nitrogen deficiencies. This, in combination with the higher flowering potential of female plants, could be an indication of differential function in phenology of Palmer amaranth genders that merits further investigation, as it can be used as a long-term tool to modify Palmer amaranth population.
Effects of Gender and Abiotic Stress on Palmer Amaranth Photochemistry
The application of chlorophyll fluorescence in plant physiology research under stress conditions such as nutrient deficiencies and shade has been proven to be an invaluable tool for the quantification of plant responses to abiotic stresses (Dai et al. Reference Dai, Shen, Liu, Wang, Hannaway and Lu2009; Long et al. Reference Long, Ma, Wan, Song, Sun and Qin2013). The employment of the chlorophyll fluorescence technique in this work revealed significant differences in Palmer amaranth photochemistry and subsequent differential response of Palmer amaranth gender due to nutrient and light-stress treatments, including ETR and chlorophyll content (Figure 4a and b). ETR is a measure of a leaf’s photosynthetic capacity while photosynthesis occurs (Schreiber Reference Schreiber2004) and is estimated as a fraction of the variable fluorescence (FV):maximum fluorescence (FM) ratio, which evaluates the maximum quantum yield in PS II (Zhang et al. Reference Zhang, Liu, Tao and Chen2014) (Eq. 3). The greater photosynthetic capacity of female compared with male plants is demonstrated in Figure 4a. An exception from this trend was observed only under nitrogen and phosphorus deficiencies at high light intensity (Figure 4a). As reported by Cheng et al. (Reference Cheng, Fuchigami and Breen2000), decreased leaf nitrogen content, which usually occurs under nitrogen-deficient conditions, was associated with decreased Fv/FM ratio. Zhang et al. (Reference Zhang, Liu, Tao and Chen2014) observed decreases in chlorophyll content and FV/FM ratio under phosphorus deficiency. FV/FM ratio was found to be reduced (α=0.001) in females compared with male Palmer amaranth plants under phosphorus and nitrogen deficiencies. More particularly, FV/FM ratio in females under phosphorus, nitrogen, and potassium deficiencies was equal to 0.50, 0.53, and 0.51, whereas for males it was 0.56, 0.54, and 0.51, respectively (standard error of mean=0.006). Bjorkman and Demming (Reference Bjorkman and Demming1987) have shown that the value of this ratio in plants with full photosynthetic capacity is around 0.83; the lower the value of this ratio, the less efficient the photochemistry (Hall and Rao Reference Hall and Rao1994).

Figure 4 Effects of nutrient deficiency by Palmer amaranth gender on (a) electron transport rate and (b) total chlorophyll content. Results are expressed as percentages of pooled data from both male and female controls. Arrows indicate the reversibility of the trend in favor of male Palmer amaranth plants.
The reversibility of female ETR under phosphorus and nitrogen deficiency at high light intensity (Figure 4a) may be an indication of a negative-energy feedback that denotes a photoinhibition symptom. The reversibility of female ETR is possibly due to Calvin cycle limitations for NADP or ATP and/or reduced carboxylation capacity (Buckley and Farquhar Reference Buckley and Farquhar2004). During photosynthetic CO2 assimilation, phosphorus-containing compounds are required for physiological processes, such as the biosynthesis of ATP from ADP or the regeneration of ribulose-1,5-bisphosphate (Hidaka and Kitayama Reference Hidaka and Kitayama2013). Under photoinhibitory conditions, the oxygenase activity of RuBisCo is favored at the expense of its carboxylase activity, giving rise to the cellular photorespiratory cycle and, consequently, dissipation of excess excitation energy, a state that is enhanced under high light intensity, by using ATP to oxidize RuBP to CO2 and 2-phosphoglycolate (Foyer et al. Reference Foyer, Bloom, Queval and Noctor2009). Shortage of available ATP, ADP, or NADP due to phosphorus or nitrogen deficiency (Gan et al. Reference Gan, Jiao, Jia, Wang, Li, Shi, Peng, Polle and Luo2013) could have caused significant restrictions of ETR in female Palmer amaranth plants, despite the greater photosynthetic capacity exhibited under high light intensity, when compared with male plants. Nitrogen is an essential component in chlorophyll (Duque et al. Reference Duque, de Almeida, da Silva, da Silva, Farinha, Santos, Fevereiro and de Sousa Araujo2013; Gan et al. 2015; Zhua et al. Reference Zhua, Fana, Houa, Wua and Wang2014). Zhao et al. (Reference Zhao, Reddy, Kakani and Reddy2005) reported significant reductions of chlorophyll content in sorghum leaves due to nitrogen deficiency. Chlorophyll content under nitrogen and phosphorus deficiencies at medium and high light intensities were lower in female (α=0.001) compared with male plants (Figure 4b), a factor that could have enhanced the reversibility of female plants’ photochemistry in favor of males.
