Introduction
Nightmares are typically characterized by awakening from rapid eye movement (REM) sleep with recollection of disturbing mental activity (Nielsen & Levin, Reference Nielsen and Levin2007). While the prevalence of weekly nightmares is relatively low in the general population (0.9–6.8%; Janson et al. Reference Janson, Gislason, De Backer, Plaschke, Björnsson, Hetta, Kristbjarnason, Vermeire and Boman1995; Nielsen & Levin, Reference Nielsen and Levin2007; Li et al. Reference Li, Zhang, Li and Wing2010), they are a common experience for those with diagnoses such as post-traumatic stress disorder (PTSD; Neylan et al. Reference Neylan, Marmar, Metzler, Weiss, Zatzick, Delucchi, Wu and Schoenfeld1998; Leskin et al. Reference Leskin, Woodward, Young and Sheikh2002) and borderline personality disorder (Semiz et al. Reference Semiz, Basoglu, Ebrinc and Cetin2008). A more recent cross-sectional study identified that nightmares were a weekly problem for 55% of a sample of 40 patients experiencing psychotic symptoms and that the distress associated with them was associated with more severe delusional beliefs, depression, anxiety, stress and impaired working memory (Sheaves et al. Reference Sheaves, Onwumere, Keen, Stahl and Kuipers2015). There was no relationship between nightmare frequency or distress and PTSD symptoms (Sheaves et al. Reference Sheaves, Onwumere, Keen, Stahl and Kuipers2015). If nightmare distress is hypothesized to impact on daytime psychotic and affective symptoms, one would expect to see treatment-related reductions in nightmare distress to improve these daytime symptoms. As such, nightmares might present themselves as a promising target for therapeutic interventions.
Imagery rehearsal (IR) therapies are recommended for the treatment of nightmare disorder by a task force commissioned by the Standards of Practice Committee of the American Academy of Sleep Medicine (Aurora et al. Reference Aurora, Zak, Auerbach, Casey, Chowdhuri, Karippot, Maganti, Ramar, Kristo, Bista, Lamm and Morgenthaler2010). While different IR protocols exist, the basic elements include a psychoeducation phase, rescripting a change of ending to the nightmare and daily imaginal rehearsal of the new dream narrative (Casement & Swanson, Reference Casement and Swanson2012). A meta-analysis of 13 IR studies carried out in samples with PTSD, report large reductions in nightmare frequency [standardized mean difference (SMD) effect size = 0.69, 95% confidence interval (CI) = 0.50–0.88] and sleep quality (SMD effect size = 0.68, 95% CI = 0.34–1.03) from pre- to post-intervention (Casement & Swanson, Reference Casement and Swanson2012). The analysis showed that the effects of treatment were durable; improvements were sustained at 6 and 12 months follow-up. PTSD symptoms also reduced as a result of IR suggesting that nightmares might serve as a maintaining factor for distressing daytime symptoms.
To our knowledge there has been no study investigating the feasibility of using IR to treat nightmares in the context of psychosis. However, imagery rescripting has been used as a technique for treatment of intrusive daytime images for those with psychosis, as reported in individual case reports (Morrison, Reference Morrison2004; Serruya & Grant, Reference Serruya and Grant2009), small case-series for those with persecutory delusions and intrusive visual memories (Schulze et al. Reference Schulze, Freeman, Green and Kuipers2013), and for those with auditory hallucinations (Ison et al. Reference Ison, Medoro, Keen and Kuipers2014). The current study aims to add to the well-established literature of IR for the treatment of post-traumatic nightmares, and to the small literature on using imagery rescripting in the context of psychosis, by considering whether IR for nightmares might be suitably adapted for use in a population with psychosis, by assessing acceptability, feasibility and describing pre- to post-therapy changes on key outcomes.
