Introduction
Among perennial species, Cyperaceae weed species are a particular problem in conservation farming systems. Navua sedge [Cyperus aromaticus (Ridl.) Mattf. & Kuek.], a C4 perennial species, is becoming an emerging and hard to control weed in tropical parts of Australia (Chadha et al. Reference Chadha, Florentine, Dhileepan, Dowling and Turville2021; Vogler et al. Reference Vogler, Carlos, Setter, Roden and Setter2015). It has been declared a noxious weed species in Fiji (Karan Reference Karan1975) and has become a problematic weed in Samoa, the Solomon Islands, and Sri Lanka (Black Reference Black1984; Vogler et al. Reference Vogler, Carlos, Setter, Roden and Setter2015). In Australia, C. aromaticus is a problematic weed species along roadsides; in pastures; and in sugarcane (Saccharum officinarum L.), banana (Musa acuminata Colla), and rice (Oryza sativa L.) production systems (Vitelli et al. Reference Vitelli, Madigan and Van Haaren2010; Vogler et al. Reference Vogler, Carlos, Setter, Roden and Setter2015). Its prolific seed production has contributed to its spread in new areas (Benson Reference Benson1992). A previous study in Fiji estimated the production of 456 million seeds ha−1 in a lowland paddock (Karan Reference Karan1975). Mowing or slashing of C. aromaticus along roadsides has further helped its seed spread in pastures. Seeds of C. aromaticus can persist >5 yr in the soil (Vitelli et al. Reference Vitelli, Madigan and Van Haaren2010). It is not readily palatable to livestock and has low nutritional value (Karan Reference Karan1975; Vitelli et al. Reference Vitelli, Madigan and Van Haaren2010). In Fiji, C. aromaticus was found to reduce the carrying capacity of pastures by 40% (Karan Reference Karan1975). Its competitive nature can completely replace pasture species such as para grass [Urochloa mutica (Forssk.) T.Q. Nguyen]. In north Queensland in Australia, it grows well in areas receiving rainfall of 1,400 mm yr−1 (Vogler et al. Reference Vogler, Carlos, Setter, Roden and Setter2015). Infestations of C. aromaticus have been observed in sugarcane and rice crops in these areas, but there is no information on the effect of C. aromaticus on rice and sugarcane yield.
Glyphosate is used along roadsides and halosulfuron-methyl is used in pastures, but their efficacy is greatly reduced when applied on large weed plants (Vitelli et al. Reference Vitelli, Madigan and Van Haaren2010; Vogler et al. Reference Vogler, Carlos, Setter, Roden and Setter2015). Further, two applications of halosulfuron are recommended, after which livestock is not allowed to graze the treated pasture for 10 wk, making the application of halosulfuron unfeasible. These issues suggest the need to integrate mechanical and cultural methods with chemical practices for C. aromaticus. However, to develop such practices, we need to understand the biology of this weed, including its seed germination ecology.
Environmental factors such as temperature, light, heat generated through fire, seed burial depth, and flooding depth can affect germination and emergence of C. aromaticus. A recent study reported variable germination of C. aromaticus at different alternating day/night temperatures, and germination varied between populations (Chadha et al. Reference Chadha, Florentine, Dhileepan, Dowling and Turville2021). These results suggest that other populations may also respond differently to temperature regimes. Similarly, light is known to affect germination of most small-seeded weed species. Seeds of C. aromaticus collected from Fiji were tested in the United Kingdom, and no germination occurred under dark conditions (Karan Reference Karan1975). It will be interesting to understand the response of Australian populations of C. aromaticus to light and dark regimes.
Fire is used in some regions to control weeds. The heat generated from a fire can also damage weed seeds. A study conducted in controlled conditions reported that seeds of C. aromaticus subjected to a temperature of 120 C for 3 min germinated (Chadha et al. Reference Chadha, Florentine, Dhileepan, Dowling and Turville2021), suggesting the need to evaluate higher temperatures, as seeds may survive at higher temperatures. The study used dry seeds, but moist or imbibed seeds may respond differently to high temperatures. In a previous study, for example, germination responses of different plant species were different for seeds subjected to moist and dry heat (Martin et al. Reference Martin, Miller and Cushwa1975).
Seed burial in the soil creates different microclimate conditions surrounding a seed, such as temperature, light, moisture, and gas availability, among others (Chauhan and Johnson Reference Chauhan and Johnson2010), all of which can affect germination and emergence of a weed species. However, such information is limited for C. aromaticus. Recently, a study reported that C. aromaticus seedlings can emerge from the surface down to a 2-cm burial depth (Chadha et al. Reference Chadha, Florentine, Dhileepan, Dowling and Turville2021). However, the study used a potting mix rather than a field soil, and the results may not be realistic, as the bulk density of potting mix can be vastly different from field soil. The literature suggests that C. aromaticus grows best in areas receiving >2,500 mm of annual rainfall (Vogler et al. Reference Vogler, Carlos, Setter, Roden and Setter2015). Such areas may experience flooding from time to time. Flooding is known to inhibit germination and growth of many weed species (Chauhan and Johnson Reference Chauhan and Johnson2010; Civico and Moody Reference Civico and Moody1979; Kent and Johnson Reference Kent and Johnson2001). However, the response of weeds depends on the timing and depth of flooding. No information is available on the effect of flooding depth on emergence of C. aromaticus.
Similar to what has been previously observed in earlier studies (Bolfrey-Arku et al. Reference Bolfrey-Arku, Chauhan and Johnson2011; Chadha et al. Reference Chadha, Florentine, Dhileepan, Dowling and Turville2021), weed populations may respond differently to environmental factors. Therefore, a study was conducted to evaluate the effect of alternating day/night temperatures, light, high “pretreatment” temperatures, burial depth, and flooding depth on germination and emergence of two populations of C. aromaticus.
