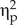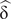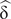In recent decades, there has been growing interest in the conceptualization of executive functions (EF) and their alterations. Since Luria (1966/Reference Luria2012) became interested in cognitive difficulties in patients with frontal damage, several authors have developed theoretical proposals regarding the mechanisms that are part of the so-called executive control functions (Diamond, Reference Diamond2013; Shimamura, Reference Shimamura2000; among others). Three of the most frequently proposed mechanisms are: Cognitive flexibility, working memory and inhibition (Anderson, Bunce, & Barbas, Reference Anderson, Bunce and Barbas2016; Diamond, Reference Diamond2013; Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000).
Inhibition is a mechanism for the control of cognitive activity that operates in different domains (attention, memory, language, etc.), both automatically and consciously, allowing to inhibit mental processes and their contents (see, among others, Anderson & Levy, Reference Anderson, Levy and Benjamin2011). Therefore, cognitive inhibition (CI) intervenes in the interruption and prioritization of cognitive processes.
In the case of memory, Hasher and Zacks (Reference Hasher and Zacks1988) differentiated three types of CI processes: a) Access processes, which control the specific information that enters working memory (WM); b) Elimination processes, which control the information that is removed from WM; and c) Restriction processes, which prevent entry of non-relevant information into WM. The alteration of these processes can cause forgetting of relevant information and could explain, in part, the decline associated with age. Anderson and colleagues (Anderson, Reference Anderson2003; Anderson et al., Reference Anderson, Bunce and Barbas2016; Anderson & Levy, Reference Anderson, Levy and Benjamin2011) emphasized the role of CI in the processes of retrieving information stored in long-term memory. The information is stored, and available, but effective recovery at a given time varies depending on its degree of accessibility. CI would act on unwanted memory traces to produce a potentially reversible and gradual change that would make them less accessible, and, as a consequence, would increase accessibility to the desired memory trace. Thus, in the future, the unwanted memory trace would be more difficult to recover. Therefore, the main function of CI is to regulate the level of accessibility of memory traces. Likewise, it is assumed that part of forgetting is a product of the action of the inhibitory control mechanisms that are put in place to stop, cancel or deactivate the recovery of interfering and/or unwanted responses (Anderson, Reference Anderson2003).
In this line, Friedman and Miyake (Reference Friedman and Miyake2004), based on a latent variable analysis, defined three inhibitory functions evaluated with different tasks: a) Inhibition of preponderant responses (evaluated, for example, with Stroop-type tasks); b) Resistance to interference from distractors (evaluated through naming tasks with interference, flanker tasks, etc.); and c) Resistance to proactive interference (assessed through the Brown-Peterson task, for example).
The previous models define an inhibition mechanism applied to the cognitive control of memory processes (cognitive memory inhibition, CMI) that would intervene, among others, in the active maintenance of relevant information in WM and in the recovery of information from long-term memory systems.
CMI has been studied in diverse populations, being populations with typical development (TD) where there is a greater number of works (Clapp, Rubens, & Gazzaley, Reference Clapp, Rubens and Gazzaley2010). In the adult population, effects of CMI have been observed depending on the length of the words, the similarity of the material to be remembered, the processing load, the maintenance time, etc. (Clapp et al., 2009). In addition, it has been found that CMI is sensitive to the decline associated with age, so that older people would have problems to solve situations of active maintenance of information relevant to the performance of certain tasks, mainly due to the lack of effectiveness to avoid irrelevant information (Solesio-Jofre et al., Reference Solesio-Jofre, Lorenzo, Gutiérrez, López-Frutos, Ruiz-Vargas and Maestú2012; Zacks, Hasher, & Li, Reference Zacks, Hasher, Li, Craik and Salthouse2000).