The primary objective was to complete a case-series to assess the acceptability and feasibility of IR for the treatment of nightmares in the context of psychosis. This type of design is recommended by the Medical Research Council (MRC) as a key stage in developing complex interventions, prior to completing a phase II trial (MRC, 2000). Acceptability was operationalized by collecting participant feedback on the experience of the intervention and assessing satisfaction. Feasibility was defined as outlining adaptations to the intervention for those with psychosis and assessing the possible impact of IR on nightmares through quantitative measures and participant feedback. In addition, measures of nightmares (frequency, distress, intensity, vividness), overall sleep quality, psychotic and affective symptoms were expected to show improvement following receipt of IR. Participants were screened for PTSD in order to describe the clinical characteristics of the sample. Given the lack of association between nightmare frequency or distress and PTSD severity (Sheaves et al. Reference Sheaves, Onwumere, Keen, Stahl and Kuipers2015), comorbid PTSD did not form part of recruitment criteria.
Method
Design
The study followed a larger study of nightmares in people experiencing psychosis (Sheaves et al. Reference Sheaves, Onwumere, Keen, Stahl and Kuipers2015). An A-B design was used in which a pre-intervention assessment was completed (week 0), immediately followed by the IR intervention (weeks 0–6, 4–6 sessions depending on clinical need), followed immediately by the post-intervention assessment (weeks 4–6, depending on length of the intervention). Two weeks later (weeks 6–8) participants received a follow-up telephone call. The case-series design was used to assess the acceptability to those with psychosis, and the feasibility of adapting the IR protocol for this population, rather than being powered to detect significant changes.
All assessments and the IR therapy were conducted by a third-year trainee clinical psychologist, with weekly supervision from a consultant clinical psychologist. The trainee clinical psychologist had received training in CBT for psychosis and imagery rescripting.
Participants
Participants for the current study formed part of a larger sample recruited for a separate study investigating the phenomenology of nightmares in the context of psychosis (Sheaves et al. Reference Sheaves, Onwumere, Keen, Stahl and Kuipers2015). Participants for that study were referred from South London National Health Service adult mental health care teams. Of the 40 participants who took part in that previous study, 22 (55%) experienced weekly nightmares. Twenty (50%) of the original 40 participants expressed an interest in being contacted about the current intervention study, 18 (45%) met inclusion criteria for the current study, seven were invited to take part and six consented. Participants were chosen pragmatically, on the basis of frequency of nightmares and their previous date of assessment. Those who were assessed first (and were eligible) were invited first, and of these, those who consented entered the study. Five of the six people who entered the study completed all the therapy sessions and the end-of-therapy assessment.
Inclusion criteria were for participants to be aged ≥18 years, experience symptoms of psychosis, experience weekly nightmares that were at least moderately distressing (≥4 on the distress scale of nightmare log), able to recall the nightmare content, proficient use of the English language for the purpose of completing questionnaires and able to provide informed consent. Exclusion criteria included a primary diagnosis of alcohol or substance dependency or an organic syndrome such as a dementia or learning disability. Those participants with comorbid PTSD were not excluded from the study.
Measures
Acceptability
Participants received a follow-up telephone call to collect feedback regarding the therapy. Participants were asked whether they could suggest any improvements to the therapy and rated their satisfaction with the therapy on a 0–10 rating scale (0 = not satisfied at all, 10 = very satisfied).
Feasibility
Participants reported changes in their nightmares in the last therapy session. They were also asked an open question, designed to elicit any adverse experiences as well as any improvements they might have noticed, in the follow up telephone call (2 weeks following cessation of therapy): ‘Have you noticed any changes following the therapy?’ Their verbatim responses to this question were written down. Adverse events are defined as harmful events that occur during a trial (Duggan et al. Reference Duggan, Parry, McMurran, Davidson and Dennis2014).
Outcome measures (completed pre-intervention and post-intervention)
A prospective ‘Dream Log’ was adapted from a previous study (Levin & Fireman, Reference Levin and Fireman2002). In particular, participants completed the log each morning for 1 week (rather than retrospectively completing it over a 2-week time-frame). This adaptation was made in order to increase reliability in recall of nightmares. Participants indicated how many nightmares they experienced each night. If the participant had experienced at least one nightmare, they were asked to pick their worst nightmare and rate it on a 7-point Likert scale for intensity, vividness, distress and also to mark whether it was recurrent (yes/no). Scores carried forward for analysis were total nightmare frequency and mean nightmare distress, intensity, and vividness across the week.