Materials and Methods
Seed Collection
Plants of C. aromaticus were collected from pasture fields in Ingham and Tablelands and brought to the Gatton Research Farm of the University of Queensland, QLD, Australia. The distance between the two locations is 160 km. These plants were grown at Gatton, and the seeds were collected separately for the Ingham and Tablelands populations. Seeds were stored at room temperature (25 C). Experiments were conducted in the weed science laboratory and screenhouse facilities of the University of Queensland.
Effect of Temperature on Germination
To determine the effect of temperature on seed germination of C. aromaticus, 25 seeds of each population were placed in 9-cm-diameter petri dishes lined with two filter papers, moistened with 5 ml of tap water, incubated in different germination incubators (ICCBOD-300, Laboratory Equipment, Marrickville, NSW, Australia) set at five alternating day/night (12/12 h) temperatures (15/5, 20/10, 25/15, 30/20, and 35/25 C). These temperature regimes were selected to reflect temperature variations in different seasons in Queensland. Petri dishes were placed in sealed polythene bags to avoid moisture loss due to evaporation. Lights were provided with cool-white fluorescent lamps that produced a photosynthetic photon flux of 80 µmol m−2 s−1. Germination data were taken at an interval of 3 d for 30 d, and seeds were considered germinated when a radicle of at least 1 mm was visible. No seeds germinated beyond 30 d after incubation. There were three replications of each treatment, and the experiment was conducted in a randomized complete block design.
Effect of Light on Germination
The effect of light on seed germination of C. aromaticus was determined by placing 25 seeds of each population in petri dishes as described earlier. Seeds were placed in the light/dark (12/12 h) condition as described earlier or in complete darkness (24 h). For the completely dark condition, three layers of aluminum foil were wrapped around the dishes. These petri dishes were placed in an incubator set at 30/20 C (12/12 h). This temperature regime was found to be the optimum for seed germination of both populations of C. aromaticus in the temperature experiment. Seed germination was evaluated at 30 d after incubation using the previously described procedure. The experiment was conducted in a completely randomized design with three replications of each treatment.
Effect of High-Temperature Pretreatment on Germination
To evaluate the effect of high temperatures on seed germination of C. aromaticus, 25 seeds of each population were placed in aluminum foil dishes that were then placed in an oven for 5 min at temperatures of 50, 100, 150, and 200 C. These temperatures were selected to reflect temperatures experienced during vegetation burning. There was also a control treatment, in which seeds stored at room temperature (25 C) were used. There were two seed statuses: dry and wet seeds. Dry and wet seeds were used to simulate an environment during the dry and wet seasons, respectively. In the wet seed treatment, seeds were placed at room temperature in petri dishes with 5 ml of tap water for 48 h. These seeds were then placed in the oven. The dry seeds were not moistened before being exposed to high temperatures. The preheated seeds were then placed in an incubator set at 30/20 C, and germination was evaluated as described previously. The experiment was conducted in a randomized complete block design with three replications of each treatment.
Effect of Seed Burial Depth on Emergence
The effect of seed burial depth on seedling emergence of C. aromaticus was determined by placing 50 seeds of each population on the soil surface in 10-cm-diameter plastic pots. Seeds were covered with the same soil to achieve burial depths of 0.2, 0.5, 1.0, 2.0, and 4.0 cm. The soil used in the experiment was a clay loam with a pH of 7.2 and organic matter of 2.7%. Before use, the soil was sieved through a 2-mm sieve. Extra pots without C. aromaticus seeds were used to ensure that weed seeds were not present in the soil. No C. aromaticus seedlings emerged in these pots. Pots were placed in trays without any holes, and subirrigation was used to water pots. Seedling emergence was counted every week for 5 wk and expressed as the percentage of the total seeds used. The pots were placed in a screenhouse at the weed science facility of the University of Queensland. The light intensity in the screenhouse was 80% of the outdoor light intensity, and the average maximum and minimum temperatures during the experiment were 35.5 and 18.7 C, respectively. The experiment was conducted in a randomized complete block design with three replications of each experiment.
Effect of Flooding Depth on Emergence
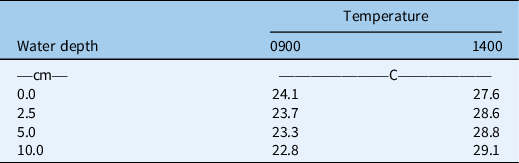
To evaluate the effect of flooding depth on seedling emergence of C. aromaticus, 50 seeds of each population were placed uniformly on the soil surface in plastic trays (10 cm by 10 cm by 5 cm). The soil used in this experiment was as described for the seed burial experiment. The soil was moistened to saturation using a fine mist sprayer, and then the trays with the soil and seeds were transferred to large containers (40 cm by 20 cm by 20 cm) to retain water and maintain the required water depth (0 [saturated], 2.5, 5.0, and 10.0 cm). Seedling emergence was counted every week for 5 wk and expressed as the percentage of the total seeds. The experiment was conducted in the screenhouse, and temperature and light conditions were as described above in the seed burial depth experiment. The experiment was conducted in a randomized complete block design with three replications of each treatment. Water temperature at 0-, 2.5-, 5.0-, and 10.0-cm flooding depths was measured at 0900 and 1400 hours daily during the experimental duration (Table 1).
Table 1. Mean temperatures measured at different water depths at 0900 and 1400 hours during the flooding experiment.

Statistical Analyses
Each experiment was repeated once over time, and the second run was started within 2 wk after the termination of the first run. Data were combined over experimental runs, as there was no interaction between the treatments and runs, and the run effect was nonsignificant. Data variance was inspected by plotting residuals to confirm the homogeneity of variance before statistical analysis (Genstat 2019). Nonlinear regression was used for analysis where appropriate; otherwise, means were separated using the LSD at the 5% level of significance.
