Introduction
Watermelon [Citrullus lanatus (Thunb.) Matsum & Nakai] is a high-value crop with a worldwide production of 3.5 million hectares (FAO 2017). Although the United States only accounts for 1.5% to 2.0% of the global crop annually, U.S. watermelon production is valued at US$514 million (USDA-NASS 2017). The majority of U.S. watermelon production occurs in Florida, California, and Texas; however, with a statewide harvest valued at US$34.9 million (USDA-ERS 2013), watermelon is an important economic crop in North Carolina.
To maximize yields, watermelon growers have adopted the use of polyethylene mulch, preplant fumigation, high-yielding cultivars, transplanting rather than direct seeding, drip fertigation, and other strategic cultural practices. Polyethylene mulch facilitates preplant fumigation, controls or suppresses many weeds, reduces evaporation, and can increase yields (Lament Reference Lament1993). Transplanting watermelon results in earlier and increased yields compared with direct-seeded watermelon (Olson et al. Reference Olson, Hochmuth and Hochmuth1994). In addition, transplanting has been widely adopted due to the popularity of triploid (seedless) watermelon, which has specific germination requirements and may not emerge uniformly under field conditions when direct seeded (Maynard Reference Maynard1989; Maynard and Elmstrom Reference Maynard and Elmstrom1992). Thus, watermelon production involves substantial investment on the part of growers, and losses due to weeds or other agricultural pests should be limited or prevented.
Watermelon growers in East Asia and in Europe have shifted from open-field production to high-tunnel production, which reduces or eliminates the potential for traditional crop rotation (Lee and Oda Reference Lee and Oda2003). These practices represent an intensification of watermelon production with repeated plantings in the same beds from year to year. Repeated plantings lead to increased disease pressure, particularly from soilborne pathogens that can become problematic with successive crops (Zitter et al. Reference Zitter, Hopkins and Thomas1996). Intensification of watermelon production and the loss of methyl bromide as a soil fumigant have caused growers to seek alternative measures such as grafting as part of an integrated pest management approach (Louws et al. Reference Louws, Rivard and Kubota2010; Sakata et al. Reference Sakata, Ohara and Sugiyama2007).
Grafting is a technique that combines the shoot of one plant (the scion) with the rootstock of another cultivar or species. The scion serves as the fruiting portion and is selected for high yield and/or high-quality fruit, while the rootstock is selected for resistance to diseases caused by soilborne pathogens, increased nutrient uptake, or tolerance of environmental stress (Keinath and Hassell Reference Keinath and Hassell2013; Miguel et al. Reference Miguel, Maroto, Bautista, Baixauli, Cebolla, Pascual, Lopez and Guardiola2004; Yetisir et al. Reference Yetisir, Çaliskan, Soylu and Sakar2006). Modern vegetable grafting was first introduced in watermelon production in the 1920s, when Japanese growers grafted watermelon to crookneck squash (Cucurbita moschata Duchesne) rootstocks to provide resistance to Fusarium wilt (Fusarim oxysporum f. sp. niveum) (Sakata et al. Reference Sakata, Ohara and Sugiyama2007; Tateishi Reference Tateishi1927). Grafting watermelon has become a common practice in many parts of the world, including China, Spain, Italy, and Israel, but it has yet to be explored as a viable management tactic on a wide scale in the United States (Kubota et al. Reference Kubota, McClure, Kokalis-Burelle, Bausher and Rosskopf2008).
Historically, growers have adopted grafting for disease resistance benefits rather than potential yield increases or increased vigor (Lee and Oda Reference Lee and Oda2003). Grafting onto vigorous rootstocks may affect the crop–weed competitive relationship. Grafted plants reportedly grow at different rates than nongrafted plants (Lee Reference Lee1994), which may affect the duration of the critical period for weed control (CPWC) or cause the CPWC to occur at different times within the season (Chaudhari et al. Reference Chaudhari, Jennings, Monks, Jordan, Gunter, McGowen and Louws2016). If left uncontrolled, weeds can cause substantial reductions in watermelon yield. Season-long interference by large crabgrass [Digitaria sanguinalis (L.) Scop.] in bareground watermelon production can cause yield loss up to 82% (Monks and Schultheis Reference Monks and Schultheis1998). In a polyethylene mulch system, interference of American black nightshade (Solanum americanum Mill.) can cause up to 60% yield loss of marketable fruit (Adkins et al. Reference Adkins, Stall, Santos, Olson and Ferrell2010). Watermelon yields were reduced by 40% when exposed to season-long interference by yellow nutsedge (Cyperus esculentus L.) at a density of 12 C. esculentus plants m−2 (Buker et al. Reference Buker, Stall, Olson and Schilling2003).