The Pittsburgh Sleep Quality Index (PSQI; Buysse et al. Reference Buysse, Reynolds III, Monk, Berman and Kupfer1989)
The PSQI measured sleep quality over the month prior to assessment. The sleep efficiency (number of hours asleep divided by number of hours in bed) and overall sleep quality scales were used for analysis. The seven PSQI component scores have good internal consistency (Cronbach's α = 0.83) and yield acceptable test–retest reliability (Buysse et al. Reference Buysse, Reynolds III, Monk, Berman and Kupfer1989). The measure has previously been used in samples with psychosis (Wulff et al. Reference Wulff, Dijk, Middleton, Foster and Joyce2012; Afonso et al. Reference Afonso, Figueira and Paiva2014).
Psychotic Symptoms Rating Scale (PSYRATS; Haddock et al. Reference Haddock, McCarron, Tarrier and Faragher1999)
This scale measures the severity of 11 dimensions of auditory hallucinations and six dimensions of delusions via 5-point scales (0–4). Evaluation of the PSYRATS delusions and hallucinations scales indicates good inter-rater reliability (Haddock et al. Reference Haddock, McCarron, Tarrier and Faragher1999) and concurrent validity with the Positive and Negative Syndromes Scale (Drake et al. Reference Drake, Haddock, Tarrier, Bentall and Lewis2007).
Voice Power Differential (VPD; Birchwood et al. Reference Birchwood, Meaden, Trower, Gilbert and Plaistow2000)
Participants completed this measure only if they reported hearing voices. The VPD assesses the power differential between the voice and the voice hearer through seven dimensions: power, strength, confidence, respect, ability to inflict harm, superiority, and knowledge (Birchwood et al. Reference Birchwood, Meaden, Trower, Gilbert and Plaistow2000). Each dimension is measured on a 5-point Likert scale. Scores on the seven dimensions of power are summed together. The scale has been shown to have good internal reliability (Cronbach's α = 0.85) and good 1-week test–retest reliability (r = 0.82; Birchwood et al. Reference Birchwood, Meaden, Trower, Gilbert and Plaistow2000).
Persecutor Power Differential (PPD; adapted from the VPD; Birchwood et al. Reference Birchwood, Meaden, Trower, Gilbert and Plaistow2000)
Participants completed this measure only where they indicated the presence of a persecutory delusion. The PPD has been adapted from the VPD. It assesses the same seven power dimensions of the VPD (power, strength, confidence, respect, ability to inflict harm, superiority, knowledge), although wording of the items is changed so that voice is replaced with a persecutor. The psychometric properties of the scale have not been described.
Clinical Outcomes in Routine Evaluation (CORE-10; Connell & Barkham, Reference Connell and Barkham2007)
CORE-10 was used as a screening measure of psychological distress. This is a short form of the CORE Outcome Measure (CORE-OM) which has high internal consistency and acceptability for patients in secondary care (Barkham et al. Reference Barkham, Gilbert, Connell, Marshall and Twigg2005). The CORE-10 has a strong correlation with the CORE-OM in patients with severe and enduring mental illness (Ward, Reference Ward2010). The construct of psychological distress is not linked to a particular disorder, but individual items cover anxiety (two items), depression (two items), trauma (one item), physical problems (one item) functioning (three items) and risk to self (one item). The CORE-10 has been shown to be sensitive to change and has good internal reliability with Cronbach's α = 0.82 (Connell & Barkham, Reference Connell and Barkham2007). It correlates well with measures of anxiety, depression and general mental health (Connell & Barkham, Reference Connell and Barkham2007).
Depression, Anxiety and Stress Scales (DASS-21; Lovibond & Lovibond, Reference Lovibond and Lovibond1995)
DASS-21 provided three scales of these negative emotional states, derived from 21 self-report items. The scales reflect experiences over the preceding week. The three scales have demonstrated very good internal consistency (all Cronbach's α >0.90) and good test–retest reliability for a sample with schizophrenia (Huppert et al. Reference Huppert, Smith and Apfeldorf2002). This scale was added after the first two participants in order to better assess affective changes.