Germination data of the alternating temperature and high-temperature pretreatment experiments were modeled using a three-parameter sigmoid model (Chauhan and Johnson Reference Chauhan and Johnson2009b). The model was
In the curve, G is the cumulative germination at time x, a is the maximum germination (%), T 50 is the time (d) required for 50% germination of the maximum germination, and b is the slope of the curve. As there were many curves for a single experiment, the curves are shown on separate graphs for each temperature. In addition, cumulative germination resulting from the alternating day/night temperatures and high-temperature pretreatment studies were analyzed using ANOVA.
Emergence resulting from burial- and flooding-depth treatments was modeled using an exponential decay curve (Chauhan and Johnson Reference Chauhan and Johnson2009b):
In the curve, E is cumulative emergence (%) at burial or flooding depth x, a is the maximum emergence, and b is the slope of the curve.
Results and Discussion
Effect of Temperature on Germination
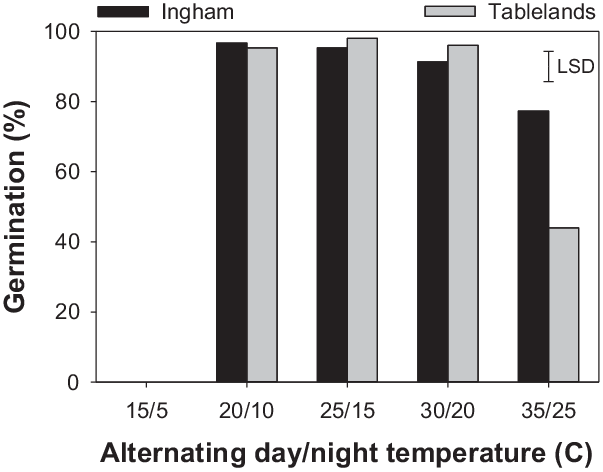
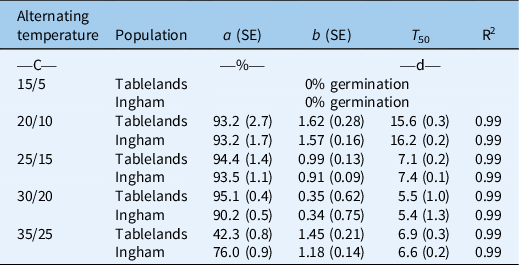
The interaction effect of alternating day/night temperature and population was significant for the cumulative germination of C. aromaticus (Figure 1). Germination was similar between the populations at 20/10, 25/15, and 30/20 C; however, at 35/25, the Ingham population had greater germination than the Tablelands population. No seeds germinated at the lowest temperature regime (15/5 C). Maximum germination (a) was similar at temperatures ranging from 20/10 C to 30/20 C (Table 2; Figure 2); however, the fastest germination (b) occurred at 30/20 C. Therefore, 30/20 C was selected for subsequent experiments. Seeds of both populations took the longest time (15 d) to achieve 50% germination (T 50) at 20/10 C and the shortest time (5 to 6 d) at 30/20 C.
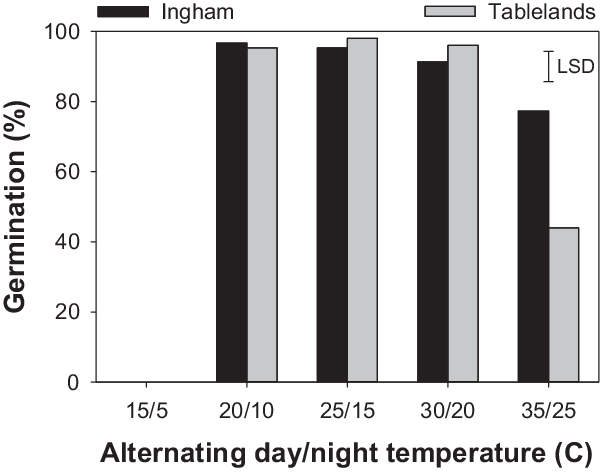
Figure 1. Effect of alternating day/night (12/12 h) temperatures on cumulative germination of freshly harvested seeds of Ingham and Tablelands populations of Cyperus aromaticus. The error bar is the LSD at P = 0.05.
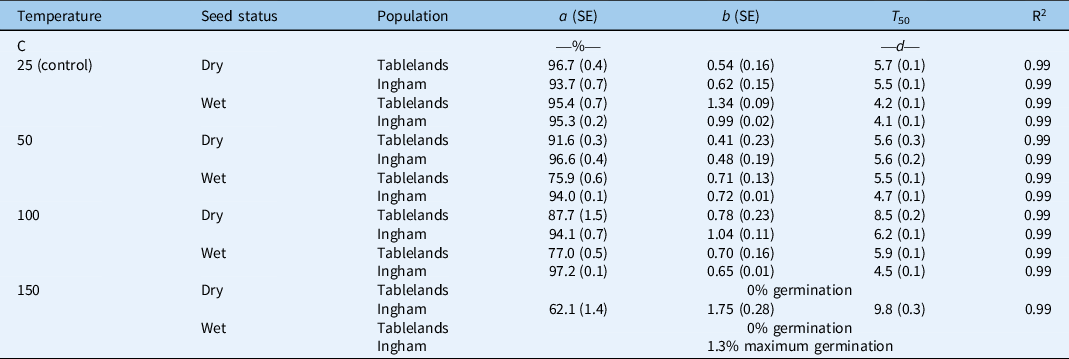
Table 2. Parameter estimates (a, maximum germination [%]; b, slope; and T 50, time to reach 50% of maximum germination [d]) of a three-parameter sigmoid model fit to seed germination of Tablelands and Ingham populations of Cyperus aromaticus as shown in Figure 2. a
a Values in parentheses represent ± standard error of the mean (SE).