In all cropping systems, a CPWC exists during which weeds must be controlled to prevent crop yield loss. The CPWC coincides with two experimentally determined periods of weed interference: (1) the critical time of weed removal or maximum duration of time that weeds can grow and interfere with the crop before unacceptable yield and quality loss occurs and (2) the critical weed-free period or minimum length of time that crops must be maintained weed free to prevent loss in crop yield or quality (Knezevic et al. Reference Knezevic, Evans, Blankenship, Van Acker and Lindquist2002). The CPWC can vary depending on the competitive nature of the crop and weed species, environmental conditions, and crop management, such as row spacing, planting density, or mulching (Ahmadvand et al. Reference Ahmadvand, Mondani and Golzardi2009; Knezevic et al. Reference Knezevic, Evans, Blankenship, Van Acker and Lindquist2002; Radosevich et al. Reference Radosevich, Holt and Ghersa1997; Tursun et al. Reference Tursun, Datta, Tuncel, Kantarci and Knezevic2015, Reference Tursun, Datta, Budak, Kantarci and Knezevic2016). If grafted watermelon exhibit greater early-season growth, more rapid canopy closure, or increased vigor, it would be expected that grafted plants have a shorter CPWC due to an enhanced weed competitive ability of the grafted plants. The duration of the CPWC was similar for grafted (2.3 wk) and nongrafted (2.5 wk) tomato (Solanum lycopersicum L.); however, the CPWC began and ended 1 wk earlier in the grafted treatment (Chaudhari et al. Reference Chaudhari, Jennings, Monks, Jordan, Gunter, McGowen and Louws2016). Thus, grafting did not eliminate or reduce the need for timely weed control in tomatoes; instead, weed control must be enacted 1 wk earlier in grafted plants relative to nongrafted plants. The purpose of the current study was to determine whether grafting influenced the CPWC of watermelon grown in plasticulture.
Materials and Methods
Field studies were conducted at the Horticultural Crops Research Station (35.028°N, 78.288°W) near Clinton, NC, in 2015 and 2016. Soil was an Orangeburg loamy sand (fine-loamy, kaolinitic, thermic Typic Kandiudults) with pH 5.9 and 0.9% organic matter and pH 6.1 and 0.8% organic matter in 2015 and 2016, respectively.
Grafting treatments included two cucurbit rootstocks and a nongrafted control. ‘Exclamation’ (Syngenta Seeds, Greensboro, NC) triploid watermelon was used as the scion for all grafted plants as well as the nongrafted control. Rootstocks included ‘Carnivor’ and ‘Kazako’ (Syngenta Seeds), which are interspecific hybrids (ISHs) derived from a cross between two squash species (Cucurbita maxima Duchesne × Cucurbita moschata Duchesne). These rootstocks are used to impart resistance to Fusarium wilt of watermelon (Kleinhenz Reference Kleinhenz2015), although Carnivor reportedly produces higher yields and grows more vigorously than Kazako (D Liere, personal communication). All seeds were coated with thiram fungicide (42-S Thiram, Bayer CropScience, Research Triangle Park, NC), applied by the seed company (Syngenta Seeds). Watermelon and ISH seeds were sown in premoistened Tobacco Soil Mix (Carolina Soil Company, Kinston, NC) in 72-cell trays (T.O. Plastics, Clearwater, MN), and the germination procedure was similar to that of Hassell and Schultheis (Reference Hassell and Schultheis2004). To ensure scions and ISH rootstocks were the same size at the time of grafting, the seeds for Exclamation scions were sown 4 d before the seeds for rootstocks Carnivor and Kazako. To prevent in-season growth of rootstock vines (or “growback”) in the field, rootstock apical meristems were treated with a 6.25% dilution of Fair 85 ® fatty alcohol solution (Fair Products, Cary, NC) (Daley and Hassell Reference Daley and Hassell2014). At 24 h before grafting, plants were brought indoors to a cool, dark room to halt photosynthesis and reduce respiration. Grafting was conducted by hand using the one-cotyledon grafting method (Hassell et al. Reference Hassell, Memmott and Liere2008). Immediately following grafting, plants were placed in a shaded healing chamber to prevent desiccation of the scion. The healing chamber maintained a low-light and high-humidity (>95% relative humidity, 23 to 27°C) environment that slowed photosynthesis and allowed the graft union to heal. To account for delayed growth while grafted plants were in the healing chamber, Exclamation seeds for the nongrafted plants were sown 6 d after sowing Exclamation scions for grafting. All plants had 3 to 5 true leaves at the time of transplanting. In 2016, grafted plants were purchased from Tri-Hishtil (Mills River, NC), and nongrafted Exclamation plants were prepared in the same manner as in 2015.
Digitaria sanguinalis, common purslane (Portulaca oleracea L.), and C. esculentus were selected for use in this study because they are among the 10 most common weeds in North Carolina watermelon fields (Webster Reference Webster2010). Rather than determining the CPWC for each individual weed, this trial was designed to measure the CPWC for the mixed community of weeds, which is more representative of a grower’s field. Weed seedlings were propagated in a greenhouse before being transplanted in the field to ensure uniform size and density. Weed seeds and tubers were sown into 288-cell trays using Fafard® 4P potting mix (Sun Gro, Agawam, MA) and managed until transplanting following a protocol established by Chaudhari et al. (Reference Chaudhari, Jennings, Monks, Jordan, Gunter, McGowen and Louws2016). Seeds of D. sanguinalis and P. oleracea were sown 21 d before the date of transplanting, and C. esculentus tubers were sown 14 d before transplanting. At the time of transplanting, average heights of D. sanguinalis, P. oleracea, and C. esculentus were 13.2, 7.8, and 11.8 cm (2015) and 14.4, 9.2, and 14.4 cm (2016), respectively.