Baseline measures only
Socio-demographic data, diagnosis, prescribed medication, alcohol and drug use data were collected. Where participants were unsure of their diagnosis, prescribed medication name or dose this was taken from medical notes.
Post-traumatic Diagnostic Scale (PDS; Foa et al. Reference Foa, Cashman, Jaycox and Perry1997)
This scale was used as a self-report screen of PTSD. The PDS can be used to yield a preliminary PTSD diagnosis according to DSM-IV criteria as well as mark symptom severity. It has been shown to have high internal consistency, test–retest reliability and high diagnostic validity compared to the Structured Clinical Interview and good sensitivity and specificity (Foa et al. Reference Foa, Cashman, Jaycox and Perry1997).
Treatment
The method of IR was adapted from that described in Nappi et al. (Reference Nappi, Drummond, Thorp and McQuaid2010). The key techniques were psycho-education, collaboratively planning a rescript of the nightmare, elaborating the rescript with sensory detail through guided imagery and daily practice of the new dream script. All sessions were completed through individual therapy with author B.S. Session numbers varied between four and six, with a longer session number being dependent on clinical need.
Psycho-education topics included the prevalence of problems with nightmares, normalizing strong emotional reactions to vivid negative content and discussion about possible causes of nightmares (related to life experiences or idiopathic). In some cases, the basic architecture of sleep cycles was discussed in order to normalize night-time awakenings.
Participants were asked to pick a nightmare with which to target for treatment, usually the most distressing (in practice the most distressing the participant felt able to tolerate). Participants provided a short sentence describing each of the ‘scenes’ of the nightmare and rated the distress of each scene out of 10. This very brief description avoided exposure to (reliving of) detailed nightmare content. There was no exposure element to the intervention unlike other nightmare treatment protocols (e.g. Davis et al. Reference Davis, Rhudy, Pruiksma, Byrd, Williams, McCabe and Bartley2011; Long et al. Reference Long, Hammons, Davis, Frueh, Khan, Elhai and Teng2011). This was an adaptation of the protocol in light of the known sensitivity of those with ongoing psychosis symptoms to high levels of affect, which is contra-indicated in cognitive behavioural interventions for psychosis (CBTp; Fowler et al. Reference Fowler, Garety and Kuipers1995). The distress rating of each nightmare scene provided an opportunity to decide where the new alternative ending should be inserted; prior to the point of maximum affect. Alternative endings were considered carefully for their ability to neutralize/sooth previous negative affect, feel acceptable to the individual and their ability to link with distressing themes from the specific nightmare content (e.g. a nightmare characterized by fear might be rescripted to end in a place of safety). Endings were encouraged to be positive (rather than neutral).
The rescript was elaborated through guided imagery. The participants were encouraged to close their eyes, or fix their gaze at a single point on the floor. They then provided a detailed description, using the first person and present tense. Sensory details, thoughts and feelings were prompted by the therapist where appropriate and participants were gently prompted towards the next sequence in the previously agreed rescript, if this did not occur naturally through their description. At the end of the imagery participants were asked to sit with the positive emotion and then to ‘return’ to the room. Following the guided imagery, participants provided feedback about their experience of the rescript, with a particular emphasis on the change in affect from start to finish.
Following this session, the therapist used therapy recordings to type up the re-script. The rescript was also recorded onto a CD. These were posted to participants, who were encouraged to rehearse the rescript at least once per day preferably prior to going to bed. In the following session participants provided feedback about their experience of rehearsing the rescript. Adaptations were made to the rescript where necessary and were once again elaborated through guided imagery.