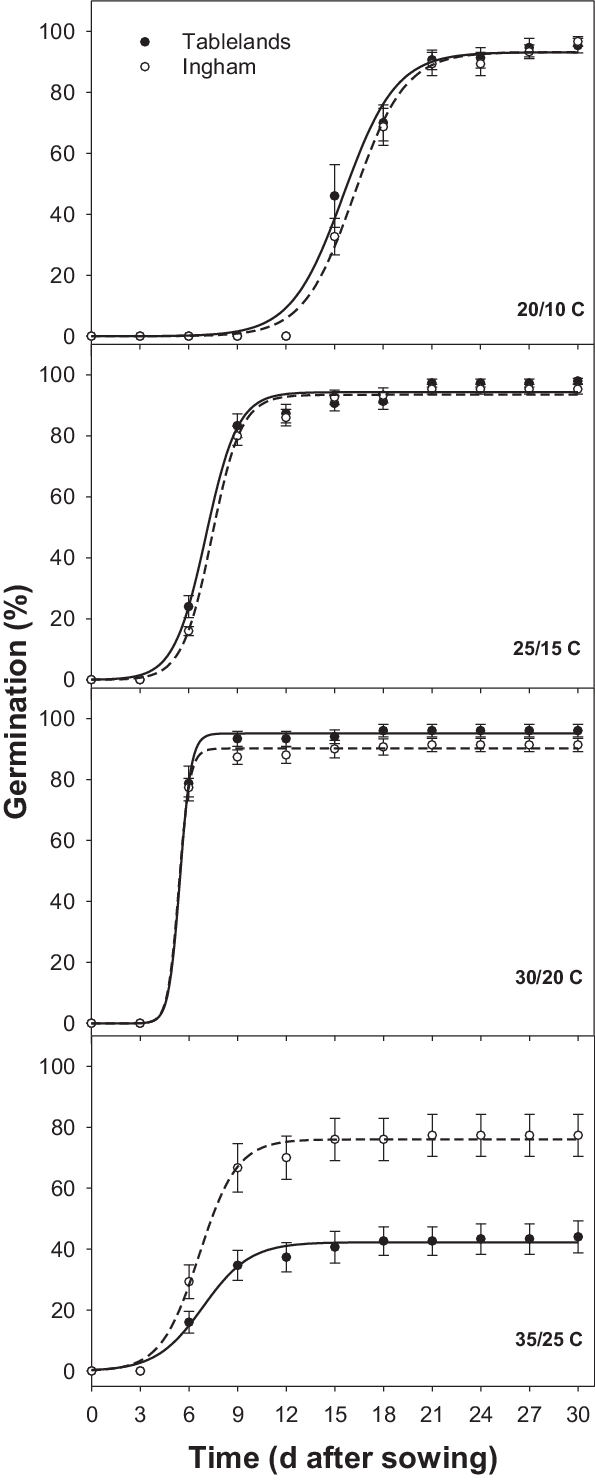
Figure 2. Effect of alternating day/night (12/12 h) temperatures on germination of freshly harvested seeds of Ingham and Tablelands populations of Cyperus aromaticus in light/dark conditions, modeled with the use of the equation G = a/(1 + e [−(x – T 50)/b]), with estimated parameters given in Table 2. Error bars are standard error of the mean.
A previous study reported 99% germination of C. aromaticus at 25/15 C (16/8 h or 8/16 h) day/night temperature and 98% germination at 25/5 C (16/8 h) (Karan Reference Karan1975). However, the study did not use higher or lower temperatures. A recent study reported that C. aromaticus populations can germinate at temperatures ranging from 17/7 to 35/25 C; however, the optimum temperature varied between populations (Chadha et al. Reference Chadha, Florentine, Dhileepan, Dowling and Turville2021). The optimum temperature regime in the present study was the same (30/20 C) for both populations. Populations responded differently at the highest temperature regime (35/25 C). The greater germination ability of the Ingham population at 35/25 C suggests that this population is more heat tolerant than the Tablelands population and can germinate at the high temperatures that occur in the hot summer months. Fresh seeds were used in this study, and the >90% germination that was recorded immediately after maturity suggests that this species does not have innate dormancy. The results suggest that most seeds can germinate immediately after maturity if there is enough moisture. These results also suggest that the stale seedbed technique can be used to stimulate germination of freshly matured seeds and then kill emerged seedlings using tillage or herbicides. Seeds of either population did not germinate at 15/5 C, suggesting that the seed will remain ungerminated in the cold months in Queensland and will germinate when the weather starts warming up. High (>90%) germination at higher temperature regimes suggests that seeds of C. aromaticus can germinate most time of the year in north Queensland.
Effect of Light on Germination
Seeds of both populations of C. aromaticus did not germinate in complete darkness, while seed germination in the light/dark regime was 93% for the Ingham population and 95% for the Tablelands population (data not shown). Similar results were reported in the study conducted in Fiji, in which no germination occurred in darkness (Karan Reference Karan1975). A recent study in Australia also reported that light is absolutely required to stimulate germination in C. aromaticus (Chadha et al. Reference Chadha, Florentine, Dhileepan, Dowling and Turville2021). Other Cyperaceae species that showed no germination in the dark are variable flatsedge (Cyperus difformis L.), rice flatsedge (Cyperus iria L.), and forked fringerush [Fimbristylis miliacea (L.) Vahl.] (Chauhan and Johnson Reference Chauhan and Johnson2009a).