To prepare fields, beds (15-cm high by 76-cm wide) were formed on 3-m centers, drip tape was laid (8-cm depth), and beds were covered with black polyethylene mulch. As beds were formed, Pic-Clor 60 fumigant (TriEst Ag Group, Greenville, NC) was applied using a 1-row Reddick Fumigant Mulch Layer (TriEst Ag Group), delivering chloropicrin and 1,3-dichloropropene at rates of 174 kg ai ha−1 and 114 kg ai ha−1, respectively, on April 29, 2015, and April 19, 2016. Soil moisture was appropriate for fumigation according to the USDA feel and appearance method (USDA-NRCS 1998), and soil and air temperatures did not reach 37.8 C for a minimum of 3 d before fumigation. Beds were formed a minimum of 21 d before watermelon transplanting, which occurred on May 19, 2015, and May 17, 2016. At 24 h before transplanting, holes were punched in polyethylene mulch using a water wheel. Each experimental unit was a 7.6-m plot with 10 watermelon seedlings transplanted at 76-cm in-row spacing with 3-m between-row spacing and a 3-m alley separating plots within a bed. Due to less success with grafting using Kazako rootstock, 8 and 7 seedlings were transplanted in plots in 2015 and 2016, respectively. SP-6 pollenizer seedlings (Syngenta Seeds) were transplanted within the plots, between every third triploid seedling, to ensure proper fruit set for the triploid watermelons (Dittmar et al. Reference Dittmar, Monks and Schultheis2010). Fertilizer was applied through drip irrigation in accordance with conventional watermelon production in North Carolina (Kemble Reference Kemble2017). Foliar applications of insecticides and fungicides were made as necessary based on disease forecasting and field scouting in accordance with conventional watermelon production in North Carolina (Kemble Reference Kemble2017). Row middles were maintained weed free by shallow cultivation between rows, hoeing, and hand weeding throughout the season.
For weed treatments, a 15 by 15 cm square was cut from the polyethylene mulch and centered on each triploid watermelon plant, and two seedlings of each weed species (D. sanguinalis, P. oleracea, and C. esculentus) were transplanted alongside each watermelon plant in an alternating pattern, totaling 6 weed seedlings per watermelon plant. Weeds were not established alongside SP-6 pollenizers. In the establishment study (EST), the three weed species were established at 0, 2, 3, 4, and 6 wk after watermelon transplanting (WATr) and remained until the final watermelon harvest to simulate weeds emerging at different times during the season. In the removal study (REM), the three weed species were transplanted at the time of watermelon transplanting and then removed at 2, 3, 4, 6, and 11 WATr, with the bed kept weed free thereafter to simulate weed control beginning at different times during the season. Weedy and weed-free treatments were present in both studies and both years. Weedy treatments included the establishment of weeds at 0 WATr and removal of weeds at 11 WATr. Weed-free treatments had no weeds transplanted into plots and included removal of weeds at 0 WATr and establishment of weeds at 11 WATr. The treatments in the study were arranged in a randomized complete block design with 4 replications.
Fresh aboveground weed biomass was collected from 5 planting holes per plot by cutting weeds at the soil line at the time of each weed removal for the REM study or at final watermelon harvest (11 WATr) for the EST study. Weeds were then oven-dried (70 C for 4 to 7 d), and dry aboveground weed biomass was recorded.
Watermelon was harvested two times, 1 wk apart, beginning at the earliest date that ripe fruit was observed in the field. Ripeness was determined by scouting for a distinct yellow ground spot on the fruit surface, breakdown of epicuticular wax, and a senescent tendril on the vine proximal from the fruit. The first harvest of each season occurred on July 28, 2015, and on July 27, 2016, respectively. At harvest, each fruit was weighed individually. Any fruit weighing ≥4 kg was classified as marketable (Schultheis and Thompson Reference Schultheis and Thompson2014). Yield and fruit count for Kazako, which had a lower graft success, were adjusted based on number of watermelon plants per plot. Before analysis, total yield, marketable yield, total fruit count, and marketable fruit count were summed across harvests for each year.
At each harvest, 5 marketable fruits from each plot were cut longitudinally to score for hollow heart and were rated on a 0 to 4 scale (0 representing no hollow heart and 4 representing severe hollow heart [>3-cm width, >10.2-cm depth]) (Dittmar et al. Reference Dittmar, Monks and Schultheis2010). Three ripe fruits per plot were measured for determination of soluble solids content (SSC) with a handheld refractometer (Atago 3810 Pal-1 digital refractometer, Atago, Bellevue, WA).
EST and REM studies were conducted in separate but adjacent fields similar to other CPWC study designs (Chaudhari et al. Reference Chaudhari, Jennings, Monks, Jordan, Gunter, McGowen and Louws2016). The two studies were designed with the intention to overlay the data onto one graph to determine the CPWC, the period when weeds must be controlled to reach a yield threshold below the acceptable yield loss (AYL). Relative to weed-free controls, the conventional 5% AYL was used to determine the dates for the CPWC (Knezevic et al. Reference Knezevic, Evans, Blankenship, Van Acker and Lindquist2002).
ANOVA was conducted separately for EST and REM studies using PROC MIXED in SAS (v. 9.4, SAS Institute, Cary, NC) with year, transplant type, weed treatments, and all interactions treated as fixed effects, and replicate nested within year treated as a random effect. Due to evidence of variation in the field that was not accounted for by blocks, the ANOVA for marketable yield included a REPEATED statement with option Type = SP(POWA) to allow plot errors to follow an anisotropic autoregressive covariance structure within each study and year. All dependent variables (watermelon marketable yield, total yield, fruit count, marketable fruit count, average fruit size, weed aboveground biomass, hollow heart, SSC) were checked for signs of heteroscedasticity by plotting residuals using output from PROC MIXED. Weed aboveground biomass required a log transformation to meet the ANOVA assumptions of normality and homoscedasticity, and the model included a REPEATED statement with option Group = WATr to allow error variance to differ with WATr. Interactions of weed treatment, year, and transplant type were checked for all dependent variables, and least-squares means for transplant types at each weed treatment were averaged over years when interactions were not significant at the 0.05 significance level. However, with some dependent variables, significant interactions (P ≤ 0.05) between year and transplant type or between year and weed treatment were observed in EST and REM studies. In these cases, graphs of interaction means were assessed; if interactions with year were deemed to be biologically unimportant and uninformative relative to the strong main effects of transplant type and weed treatment, then means for transplant type and weed treatment combinations were obtained by averaging over years. A combined ANOVA was carried out on the treatments common to the EST and REM studies, specifically season-long weedy (EST = 0 WATr, REM = 11 WATr) and weed-free (EST = 11 WATr, REM = 0 WATr) treatments, to compare the effects of transplant type and season-long weed interference on marketable yield, marketable fruit count, total yield, total fruit count, and average marketable fruit size. Mean separation was performed using Fisher’s protected LSD at the 0.05 significance level.