Thus, key adaptations to the Nappi et al. (Reference Nappi, Drummond, Thorp and McQuaid2010) protocol for the psychosis population were: (1) Provision of a CD recording of the rescripted nightmare in order to provide an alternative means of script rehearsal for those who hear voices. (2) All therapy sessions were delivered in an individual (not group) format, given that the heterogeneity in presentation was unknown at the start of the study. (3) Increased time was spent considering alternative rescripted endings, particularly if the nightmare was related to a memory. (4) The session length and number was flexible, as recommended in CBTp manuals (Fowler et al. Reference Fowler, Garety and Kuipers1995)
Statistical analysis
Means and standard deviations (s.d.) are presented and for standardized measures a Reliable Change Index (RCI; Jacobson & Truax, Reference Jacobson and Truax1991) was calculated to assess for a reliable change in symptoms. Reliable change refers to the extent to which change from pre-intervention to post-intervention falls beyond what would be expected on the basis of measurement variability. The equation uses test–retest reliability of the measure itself, as well as a measure of the variance of the sample (s.d.). The reliable change criterion is 1.96 times the standard error of the difference (Evans et al. 1998). If the participant falls beyond the reliable change criteria specified, it can be concluded with 95% certainty that they have evidenced a statistically reliable change in score, rather than that change occurring due to chance. The RCI was calculated for standardized measures (PSQI, PSYRATS for hallucinations and delusions, VPD, CORE-10).
Results
Baseline demographic and clinical characteristics
A summary of the clinical and demographic information of the six participants who entered the study is presented in Table 1. Five participants had previous experience of CBTp and one participant had concurrent CBTp delivered by a specialist outpatient psychosis clinic.
Table 1. Clinical and demographic information of participants entering treatment: means (s.d.) and number of participants (n = 6)

PSQI, Pittsburgh Sleep Quality Index; PTSD, Post-traumatic Stress Disorder; PDS, Post-traumatic Diagnostic Scale.
All six participants had ongoing symptoms of psychosis. All six experienced delusional beliefs and five additionally experienced hearing voices. Three participants experienced command hallucinations. The delusional beliefs included persecutory and grandiose delusions. The mean level of global distress at baseline, measured by the CORE-10 fell within the severe range (Connell & Barkham, Reference Connell and Barkham2007). The mean level of depression and anxiety fell within the extremely severe range and mean levels of stress fell within the severe range (Lovibond & Lovibond, Reference Lovibond and Lovibond1995). Three participants screened positive for PTSD. Number of traumatic events experienced by these three participants ranged between one and six. The chosen index event (the event that bothered the participant the most) included experience of childhood sexual abuse (n = 2) and the sudden unexpected death of a family member in adulthood (n = 1). None of the nightmares chosen for the intervention were a replication (re-experiencing) of the content of these index events. Two participants acknowledged some similarity between their nightmare and past trauma, including seeing the same people, and experiencing the same emotional response (e.g. guilt or disgust).
Two participants experienced nightmares every night of the week, three participants experienced them more than half of the nights per week and one participant had one nightmare over the previous week. Table 2 shows a descriptive summary of the nightmare content for those who engaged in the intervention. All participants had at least three frequent, recurrent and distressing nightmares.
Table 2. Nightmare content across five participants who completed the intervention
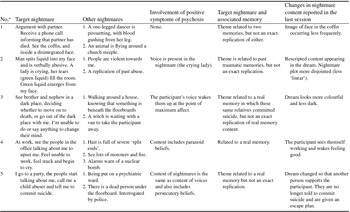
*Participant number.
Of the six participants who entered the intervention, care teams were contacted for four participants in order to assess and manage any risk that arose during the course of the study as a result of the detailed assessments. In all four cases, this risk was unrelated to the intervention process, but in two cases had not previously been discussed by the participants with their care team. Two participants disclosed voices commanding them to hurt others and two participants disclosed a significant risk of suicide. No participants reported attempting suicide or carrying out a violent incident during the intervention.
Change in score from baseline to post-intervention
Five of the six participants who entered the therapy completed all sessions and follow-up questionnaires. One participant withdrew from the study. His recurrent nightmare described at screening had stopped occurring. While he continued to experience highly frequent nightmares at the start of therapy, he had difficulty recalling them and did not consider any to be recurrent. His data is included in the clinical and demographic information collected at baseline (Table 1), but not follow-up data.