The germination response to light is species specific. Some seeds germinate equally in light and dark (Baskin and Baskin Reference Baskin and Baskin1998) and others, for example, C. aromaticus, have an absolute light requirement for germination. A light-absorbing pigment is thought to control the germination response to light. In seeds such as C. aromaticus, light may convert inactive phytochrome red to the active phytochrome far-red (Rollin Reference Rollin1972). The absolute light requirement for germination means seeds of C. aromaticus present on the soil surface will have greater germination than buried seeds. Conservation farming systems that practice no or minimum tillage, for example, pastures, fruit plantations, or sugarcane fields, will have greater infestations of C. aromaticus compared with conventional tillage farming systems. The results also suggest that seeds may not germinate under a dense crop canopy or when buried deep in the soil.
Effect of High-Temperature Pretreatment on Germination
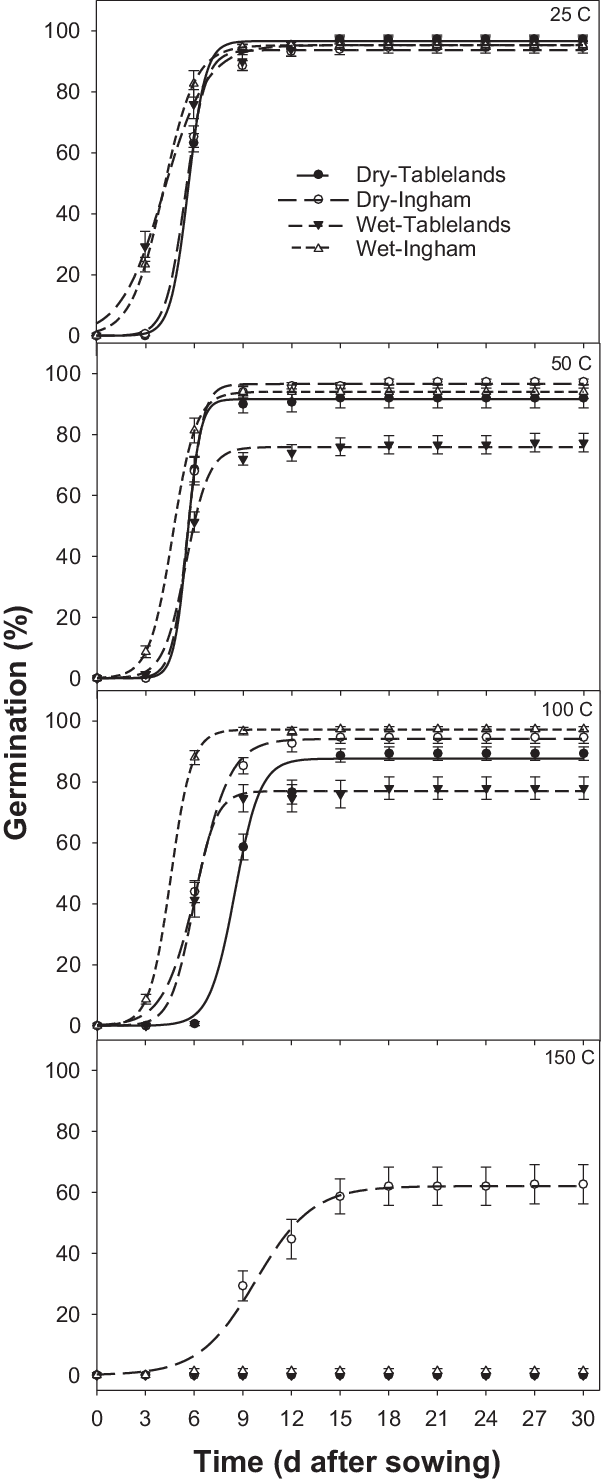
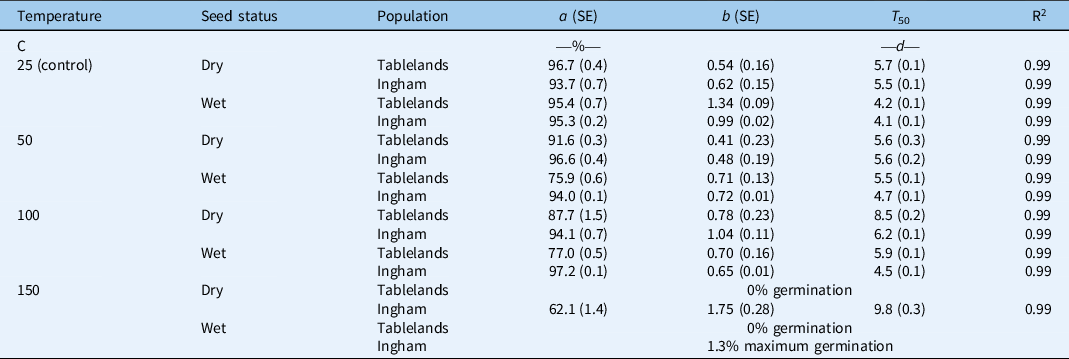
The interaction effect between population, seed status (dry or wet), and pretreatment temperature was significant on cumulative germination of C. aromaticus (Figure 3). In general, high pretreatment temperature affected germination of wet seeds more than dry seeds. Germination was similar between the population and seed status for seeds placed at room temperature (25 C, control treatment) (Figures 3 and 4; Table 3). The pretreatment temperature of 50 and 100 C did not affect germination (94% to 97%) of the Ingham population for both dry and wet seeds. However, germination of wet seeds (76% to 77%) from the Tablelands population was lower at 50 and 100 C than germination of dry seeds (88% to 92%). Germination of the Tablelands population was completely inhibited at pretreatment of ≥150 C, but the Ingham population germinated after being exposed to 150 C. More than 60% of the dry seeds of the Ingham population germinated following the pretreatment temperature of 150 C, and 1% of wet seeds germinated at this temperature. The fitted model suggested that the Tablelands population took longer to achieve 50% germination (T 50) of the maximum germination than the Ingham population. Irrespective of population, dry seeds took longer to achieve 50% germination (T 50) than the wet seeds.