Nonlinear regression of least-squares means for transplant type and weed treatment combinations was conducted in SAS using PROC NLIN to estimate coefficients for models to describe weed aboveground biomass and watermelon marketable yield as a function of weed EST and REM treatments (Knezevic et al. Reference Knezevic, Evans, Blankenship, Van Acker and Lindquist2002). The effect of EST treatments on weed aboveground biomass accumulation was described by the two-parameter decay equation:
where Y is the aboveground weed biomass, a is the y-intercept, b is the asymptote of the curve, and T is the time (x-axis expressed in WATr). The effect of REM treatments on weed aboveground biomass accumulation was described by the following three-parameter logistic equation:
where Y is the aboveground weed biomass, a is the upper weed biomass asymptote, T is time (x-axis expressed in WATr), and b and k are constants. The effect of REM treatments on watermelon marketable yield for each transplant type is described by the three-parameter logistic equation proposed by Knezevic et al. (Reference Knezevic, Evans, Blankenship, Van Acker and Lindquist2002):
where Y is the marketable yield (% of season-long weed-free marketable yield), T is time (x-axis expressed in WATr), d is the point of inflection (WATr), and c and f are constants. The effect of EST treatments on watermelon marketable yield for each transplant type is described by the Gompertz equation:
where Y is the marketable yield (% of season-long weed-free marketable yield), a is the upper yield asymptote, b and k are constants, and T is time (x-axis expressed in WATr).
Results and Discussion
Weed Aboveground Biomass
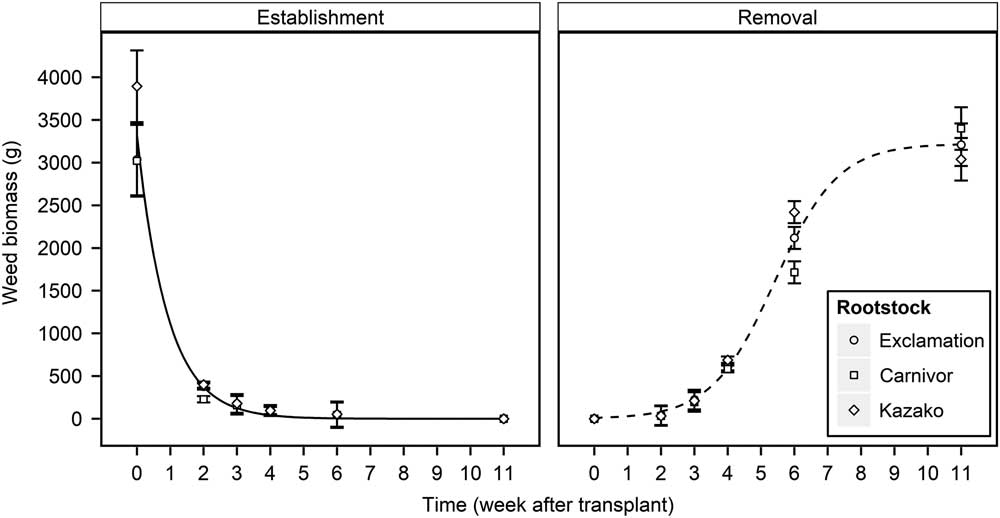
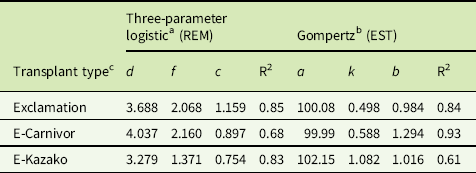
Weed EST and REM treatments (WATr) both had a significant (P<0.001) effect on weed aboveground biomass. Watermelon transplant type had a significant effect on weed aboveground biomass in the EST study (P<0.001) but not in the REM study (P = 0.100). While a significant effect of transplant type was observed in the EST study, the effect of EST timing was markedly stronger than that of transplant type (F = 2830.5 and 2.3, respectively). Thus, the effect of weed EST treatments on the weed aboveground biomass was fit to a single exponential decay curve for the three watermelon transplant types (Equation 1). Graphs of interaction means demonstrated that averaging treatments over years was appropriate (unpublished data), despite a significant weed treatment by year interaction (P<0.001 and P = 0.014 for the EST and REM studies, respectively). Weed aboveground biomass was averaged across years for all transplant types, and the exponential decay curve was fit to the combined data (Figure 1). Weed aboveground biomass decreased at an exponential rate as weeds were established later in the field, with the greatest decrease occurring between 0 and 3 WATr. When weed establishment was delayed until 3 WATr, predicted weed biomass accumulation decreased by 96%, from 3,316 to 125 g per 5 watermelon planting holes (Figure 1). Weed aboveground biomass increased as REM treatments were delayed as described by a three-parameter logistic equation (Equation 2), with the predicted biomass accumulation approaching a maximum asymptote of 3,213 g at 11 WATr (Figure 1). The most rapid increase in biomass weed accumulation occurred when weed removal was delayed from 4 to 6 WATr, when weed biomass accumulation was predicted to increase from 593 to 2,094 g per 5 watermelon planting holes. Weed biomass accumulation for all transplant types followed similar trends in EST and REM studies, with the largest disparities observed at the 0 WATr establishment (873 g) and the 6 WATr removal (705 g) (Figure 1). Grafted watermelon plants are reported to be more vigorous, which may allow them to grow more rapidly than nongrafted plants; however, weed biomass accumulation was similar between grafted and nongrafted transplant types (Lee Reference Lee1994; Lee and Oda Reference Lee and Oda2003; Sakata et al. Reference Sakata, Ohara and Sugiyama2007). Similar findings were reported in grafted tomato, with grafted plants showing no advantage or disadvantage over self-rooted plants with regard to weed biomass production (Chaudhari et al. Reference Chaudhari, Jennings, Monks, Jordan, Gunter, McGowen and Louws2016; Ghosheh et al. Reference Ghosheh, Al-Kawamleh and Makhadmeh2010). As suggested by Chaudhari et al. (Reference Chaudhari, Jennings, Monks, Jordan, Gunter, McGowen and Louws2016), it is possible that the plasticulture system provided sufficient water and nutrients such that resources were nonlimiting and resulted in similar weed aboveground biomass production in all transplant types.