The number of studies on CMI in populations with TD contrasts with those carried out on populations with intellectual disability (ID). In the only meta-analysis on CMI in intellectual disability that, as far as we know, has been carried out, there are 11 articles that evaluate CMI in people with ID (Baker et al., Reference Baker, Hooper, Skinner, Hatton, Schaaf, Ornstein and Bailey2011; Belacchi et al., Reference Belacchi, Passolunghi, Brentan, Dante, Persi and Cornoldi2014; Borella, Carretti, & Lanfranchi, Reference Borella, Carretti and Lanfranchi2013; Brega et al., Reference Brega, Goodrich, Bennett, Hessl, Engle, Leehey and Grigsby2008; Carretti, Belacchi, & Cornoldi, Reference Carretti, Belacchi and Cornoldi2010; Danielsson, Henry, Rönnberg, & Nilsson, Reference Danielsson, Henry, Rönnberg and Nilsson2010; Lanfranchi, Cornoldi, & Vianello, Reference Lanfranchi, Cornoldi and Vianello2004; Lanfranchi, Jerman, & Vianello, Reference Lanfranchi, Jerman and Vianello2009; dl & Taube, Reference Merrill and Taube1996; O'Dekirk & Merrill, Reference O'Dekirk and Merrill2006; Sampaio, Sousa, Fernández, Henriques, & Gonçalves, Reference Sampaio, Sousa, Férnandez, Henriques and Gonçalves2008, for an exhaustive review see Palomino, López-Frutos, Botella, & Sotillo, Reference Palomino, López-Frutos, Botella and Sotillo2019). People with ID of different etiologies participated in the articles and different experimental paradigms and evaluation procedures were used. Overall, the effect size showed that, in the population with ID, difficulties are observed in the control of CMI in all chronological stages (Belacchi et al., Reference Belacchi, Passolunghi, Brentan, Dante, Persi and Cornoldi2014; Brega et al., Reference Brega, Goodrich, Bennett, Hessl, Engle, Leehey and Grigsby2008; Danielsson et al., Reference Danielsson, Henry, Rönnberg and Nilsson2010 ; Lanfranchi et al., Reference Lanfranchi, Cornoldi and Vianello2004), with the exception of the adult stage -19 to 45 years old- where empirical evidence is scarce. In a single study, CMI processes were evaluated in people with ID aged between 19 and 45 years (Carretti et al., Reference Carretti, Belacchi and Cornoldi2010), with no significant difficulties being observed. This age range is especially relevant as several works (Das & Mishra, Reference Das and Mishra1995; Hawkins, Eklund, James, & Foose, Reference Hawkins, Eklund, James and Foose2003) detected early onset of aging among the population with ID from the age of 35, with a greater probability of manifesting more prominent functioning difficulties after the age of 40 years (Esbensen, Seltzer, & Krauss, Reference Esbensen, Seltzer and Krauss2008; Ghezzo et al., Reference Ghezzo, Salvioli, Solimando, Palmieri, Chiostergi, Scurti and Franceschi2014; Pearlson et al., Reference Pearlson, Warren, Starkstein, Aylward, Kumar, Chase and Folstein1990). The results of the studies reviewed with samples of adult individuals -45 to 65 years- and older individuals -over 65 years- with ID show significant difficulties in CMI (Brega et al., Reference Brega, Goodrich, Bennett, Hessl, Engle, Leehey and Grigsby2008; Danielsson et al., Reference Danielsson, Henry, Rönnberg and Nilsson2010). Despite this, the limitations in the results of the CMI meta-analysis in people with ID reflect the need for studies that evaluate the CMI in people with specific ID etiologies, with samples of different ages and using the same experimental tasks.
Taking into account that CMI is part of executive control functions and its relationship with forgetting and aging, the objectives of this study are:
1. To observe the functioning of CMI processes in an adult population with ID. People with Down syndrome (DS) were selected as DS is considered the most prevalent cause of ID, with an unquestioned genetic diagnosis and a relatively well-defined neuropsychological profile (Lanfranchi et al., Reference Lanfranchi, Jerman and Vianello2009).
2. To analyze the functioning of CMI in adults with ID of different age groups. Taking into account that in people with DS, a greater probability of age-related premature aging has been observed (Zigman, Reference Zigman2013), specifically, after 35 years of age, we decided to establish two age groups: Young people between 18 and 35 years old, and older people aged over 35 years and one month.
The relevance of the research and the objectives set out were determined for several reasons: a) The lack of research on CMI processes in people with DS through a paradigm of delayed visual recognition; b) the selection of participants with a single etiology and with a wide age range. Thus, the bias of previous CMI studies regarding the etiology and age of the samples is avoided; c) the use of a paradigm based on theoretical models of executive control with broad consensus; d) the results will allow comparison with other populations with and without intellectual disabilities in which CMI has been studied.
Method
Participants
Thirty-six people with Down syndrome (mean age = 33.44 years; standard deviation = 7.54 years; range 20–48 years; 18 men and 18 women) and thirty-six people with typical development (mean age = 33.55 years; standard deviation = 7.52 years; range 21–47 years; 18 men and 18 women) participated in the study.
All participants in the experimental group were matched one by one with the participants of the control group in gender, chronological age (interval +/- six months) and hand dominance.