A summary of mean data from the five participants who completed the intervention is presented in Table 3 and individual data is shown in Figure 1. Overall, mean nightmare frequency increased from 4.40 nightmares per week (s.d. = 2.30) to 5.00 (s.d. = 3.46); one of the five participants had decreased nightmare frequency, two remained unchanged and two increased. However, mean nightmare-related distress across the week decreased from 5.43 (s.d. = 0.92) to 4.28 (s.d. = 0.94); four (of five) participants had a decreased mean distress rating, while one participants’ score remained unchanged (Fig. 2). The mean vividness of nightmares decreased in all five cases from 5.57 (s.d. = 0.85) to 4.50 (s.d. = 0.37) and the intensity of nightmares decreased for four out of five participants from 5.33 (s.d. = 0.41) to 4.11 (s.d. = 0.52) (Fig. 2). Overall sleep quality improved from 13.00 (s.d. = 5.34) to 12.00 (s.d. = 4.64). Four out of five participants showed a positive improvement in sleep quality score, while one participant's sleep quality worsened.
Table 3. Mean scores (s.d.) at baseline and post-intervention for participants who completed the intervention

PSQI, Pittsburgh Sleep Quality Index; PSYRATS, Psychotic Symptoms Rating Scale; PPD, Persecutor Power Differential VPD, Voice Power Differential; CORE-10, Clinical Outcome in Routine Evaluation-10; DASS-21, Depression Anxiety and Stress Scale (21-item version).

Fig. 1. Frequency of nightmares from a weekly prospective nightmare log completed at baseline and immediately following the Imagery Rehearsal intervention, for each of the five participants (P1–P5).

Fig. 2. Likert ratings (1–7) of nightmare distress, intensity and vividness at baseline and immediately following the Imagery Rehearsal intervention, for each of the five participants participants (P1–P5).
Scores on the PSYRATS for delusions decreased in four out of five participants, and increased for one participant (baseline mean = 18.00, s.d. = 1.87; post-intervention mean = 16.20, s.d. = 2.77). Two participants completed the PPD (baseline mean = 23.50, s.d. = 10.61), both evidenced a decrease in score following the intervention (post-intervention mean = 20.50, s.d. = 9.19). Severity of hallucinations measured by PSYRATS decreased in four out of five cases, while one participant's score increased (baseline mean = 28.80, s.d. = 2.86; post-intervention mean = 27.40, s.d. = 3.97). Three participants evidenced a decrease in score on the VPD scale; one participant's score increased and one remained unchanged (baseline mean = 25.67, s.d. = 1.53; post-intervention mean = 23.67, s.d. = 4.04).
CORE-10 scores revealed that four out of five participants’ global distress scores decreased, while one increased. There was little overall change in mean scores (baseline mean = 2.50, s.d. = 0.72; post-intervention mean = 2.16, s.d. = 0.85). Data from three participants for depression indicated a decrease in depression symptoms for two of them and a stable score for the remaining one (baseline mean = 28.67, s.d. = 14.05; post-intervention mean = 24.67, s.d. = 15.14). Stress scores decreased for one participant, one participant's stress score increased and the last participant's score remained unchanged (baseline mean = 27.33, s.d. = 11.72; post-intervention mean = 24.67, s.d. = 15.14). Anxiety scores showed the greatest decrease for the three participants for whom data was available (baseline mean = 28.67, s.d. = 9.45; post-intervention mean = 21.33, s.d. = 12.06).
RCI (Jacobson & Truax, Reference Jacobson and Truax1991)
Despite four participants reporting improved sleep quality (PSQI), none of them achieved a 6-point change in score indicative of a statistically RCI (with 95% certainty). Two participants (participants 1 and 3) showed statistically reliable improvements in delusional severity (with 95% confidence) as evidenced by the RCI. Two of five participants (participants 1 and 3) evidenced a statistically reliable improvement in PSYRATS for hallucination score, as evidenced by the RCI (95% confidence). Participant 2 was the only participant to have evidenced an increase in the severity of hearing voices; this was also a statistically reliable change as calculated by the RCI.