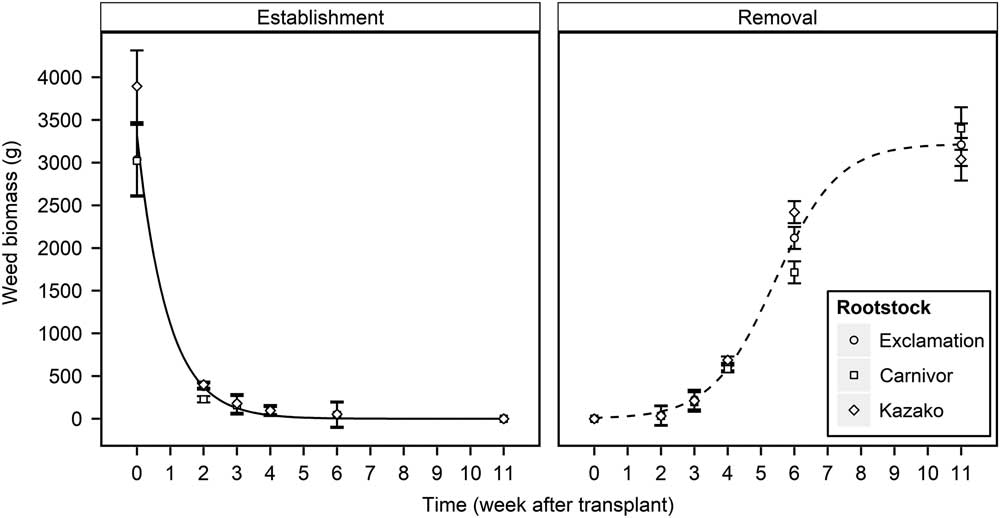
Figure 1 Influence of weed establishment (EST) and removal (REM) treatments on weed aboveground biomass production of nongrafted Exclamation as well as Exclamation grafted on Carnivor and Kazako interspecific hybrid squash rootstocks combined over 2015 and 2016, Clinton, NC. Points represent observed means±SE. Lines represent predicted values for all transplant types in each study. EST predicted values are defined by the two-parameter exponential decay equation Y = 3316.21 × exp(−1.09 × WATr); R2 = 0.98. REM predicted values are defined by the Gompertz equation Y = 3222.18/[1 + 300.5 × exp(−1.05 × WATr)]; R2 = 0.99. WATr, weeks after transplant.
Watermelon Yield and Fruit Count
Means separation was conducted to compare transplant types at the season-long weedy (EST = 0 WATr, REM = 11 WATr) and weed-free (EST = 11 WATr, REM = 0 WATr) treatments for marketable yield, marketable fruit count, total yield, total fruit count, and average marketable fruit size (Table 1). For all dependent variables, the main effects of weed treatment and transplant type were significant at the α = 0.05 level, and no significant interactions of transplant type by weed treatment were observed. A significant year by weed treatment interaction was observed for marketable yield (P<0.0001), marketable fruit count (P<0.0001), total yield (P = 0.0001), and total fruit count (P = 0.002). Graphs of these interaction means were assessed and were deemed to be unimportant relative to the main effects of transplant type and weed treatment (unpublished data), and the data were averaged across years for means separation.