The participants of the experimental group were recruited from different occupational centers of the Community of Madrid.
The inclusion criteria were: (I) Being over 18 years old, (II) having an IQ ≥ 35, (III) having sufficient attention and auditory comprehension abilities to understand the instructions of the cognitive tests and experimental tasks (evaluated through the Comprehensive language section of the CAMCOG-DS), (IV) not having a hearing loss greater than 30 decibels, V) not having any chronic neurological disease (e.g. epilepsy or dementia), (VI) not having ingested first-generation antipsychotics.
The ethics committee of the university approved the research project. All participants had signed an informed consent before the evaluation.
Cognitive Assessment
In order to observe the cognitive functioning of the participants, a cognitive assessment was applied to all participants consisting of: the Go/No-Go Test (Bezdjian, Baker, Lozano, & Raine, Reference Bezdjian, Baker, Lozano and Raine2009); Functional Activities Questionnaire, FAQ (Pfeffer, Kurosaki, Harrah, Chance, & Filos, Reference Pfeffer, Kurosaki, Harrah, Chance and Filos1982); Global Deterioration Scale, GDS (Reisberg, Ferris, de Leon, & Crook, Reference Reisberg, Ferris, de Leon and Crook1982); Subjective Memory Complaints Questionnaire, QSM (Montejo, Montenegro, Reinoso, De Andrés, & Claver, Reference Montejo, Montenegro, Reinoso, De Andrés and Claver2003); Geriatric Depression Scale (Yesavage, Brink, Rose, & Rush, Reference Yesavage, Brink, Rose and Rush2000). In addition to these tests, the following tests were applied to the group of people with DS: Cambridge Cognitive Assessment test for the Assessment of Mental Disorders in Adults with Intellectual Disability, CAMCOG-DS (Holland & Ball, Reference Holland, Ball and Prasher2009); Wechsler Intelligence Scale for Adults Fourth Edition, WAIS–IV (Wechsler, 2008/2012). On the other hand, the revised Cambridge Cognitive Assessment Test for the Assessment of Mental Disorders in Old Age, CAMCOG-R (Roth, Huppert, Mountjoy, Tym, & López-Pousa, 1999/Reference Roth, Huppert, Mountjoy, Tym, López-Pousa and López-Pousa2003) was applied to the group with TD.
The cognitive battery was administered to the group with DS in three sessions of approximately 50 minutes each. In each session, breaks were ensured to avoid the fatigue of the participant. Subsequently, the family filled out the FAQ and GDS questionnaires (approximate time, 15–20 minutes). The participants with TD completed the cognitive battery in a session of approximately 75 minutes, with breaks every half hour. Later, at home, participants with TD filled out the FAQ and GDS questionnaires.
All participants were evaluated individually in a comfortable environment without distractions.
Stimuli and tasks
In both groups, the experimental session to evaluate CMI was carried out after the cognitive evaluation, with a duration of 14 minutes. With the objective of evaluating the functioning of CMI processes, a visual recognition paradigm (Clapp et al., 2009; Solesio-Jofre et al., Reference Solesio-Jofre, Lorenzo, Gutiérrez, López-Frutos, Ruiz-Vargas and Maestú2012) was used. The task consisted of 40 trials distributed in two blocks of 6 minutes each (20 trials per block) and separated by a rest interval of 2 minutes. The task consisted of three phases: Coding, maintenance and recovery (recognition). The stimuli were presented using E-Prime 2.0.
A total of 80 color images of food taken from the FoodCast research image database (Foroni, Pergola, Argiris, & Rumiati, Reference Foroni, Pergola, Argiris and Rumiati2013), Food-pics (Blechert, Meule, Busch, & Ohla, Reference Blechert, Meule, Busch and Ohla2014) and Utrecht standardized food images were selected (Charbonnier, van Meer, van der Laan, Viergever, & Smeets, Reference Charbonnier, van Meer, van der Laan, Viergever and Smeets2016). Explicit authorization from the authors to use these databases was obtained.
The images were cropped on a white background. In each trial, it was prioritized that the stimuli belong to the same database. When it was not possible, care was taken that they had similar visual characteristics. The selection was made through an inter-judge task where they were asked to name the food in each picture. Only the stimuli with a 100% inter-judge coincidence were selected.
The experimental task was presented through a HP personal computer. The images were presented at a distance of 50 cm and focused on the fovea.