Weed Management Implications
Nitrogen deficiency was found to be the most important factor that affects the performance of both genders, particularly under high light intensities (Tables 1 and 2). The growth of weeds under increasing rates of nitrogen is species dependent (Blackshaw et al. Reference Blackshaw, Brandt, Jazen, Entz, Grant and Derksen2003), and amaranths have been found to thrive in nitrogen-enriched environments (Korres et al. Reference Korres, Norsworthy, Brye, Skinner, Mauromoustakos and Bagavathiannan2017). Nitrogen accumulation, for example, was greater in redroot pigweed compared with corn when grown in an nitrogen-enriched environment (Teyker et al. Reference Teyker, Hoelzer and Liebl1991). It has been discussed by Liebman and Davis (Reference Liebman and Davis2000) that high initial growth rate, as in the case of Palmer amaranth female plants, is negatively correlated with seed mass and seed reserves. They also discussed the importance of nitrogen application timing on weed growth, particularly with respect to the weed species’ ability to absorb nutrients earlier and more rapidly than the crop, and suggested that delayed fertilization is likely to be most useful for managing small-seeded weeds in large-seeded crops. Female Palmer amaranths fulfill all requirements stated by these authors, as they exhibit high early growth rates (Figure 2), produce small seeds (Ward et al. Reference Ward, Webster and Steckel2013), and are the most agronomically important weed species in cotton and corn (Korres et al. Reference Korres, Norsworthy, Bagavathiannan and Mauromoustakos2015a; Riar et al. Reference Riar, Norworthy, Steckel, Stephenson, Eubank and Scott2013; Webster and Nichols Reference Webster and Nichols2012), both of which are large-seeded crops.
Alternatively, the use of nitrogen-demanding winter cereal cover crops (Dabney et al. Reference Dabney, Delgado and Reeves2001; Kaspar et al. Reference Kaspar, Kladivko, Singer, Morse and Mutch2008) prior to crop planting can be an invaluable tool in integrated Palmer amaranth control methods. Furthermore, the crop density and cultivar selection are known to influence weed suppression (Korres et al. Reference Korres, Norsworthy, Green, Godwin, Martin and Lancaster2015b, Reference Korres, Norsworthy, Brye, Skinner, Mauromoustakos and Bagavathiannan2017). It is therefore important to investigate whether the manipulation of soil fertility and light environment at the field level can alter/suppress Palmer amaranth population, a species prone to easily developing herbicide resistance.
Future Research
The use of monocultures, the overreliance on a single herbicide mode of action, and negligence in the area of other weed control measures are the major reasons for rapid herbicide resistance evolution (Mortensen et al. Reference Mortensen, Egan, Maxwell, Ryan and Smith2012). Most experts agree that weed management systems in the days ahead will be more complicated (Thompson Reference Thompson2012). If problems with herbicide-resistant weeds are addressed only with herbicides, herbicide resistance evolution will most likely prevail (Mortensen et al. Reference Mortensen, Egan, Maxwell, Ryan and Smith2012). The results presented here offer a wide range of alternatives and new openings in current research for improving management of herbicide-resistant Palmer amaranth. Therefore, the differential performance of Palmer amaranth gender deserves further investigation in relation to Palmer amaranth population manipulation through management strategies that could alter canopy microenvironments at field scale. Questions related to this may offer substantial solutions for integrated weed management. Is the manipulation of Palmer amaranth population favoring either male or female dominance possible? Could various management strategies to alter the microenvironment at the field level favor expression of a particular sex? What are the long-term implications of this approach on Palmer amaranth population dynamics, and how would the soil seedbank be affected?
Moreover, the effects of aminooxyacetic acid on Palmer amaranth flowering merit further attention, particularly for late-season emergence of Palmer amaranth. The application of aminooxyacetic acid, an inhibitor of PAL (Koshio et al. Reference Koshio, Hasegawa, Okada and Takeno2015), could suppress the accumulation of phenylpropanoids (Hirai et al. Reference Hirai, Kuwano, Kojima, Koshimizu, Shinozaki and Takimoto1995) and induce flowering in Palmer amaranth. This deserves special attention, as seed prevention for Palmer amaranth escapes is of crucial importance, as it can affect the long-term population dynamics of the weed and, consequently, its soil seedbank reserves.
In addition, PAL as a regulator of phenolic compound production is indirectly related to plant chemical defensive mechanisms against insects and fungi through stilbene synthesis (Hopkins and Hüner Reference Hopkins and Hüner2009). Future research also needs to investigate whether blockage by the application of aminooxyacetic acid will increase Palmer amaranth predation. Finally, could the efficacy of photoporphyrinogen oxidase inhibitors (PPO) herbicides be improved if Palmer amaranth’s chlorophyll content under abiotic stress conditions is suppressed? Can the use of chlorophyll biosynthesis modulator herbicides alter Palmer amaranth population in relatively open crop canopies? As stated by Rebeiz et al. (Reference Rebeiz, Montazer-Zouhoor, Hopen and Wu1984), dicotyledonous weeds, including redroot pigweed, are very susceptible to photodynamic herbicides. These are some of the questions that merit further attention as the chemical solutions for Palmer amaranth control continue to narrow significantly.
Acknowledgments
The authors would like to thank Stephanie Williamson for her assistance in nutrient solution preparations.