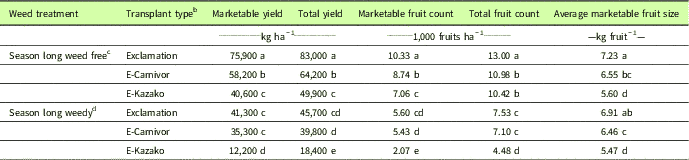
Table 1 Influence of weedy and weed-free treatments on yield, marketable yield, fruit count, marketable fruit count, and average marketable fruit size by transplant type from 2015 and 2016, Clinton, NC.Footnote a
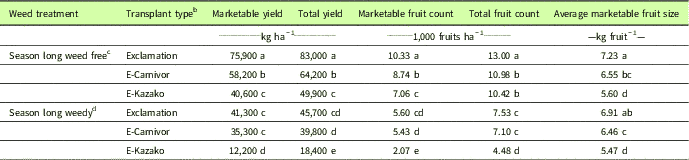
a Data pooled over 2015 and 2016. Means separation using Fisher’s protected LSD test, P≤0.05.
b E-Carnivor, Exclamation scion grafted onto Carnivor rootstock; E-Kazako, Exclamation scion grafted onto Kazako rootstock.
c Season-long weed-free means were calculated using combined values from weed-free plots in establishment (EST = 11 WATr) and removal (REM = 0 WATr) studies.
d Season-long weedy means calculated using combined values from weedy plots in establishment (EST = 0 WATr) and removal (REM = 11 WATr) studies.
In the absence of weed interference, marketable and total yields, marketable and total fruit counts, and average marketable fruit size were greater in nongrafted Exclamation than Exclamation grafted onto Carnivor (E-Carnivor) or Kazako (E-Kazako) (Table 1). Under season-long weed interference, nongrafted Exclamation and E-Carnivor produced higher marketable and total yields, higher marketable and total fruit counts, and larger average marketable fruit size than E-Kazako (Table 1). Compared with weed-free treatments, season-long interference by this mixed population of weeds (D. sanguinalis, P. oleracea, and C. esculentus) reduced marketable yield by 46%, 39%, and 70% for Exclamation, E-Carnivor, and E-Kazako, respectively. While nongrafted Exclamation was the highest-yielding transplant type under weed-free conditions, the differences in yield among grafting treatments were reduced under season-long weed interference. This suggests that weedy fields will yield similarly if planted with nongrafted Exclamation or grafted E-Carnivor, but E-Kazako would produce less yield and fewer, smaller fruits in weedy conditions. In contrast, previous studies have suggested that grafting to vigorous rootstocks will result in increased yields (Lee and Oda Reference Lee and Oda2003; Miguel et al. Reference Miguel, Maroto, Bautista, Baixauli, Cebolla, Pascual, Lopez and Guardiola2004), although some studies report no difference or higher yields of nongrafted watermelon compared with grafted watermelon (Kokalis-Burelle et al. Reference Kokalis-Burelle, Butler, Hong, Bausher, McCollum and Rosskopf2016; Miller et al. Reference Miller, Khalilian, Adelberg, Farahani, Hassell and Wells2013). Results of the season-long weed-free treatments are similar to a previous finding that nongrafted watermelon produces higher yields than watermelon grafted to Cucurbita rootstocks in a disease-free field (Yetisir et al. Reference Yetisir, Sari and Yucel2003). Disparities in the yield performance of grafted plants may be due to graft incompatibility among particular rootstock–scion combinations or to the differences in environmental conditions and production systems between studies.
Watermelon Fruit Quality
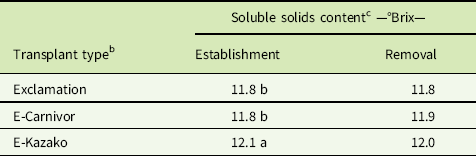
Fruit quality was determined by measuring SSC in degrees Brix (°Brix) and by evaluating fruit for hollow heart. In both EST and REM studies, there was no significant interaction of weed treatment with year (P = 0.295 and P = 0.893, respectively) or transplant type with year (P = 0.544 and P = 0.470, respectively), thus least-squares means of SSC measurements were averaged across years. Weed treatment had a significant effect on SSC in both EST and REM studies (P = 0.003 and P = 0.001, respectively), but there was no meaningful pattern of SSC increasing or decreasing in response to weed interference (unpublished data). Instead, means for transplant types were averaged across weed treatments and years and reported separately in each study. In the EST study, grafted E-Kazako had greater SSC than grafted E-Carnivor and nongrafted Exclamation. In the REM study, there was no significant (P = 0.228) difference in SSC between transplant types. While E-Kazako exhibited the highest mean SSC and was statistically different (P = 0.001) from grafted E-Carnivor and nongrafted Exclamation (Table 2), a difference of 0.3°Brix is very minor. Research in apples (Malus spp.) demonstrated that trained consumer panels are unable to distinguish any change smaller than 1.0°Brix (Harker et al. Reference Harker, Marsh, Young, Murray, Gunson and Walker2002). Hollow heart was present in very few fruits in either study. No symptoms of hollow heart were demonstrated by 93% and 94% of fruit in the EST and REM studies, respectively. These results suggest that neither grafting nor weed interference by the selected weed population had an effect on watermelon fruit quality as measured by SSC and incidence of hollow heart disorder.
Table 2 Influence of transplant type on soluble solids content in establishment and removal studies from 2015 and 2016, Clinton, NCFootnote a
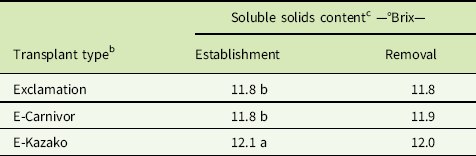
a Data pooled over 2015 and 2016.
b E-Carnivor, Exclamation scion grafted onto Carnivor rootstock; E-Kazako, Exclamation scion grafted onto Kazako rootstock.
c Means separation using Fisher’s protected LSD test, P≤0.05. Lack of letters indicates that F-statistic was not significant at α = 0.05.