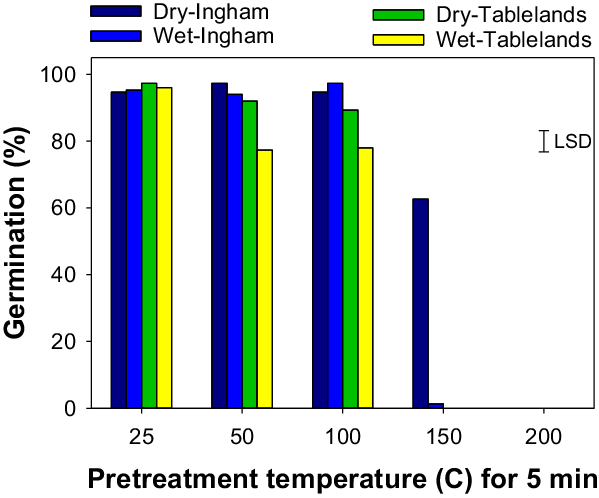
Figure 3. Effect of pretreatment temperature for 5 min on cumulative germination of Ingham and Tablelands populations of Cyperus aromaticus seeds (dry and wet) after 30 d of incubation in light/dark at 30/20 C alternating day/night temperature. The error bar is the LSD at P = 0.05.
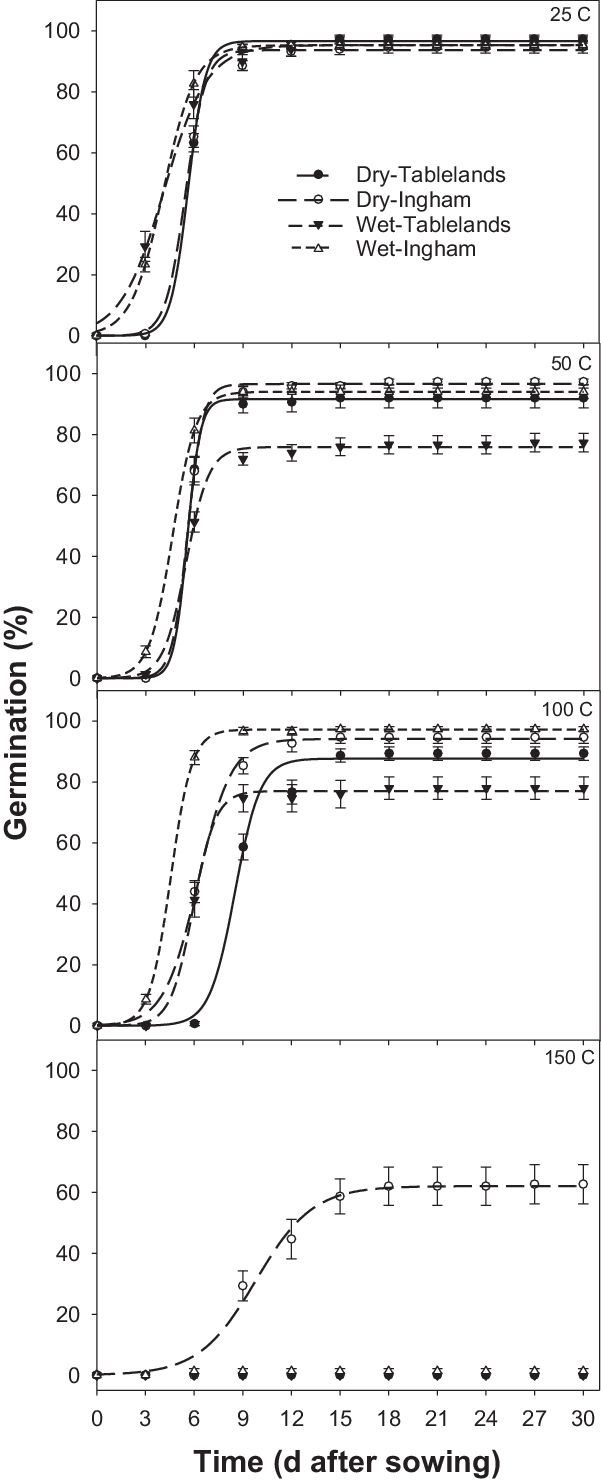
Figure 4. Effect of pretreatment temperature for 5 min on germination of freshly harvested seeds of Ingham and Tablelands populations of Cyperus aromaticus seeds (dry and wet) in light/dark at 30/20 C alternating day/night temperature, modeled with the use of the equation G = a/(1 + e [−(x – T 50)/b]), with estimated parameters given in Table 3. Error bars are standard error of the mean.
Table 3. Parameter estimates (a, maximum germination [%]; b, slope; and T 50, time to reach 50% of maximum germination [d]) of a three-parameter sigmoid model fit to seed germination of Tablelands and Ingham populations of Cyperus aromaticus as shown in Figure 4. a
a Values in parentheses represent ± standard error of the mean (SE).
A similar study in Australia reported that a pretreatment temperature of 100 C for 3 min reduced germination of C. aromaticus compared with the control treatment, and only dry seeds were used in the study (Chadha et al. Reference Chadha, Florentine, Dhileepan, Dowling and Turville2021). The current study found differential responses between the two populations. The germination of the Ingham population was not affected by the pretreatment temperature of 100 C, and seeds of this population germinated after exposure to 150 C for 5 min, suggesting that the Ingham population is more tolerant to high temperatures than the Tablelands population. The results also suggest that moist heat will affect germination more than dry heat. These results were also supported by a previous study that reported that the germination of seeds exposed to moist heat was inhibited at a lower temperature than dry heat-treated seeds (Martin et al. Reference Martin, Miller and Cushwa1975).