Two of the five participants had statistically reliable change on the VPD. Participant 1 indicated an improvement in the perceived power of her voice-hearing and participant 6 reported a worsening in the perceived power of her voices (with 95% confidence), as calculated by the RCI. Barkham et al. (Reference Barkham, Bewick, Mullin, Gilbody, Connell, Cahill, Mellor-Clark, Richards, Unsworth and Evans2013) report a RCI of 6 for the CORE-10 total score (clinical score×10), recommending a 90% false-positive rate rather than the traditional 95%. One participant achieved this statistically reliable improvement (participant 5).
Acceptability: participant feedback
Mean rating on a 1–10 scale for satisfaction with the intervention was high at 9.20 (s.d. = 1.30). When asked to suggest improvements to the intervention participants recommended adjustments to the research room (e.g. using the same room for all appointments) and adding relaxing sounds (e.g. the sea/birds) to the background of the rescript recorded onto CD. The lowest satisfaction rating (7/10) was from participant 2 who acknowledged that his voice-hearing had made it challenging to engage in the imagery. During the intervention, he had experienced a recent significant change in personal circumstances which resulted in his voice-hearing becoming generally more difficult to manage. This was reflected in his increased PSYRATS for voices score.
Feasibility: participant feedback
All participants noted changes in the content of their nightmares following the intervention (see Table 2). Participants were still experiencing nightmares at the end of the intervention (see Fig. 1). However, the feedback following the intervention indicated that all five participants who completed the intervention reported a change in their emotional response following the nightmare (see Table 4). No adverse effects were reported by participants as a result of the intervention.
Table 4. Participants’ comments about changes noticed following therapy (from follow-up telephone calls)

Discussion
This case-series is, to the best of our knowledge, the first to investigate the use of IR as an intervention for nightmares in the context of psychosis. The study was designed specifically to assess the acceptability and feasibility of delivering IR for the treatment of nightmares, to patients with active symptoms of psychosis. In addition, the study aimed to explore changes in measures of nightmares (frequency, distress, intensity, vividness), sleep quality (PSQI), psychotic (PSYRATS for hallucinations and delusions) and affective symptoms (DASS-21 and CORE-10) following receipt of IR. The study was not powered to detect whole group changes in these symptoms, but the RCI was used as a marker of statistically significant changes in symptoms, for individual participants where standardized measures were used.
Five out of six participants completed the therapy, indicating that it is possible to administer a brief intervention outside of an established therapeutic relationship. Ratings of satisfaction with therapy were high. Comments on the intervention indicated positive changes following therapy (Table 4). While participants acknowledged still experiencing nightmares following IR, many noted small changes in content (see Table 2) and changes in their response to the nightmare either in terms of reduced distress or increased ability to cope following the nightmare. The comments on IR were consistent with quantitative measures which indicated no improvement in the frequency of nightmares following IR (Fig. 1), but descriptive improvements in nightmare related distress, vividness and intensity (Fig. 2).
Contrary to previous literature (e.g. Casement & Swanson, Reference Casement and Swanson2012), from the PTSD population, there was no decrease in the frequency of nightmares following IR for any of the participants. There are a number of plausible reasons for this. First, participants reported 3–4 regular distressing nightmares, while the treatment targeted just one nightmare. It is possible that people experiencing psychotic symptoms are more prone to nightmares in general (Sheaves et al. Reference Sheaves, Onwumere, Keen, Stahl and Kuipers2015), rather than being related to discrete traumatic experiences (as is the case in PTSD). Second, it is possible that the medication participants were taking impacted on sleep architecture, resulting in changes in dream frequency.
The current sample was managing a number of distressing symptoms that had not responded adequately to previous treatment. All of the participants were engaged with NHS care teams, five of six were prescribed anti-psychotic medications and all had experience of CBTp. Despite this, baseline levels of anxiety and depression were in the extremely severe range and global distress was in the severe range. Half of the participants screened positive for PTSD. This is representative of the increased prevalence of trauma in samples with psychosis (Greenfield et al. Reference Greenfield, Strakowski, Tohen, Batson and Kolbrener1994; Shaw et al. Reference Shaw, McFarlane, Bookless and Air2002; Holowka et al. Reference Holowka, King, Saheb, Pukall and Brunet2003). It was necessary to contact care teams to assess and manage risk to self or others (unrelated to the intervention) in four out of six participants, consistent with previous research indicating that those experiencing nightmares are at increased risk of suicidal ideation (Pigeon et al. Reference Pigeon, Pinquart and Conner2012). Despite these symptoms, participants engaged in the brief intervention.