Critical Period for Weed Control
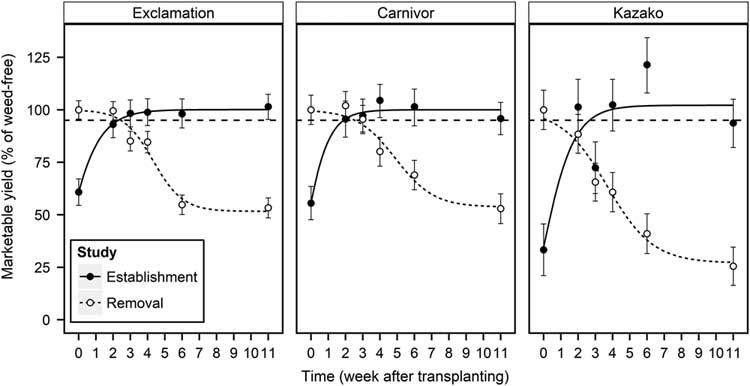
An AYL of 5% was used as the threshold to determine the CPWC. The CPWC was determined for each transplant type when marketable watermelon yield was reduced by 5% of season-long weed-free treatments. As weed establishment was delayed, yield increased for all transplant types (Figure 2). To avoid yield loss beyond 5%, weed establishment must be prevented for at least 2.3, 1.9, and 2.6 WATr for Exclamation, E-Carnivor, and E-Kazako, respectively. These timings represent the critical weed-free period when fields must be kept free of weeds to produce yields at the AYL level and after which no further yield loss due to weed interference will occur. As weed removal was delayed, marketable yield decreased for all transplant types in a relationship characterized by the three-parameter logistic equation (Figure 2; Equation 3). In the REM study, the 5% AYL occurred at 2.5, 2.6, and 0.3 WATr for Exclamation, E-Carnivor, and E-Kazako, respectively. These timings represent the critical time of weed removal, the maximum amount of time weed removal can be delayed before yield loss due to weed interference will occur. Together, predicted values (Equations 3 and 4; Table 3) from the EST and REM studies are used to define the beginning and end of the CPWC, the period of time when weeds must be controlled to avoid yield losses above a 5% AYL. Extended weed control before or after the CPWC could produce higher yields, closer to the weed-free yields (i.e., 0% AYL); but more importantly, weed control beyond the CPWC could reduce contributions to the weed seedbank, reducing weed populations in subsequent growing seasons. Typically, the beginning and end of the CPWC are determined by the removal and establishment studies, respectively (Knezevic et al. Reference Knezevic, Evans, Blankenship, Van Acker and Lindquist2002). A more competitive crop or a production system that favors the crop development over weeds may shorten the CPWC by allowing weed removal to occur at a later date (Radosevich et al. Reference Radosevich, Holt and Ghersa1997). This research estimated that E-Kazako has a CWPC from 0.3 to 2.6 WATr (2 to 18 d), meaning fields must be kept free of weeds from 2 d after transplant (DATr) until 18 DATr to prevent a 5% yield loss. For E-Carnivor, weed establishment must be prevented until 1.9 WATr (13 d), and weed removal must occur before 2.6 WATr (18 d). For nongrafted Exclamation, it was estimated that weed establishment must be prevented until 2.3 WATr (16 d), and weed removal must occur before 2.5 WATr (18 d). Marketable yield was highly correlated with marketable fruit count in both the EST and REM studies (r = 0.969 and 0.974, respectively); thus, the CPWC for each transplant type would be similar to estimates in Figure 2 if applied to marketable fruit count.
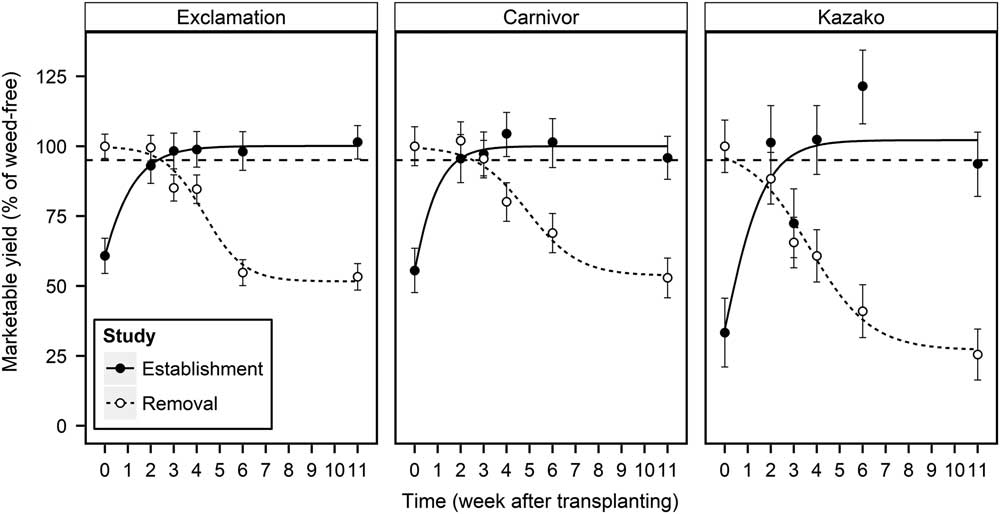
Figure 2 Influence of weed establishment (EST) and removal (REM) treatments on marketable yield (% of weed-free) of nongrafted Exclamation and Exclamation grafted on Carnivor and Kazako rootstocks combined over 2015 and 2016, Clinton, NC. Points represent observed means±SE. Solid and dashed lines represent predicted values of EST and REM studies, respectively.