Fire, or burning, is used in some tropical areas to destroy existing vegetation and weed seeds. The exposure of seeds to high temperatures generated by fire results in embryo damage (Jeffery et al. Reference Jeffery, Holmes and Rebelo1988; Willis et al. Reference Willis, Mckay, Vranjic, Kilby and Groves2003). Burning is a common practice for clearing vegetation and controlling weeds before sowing a crop in some Asian and African countries (Bolfrey-Arku et al. Reference Bolfrey-Arku, Onokpise, Carson, Shilling and Coultas2006; Roder et al. Reference Roder, Phengchanh and Keoboulapha1997). During burning, the surface temperature may rise to 500 C (Bebawi and Campbell Reference Bebawi and Campbell2002), and the data of the present study suggest that fire can be used to destroy seeds of C. aromaticus on the soil surface. It is important to point out here that crop residue burning may not increase the temperature to this extent, but the burning of trees and shrubs may be able to achieve this temperature. Soil temperature of 200 C for 5 min will be able to kill most seeds of C. aromaticus.
Effect of Seed Burial Depth on Emergence
Seed burial depth strongly affected seedling emergence in both populations of C. aromaticus, and each population responded differently (Figure 5). Germination was greatest for the seeds placed on the soil surface (78% and 71% for the Ingham and Tablelands populations, respectively). Seedling emergence declined sharply with an increase in burial depth. No seedlings of the Tablelands population emerged from 0.5 or 1.0 cm, but 4% and 2% of seedlings from the Ingham population emerged from 0.5 and 1.0 cm, respectively. Irrespective of population, seedlings of both populations did not emerge from 2-cm or greater depths.
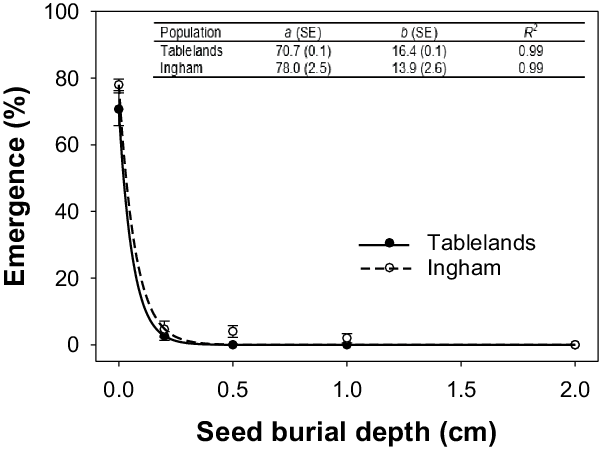
Figure 5. Effect of burial depth on seedling emergence of Ingham and Tablelands populations of Cyperus aromaticus, modeled with the use of the equation E = a × e −bx . Error bars are standard error of the mean.
The results of the present study are different from what had been reported in a recent study (Chadha et al. Reference Chadha, Florentine, Dhileepan, Dowling and Turville2021). In the present study, seedling emergence at 0.5-cm depth was reduced by 95% to 100%, but the published study reported >80% emergence of C. aromaticus from 0.5 cm and >55% from 1 cm. For one population, emergence was 6% from a burial depth of 2 cm. There could be several reasons for this difference, but the most important is the different media used in both studies. The previous study used potting mix, and the current study used field soil. Potting mix usually contains a remarkably high amount of organic matter, which can greatly reduce the bulk density of the media. Bulk density is well known to influence seedling emergence (Nasr and Selles Reference Nasr and Selles1995; Zuo et al. Reference Zuo, Kuai, Zhao, Hu, Wu and Zhou2017). Differential soil compaction by different personnel can also affect seedling emergence.
The results of the current study are consistent with the light experiment, in which no seeds germinated in darkness. Seeds buried at 0.5 or 1.0 cm receive very limited incident light (Woolley and Stoller Reference Woolley and Stoller1978), and this could be the main reason for very low or no emergence of buried seeds. Small seed size (200 mg per seed) could also be another reason for low emergence from depths, as such seeds may not have enough carbohydrate reserves to support seedling emergence from greater depths (Baskin et al. Reference Baskin, Milberg, Andersson and Baskin2004). Our data are consistent with results reported for three other Cyperaceae weeds—C. difformis, C. iria, and F. miliacea—in which their small seed size and absolute light requirement did not allow emergence from seeds buried at 1 cm (Chauhan and Johnson Reference Chauhan and Johnson2009a).
The Ingham population was able to emerge from a depth of 1 cm, but not the Tablelands population, suggesting a greater potential of the Ingham population to become more invasive. Regardless of population, the results suggest that conservation farming systems with no or limited soil disturbance will favor emergence of this weed species. These results are supported by their heavy infestations observed in pastures and along roadsides, which receive limited or no soil disturbance. Burying C. aromaticus seeds below their maximum depth of emergence could help suppress this weed. However, subsequent tillage operations should be avoided for several years, as those operations may bring the seeds back to the soil surface. The seedbank of C. aromaticus can persist more than 5 yr in the soil (Vitelli et al. Reference Vitelli, Madigan and Van Haaren2010). It should also be noted that burial will only be effective for the suppression of emergence from seeds. Cyperus aromaticus is a perennial species and produces rhizomes. The rhizomes can emerge from greater depths, and tillage operations may increase shoot emergence by breaking apical dormancy of the rhizomes.