Sheaves et al. (Reference Sheaves, Onwumere, Keen, Stahl and Kuipers2015) previously showed significant correlations between higher levels of nightmare distress and more severe delusional beliefs, depression, anxiety, stress and working memory. If nightmare distress is hypothesized to impact on daytime symptoms, one would expect to see treatment related reductions in nightmares distress to improve these daytime symptoms. Two of five participants experienced significant reductions in delusional severity, all three participants with complete measures exhibited descriptive improvements in depression scores, two out of three improved anxiety and four out of five improved global distress (one participant significantly so).
There are several limitations to the current study which would be important to address in a future evaluation. First, the nightmare log has not been validated and in particular, not for this clinical sample. Second, the sample recruited varied with regards to comorbid PTSD, type of delusion (persecutory or grandiose) and affective vs. non-affective psychosis. While this sample might be representative of the varied presentations of those experiencing psychotic symptoms, future research might benefit from a more homogenous sample to reduce heterogeneity in treatment response. Third, there was no independent assessor. The same individual provided the therapy and completed the assessments. Future research would benefit from blind, independent assessments for both qualitative and quantitative outcomes in order to avoid potential demand characteristics. Last, all participants had experience of CBTp and one person was receiving concurrent therapy. It is unclear how this might have impacted on this brief intervention both in terms of being socialized to CBTp techniques and to the outcomes.
The current pilot study was intended as a first step in developing an intervention for the treatment of nightmares for those experiencing symptoms of psychosis. Future research is clearly warranted to test the efficacy of IR, with a follow-up assessment period. This future research should consider some key adaptations to the treatment protocol. These include adding additional therapeutic techniques to further target the alleviation of nightmare-related distress (e.g. self-soothing techniques). In addition, given the lack of reduction in nightmare frequency, it might be of benefit to imbed IR within a CBT for insomnia intervention to manage night-time awakenings. Finally, a larger study could also consider the impact of antipsychotic medications on the frequency of nightmares and response to IR treatment, given that these medications are known to impact on REM sleep, within which the majority of dreaming occurs (Cohrs, Reference Cohrs2008).
The current study demonstrated that it was possible to adapt an IR protocol for the treatment of nightmares in those experiencing current symptoms of psychosis, with high satisfaction ratings from the participants, and without reported adverse effects. This is an important first step in developing an intervention for nightmares, which have been shown to be a weekly problem for around half of a community sample of those with psychosis (Sheaves et al. Reference Sheaves, Onwumere, Keen, Stahl and Kuipers2015). Participants described positive changes following the intervention, descriptive decreases in nightmare-related distress, vividness and intensity were observed for the majority of participants, and significant reductions in delusional severity for two participants. Further work should aim to develop the intervention and assess its efficacy in a study powered to detect whole group changes in nightmares and other psychotic symptoms.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and international committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was reviewed and approved by the City Road and Hampstead NHS Research Ethics Committee (11/LO/2045).
Acknowledgements
We are very grateful to the participants who gave their time to take part in the study and the teams that facilitated contact with participants within South London and Maudsley NHS Foundation Trust, particularly the Psychological Interventions Clinic for outpatients with Psychosis (PICuP) and Oxleas NHS Foundation Trust (Dr Marina Richards and Natalie Cook). This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of Interest
None.
Learning objectives
-
(1) It was possible to adapt an Imagery Rehearsal protocol and provide this as an intervention for a small sample of adults living in the community who were experiencing current symptoms of psychosis. The intervention had high satisfaction ratings and seemed to be both feasible and acceptable.
-
(2) Reductions in nightmare-related distress were noted in four of five participants following receipt of Imagery Rehearsal.
-
(3) A larger controlled study, powered to detect group changes in nightmare distress and psychotic symptoms is warranted.








Comments
No Comments have been published for this article.