Table 3 Parameter estimates for three-parameter logistic equation and Gompertz equation for relative marketable watermelon yield (% weed-free check) as a function of time (weeks after transplant, WATr) for establishment (EST) and removal (REM) studies.

a Three parameter logistic equation: Y = [(1/{exp[c × (T−d)]+f}]+[(f−1)/f] × 100, where Y is the marketable yield (% of season-long weed-free marketable yield), T is time (x-axis expressed in WATr), d is the point of inflection (WATr), and c and f are constants.
b Gompertz equation: Y = a × exp[−b × exp(−k × T)] × 100, where Y is the marketable yield (% of season-long weed-free marketable yield), a is the upper yield asymptote, b and k are constants, and T is time (x-axis expressed in WATr).
c E-Carnivor, Exclamation scion grafted onto Carnivor rootstock; E-Kazako, Exclamation scion grafted onto Kazako rootstock.
The estimated CPWC occurred early in the season and lasted 16 d for E-Kazako; however, no CPWC was observed in nongrafted Exclamation or E-Carnivor. Instead, the duration that weeds could persist from the start of the season without affecting yield lasted longer than when the first weeding must occur to prevent yield loss. Thus, results from this study estimate that a single weed control event enacted between 16 and 18 DATr and between 13 and 18 DATr is sufficient to yield within 95% of the weed-free yield for Exclamation and E-Carnivor, respectively. Similarly, no CPWC was observed in red beet (Beta vulgaris L.); instead, a single weeding event 2 to 4 wk after 50% crop emergence achieved similar yields to the weed-free treatments (Hewson and Roberts Reference Hewson and Roberts1973). Recent research in Arkansas reported a lack of a CPWC in cotton (Gossypium hirsutum L.) planted with a rye (Secale cereale L.) cover when weed densities were low, and any weed control between 20 and 52 d after planting was sufficient to yield within the 5% AYL level (Korres and Norsworthy Reference Korres and Norsworthy2015).
In contrast to the present findings, previous research (Adkins et al. Reference Adkins, Stall, Santos, Olson and Ferrell2010; Monks and Schultheis Reference Monks and Schultheis1998) in watermelon reports a CPWC of 0 to 6 and 3.3 to 3.9 WATr for D. sanguinalis and S. americanum, respectively. In peanut (Arachis hypogaea L.), which exhibits a prostrate growing habit similar to watermelon, the CPWC for a mixed population of weeds was 3 to 8 WATr (Everman et al. Reference Everman, Clewis, Thomas, Burke and Wilcut2008). The duration of the CPWC for tomato (2.5 wk) was not affected by grafting, but the CPWC occurred 1 wk earlier in the grafted tomato (Chaudhari et al. Reference Chaudhari, Jennings, Monks, Jordan, Gunter, McGowen and Louws2016). Relative to the present study, these extended durations of the CPWC may be attributed to the differences in productions systems, weed establishment techniques, crops species, and weed species.
Results of this experiment suggest that watermelon growers should control D. sanguinalis, P. oleracea, and C. esculentus in a single event between roughly 13 and 18 DATr when planting nongrafted watermelon or watermelon grafted to Carnivor rootstock. Due to reduced yield and low grafting compatibility, Kazako as a rootstock is no longer available as a commercial rootstock variety beginning in 2017 (D Liere, personal communication). Except for graminicides, there are limited POST weed control options in watermelon; therefore, to prevent yield loss to weed interference, watermelon growers should select a PRE herbicide that provides residual control for a minimum of 18 DATr. Use of a PRE herbicide is common due to the sensitivity of watermelon to cultivation as vines extend to row middles (Coolong and Granberry Reference Coolong and Granberry2017). It is important to recognize that the duration of the CPWC is estimated and is based on environmental conditions of field sites, and a high-value crop such as watermelon should be managed conservatively to minimize yield loss due to weed interference. Further, the CPWC is defined by yield loss in a single season, and it does not account for contributions to the soil seedbank by late-emerging weeds. Best management practices, particularly if herbicide resistance is suspected, should eliminate seed production by weed species. The present study demonstrates that grafted watermelon offers no benefit for weed competition or yield benefits in the presence of weeds compared with nongrafted watermelon; however, it is possible that specific rootstock–scion combinations may perform differently than those included in this work. There is still utility to vegetable grafting, including management of soilborne disease, abiotic stress tolerance, and enhanced yield and fruit quality (Davis et al. Reference Davis, Perkins-Veazie, Sakata, López-Galarza, Maroto, Lee, Huh, Sun, Miguel, King, Cohen and Lee2008; Lee and Oda Reference Lee and Oda2003; Louws et al. Reference Louws, Rivard and Kubota2010). But yield reductions, even under season-long weed-free conditions, suggest watermelon grafting should be adopted only in cases where soilborne pathogens are anticipated to be a problem, as has been suggested in previous studies (Kokalis-Burelle et al. Reference Kokalis-Burelle, Butler, Hong, Bausher, McCollum and Rosskopf2016; Taylor et al. Reference Taylor, Bruton, Fish and Roberts2008).
Acknowledgments
The authors express appreciation to the following individuals for their assistance with conducting this research: Sushila Chaudhari, David Suchoff, Brad Thompson, Keith Starke, Nicholas Basinger, Stephen Smith, Sam McGowen, Shawn Beam, Matt Waldschmidt, Wesley Hairr, and the station crew at the Horticultural Crops Research Station in Clinton, NC. The authors thank Dean Liere and Syngenta for providing seed for the experiment and the USDA-NIFA-2011-51181-30963 for providing funding for this research. No conflicts of interest have been declared.