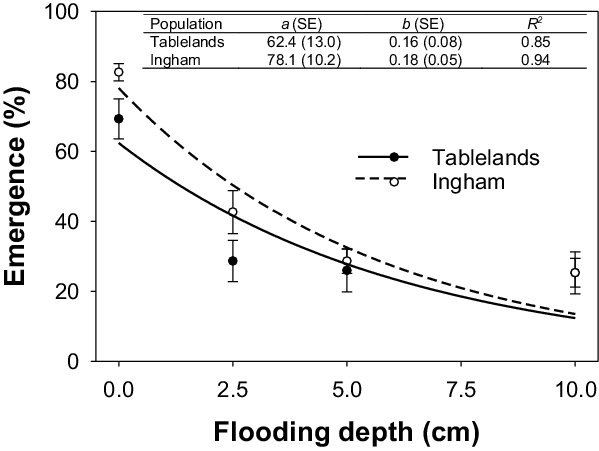
Effect of Flooding Depth on Emergence
Flooding significantly affected the seedling emergence of both populations of C. aromaticus (Figure 6). However, greater emergence was observed for the Ingham population compared with the Tablelands population. Maximum germination was observed for seeds placed in saturated conditions (i.e., 0-cm flooding depth); 78% for Ingham and 62% for Tablelands. Flooding to a depth of 2.5 cm resulted in 50% emergence of the Ingham population, whereas 42% of seedlings emerged from the Tablelands population. About 12% to 14% of seedlings emerged at the flooding depth of 10 cm.
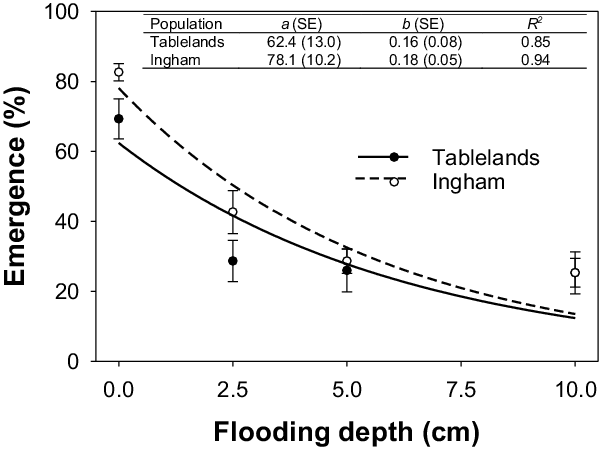
Figure 6. Effect of flooding depth (0, 2.5, 5.0, and 10.0 cm) on seedling emergence of Ingham and Tablelands populations of Cyperus aromaticus, modeled with the use of the equation E = a × e −bx . Error bars are standard error of the mean.
Flooding is known to inhibit emergence and growth of many weed species; however, the response is thought to be species specific (Chauhan and Johnson Reference Chauhan and Johnson2010). Weed species such as goose weed (Sphenoclea zeylanica Gaertn.) are not suppressed by flooding, whereas species such as C. iria and F. miliacea, can be controlled by flooding (Chauhan and Johnson Reference Chauhan and Johnson2009a; Civico and Moody Reference Civico and Moody1979). Flooding greatly reduced emergence of C. aromaticus in the current study, but a flooding depth of 10 cm was needed to reduce emergence by >80% compared with 0 cm. It must be acknowledged here that C. aromaticus seedlings remained noticeably short (visual data) and never emerged (35 d) above the water surface. These results suggest that continuous standing water will provide complete suppression of C. aromaticus biomass. However, growers may not have sufficient water to use continuous flooding as a weed control mechanism (Tuong et al. Reference Tuong, Bouman and Mortimer2005), and seedlings can grow during the intermittent flooding phase.
Flooding inhibits seedling emergence by affecting chemical and physical properties of the soil (Smith and Fox Reference Smith and Fox1973). Flooding can reduce oxygen, increase carbon dioxide, and increase toxic gaseous products. Smaller temperature fluctuations in deep water may also be responsible for lower weed germination in the water. However, this was not the case in our study (Table 1). The current study was conducted in controlled conditions using clear water, but turbid conditions in the field situations may further reduce emergence of C. aromaticus by reducing light penetration to the soil surface (Chauhan and Johnson Reference Chauhan and Johnson2010).
In conclusion, the present study evaluated the germination and emergence response of two populations of C. aromaticus to some environmental factors. Fresh seeds of C. aromaticus germinated at alternating day/night temperatures ranging from 20/10 to 35/25 C, suggesting that the weed has no innate dormancy and can germinate at any time of the year in north Queensland. Darkness completely inhibited germination, implying that shade under a crop canopy and crop residue as mulch can suppress germination of C. aromaticus. Pretreatment temperatures of 150 to 200 C completely inhibited germination of C. aromaticus, indicating that fire can be used to reduce the seedbank of C. aromaticus on the soil surface. Shallow burial of seeds to 2 cm completely inhibited emergence, suggesting the use of tillage to bury seeds below the maximum depth of emergence. Maximum germination on the soil surface and an absolute requirement of light for germination suggest that farming practices, with no or minimal soil disturbance (e.g., pastures, roadsides, fence line, fruit plantations, etc.) will favor greater germination of C. aromaticus. When and where possible, flooding can be used to suppress emergence of C. aromaticus; however, the flooding depth needs to be 10 cm or greater, and flooding needs to be continuous. In general, the Ingham population showed greater germination or emergence than the Tablelands population in response to different levels of environmental factors, suggesting that the Ingham population has a greater potential for spreading in new areas and for increasing further infestations in the same area. These observations warrant the study of more populations (seeds as well as rhizomes) of C. aromaticus from Queensland to explore the mechanism of their differential responses to environmental factors.
Acknowledgments
This research received no funding. The author declares no conflicts of interest. The author acknowledge the consultants for collecting the plants for this study.