First, the participants carried out a familiarization session of the evaluation procedure. A task was used that had the same procedure as the experimental task, but with different stimuli. In the event that the person was not able to understand the task, his/her participation was dismissed. Subsequently, they performed the experimental task (see Figure 1 and 2). The trials of the coding phase began with the black and white image of an eye, to announce the beginning and fix the participant's attention. Then, the image of a food appeared inside the figure of a house for 1500 ms. The participants were instructed to memorize the image that was inside the house. During the maintenance interval, the participant first saw a blank screen with a cross in the center (fixation point) for 1000 ms. Then, two situations could occur: In the condition without interference (no-interference condition, NIC), the blank screen was kept with the cross for another 1500 ms; In the condition with interference (interference condition, IC), another image of the same type of food (unframed inside a house) appeared for 1500 ms. In addition, to ensure interference (in this case of interruption in processing), participants were asked, in half of the trials, if the food presented was sweet, and in the other half, if it was salty. The expected response half of the time was affirmative and in the other half negative.

Figure 1. Structure of a Trial in the Condition without Interference.

Figure 2. Structure of a Trial in the Condition with Interference-Interruption-.
Afterwards, in both conditions, the screen was kept blank for 4000 ms with a cross in its center to fix the attention.
The recognition phase was identical in both conditions: First, the image of a house with two interrogations on the sides was presented for 1000 ms. Just after, for 1500 ms, the image of a food was presented inside a house between question marks. At the same time, the participant heard the phrase "Was it in the house before?" In half of the presentations, the image of the recognition phase was equal to that of the coding phase and in the other half, it was different, but of the same type of food. A randomized distribution of the trials was presented.
Subsequently, the image of the food disappeared, but the image of the house with the question marks was maintained until the participant yielded an answer (affirmative/negative).
Two buttons were available to give the answers, one green for the affirmative answers and one red for the negative answers. The participants placed the buttons so that the option "yes" (green button) was next to their dominant hand.
Once the recognition response was given, a blank screen (250 ms) appeared, followed by a screen with the drawing of an eye indicating the beginning of the next trial.
Design
Three independent variables were manipulated: Population (interparticipants), age (interparticipants) and type of interference (intraparticipants). The population variable had two levels: People with DS and people with TD. To observe the relationship between age and the ability to resolve interference in memory tasks in people with ID, two groups were established: Young adults (up to 35 years) and older adults (over 35 years). The interference variable had two levels: Without interference (during the maintenance phase no new information was presented) and with interference in the form of interruption (during the retention interval, a concurrent task was performed). Therefore, the design used was a 2 x 2 x 2 factorial design. The dependent variable was the number of hits.
Procedure
The participants of both groups first performed the cognitive assessment and then the experimental tasks. The experimental conditions were always carried out in the same order: First without interference and then the condition with interference -interruption-. Thus, it was possible to establish the performance of the groups in delayed visual recognition tasks where interference inhibition does not necessarily have to take place at the beginning.
Statistical Analyses
Data analyses were performed with version 20 of the statistical package for social sciences (SPSS).
First, the samples were analyzed to verify if they met the assumptions of normality (Kolmogorov-Smirnov Test) and homocedasticity of variances (Levene's Test) for the tests performed. Parametric statistics were used with those tests that met the first assumption and non-parametric statistics were used with the rest. In addition, the effect size was calculated with the partial square eta statistic ( $ {\upeta}_{\mathrm{p}}^2\Big). $ Following conventions, a small effect size was considered for
$ {\upeta}_{\mathrm{p}}^2\Big). $ Following conventions, a small effect size was considered for  $ {\upeta}_{\mathrm{p}}^2 $= .01, a medium effect for
$ {\upeta}_{\mathrm{p}}^2 $= .01, a medium effect for  $ {\upeta}_{\mathrm{p}}^2 $= .06, and a large effect for
$ {\upeta}_{\mathrm{p}}^2 $= .06, and a large effect for  $ {\upeta}_{\mathrm{p}}^2 $= .14. In the analysis with a single sample, Cohen's d (
$ {\upeta}_{\mathrm{p}}^2 $= .14. In the analysis with a single sample, Cohen's d ( $ \hat{\updelta\ } $) was used. For its interpretation, the conventions d = 0.2 small, d = 0.5 medium and d = 0.8 large were used. With respect to the direction, the positive sign in both statistics and in the effect sizes indicated, depending on the analysis: More memory in the condition without interference, more memory in the group without disability or more memory in the group of young adults. Moreover, the original data of the intergroup and intragroup analyses can be consulted in the supplementary material file (Tables S1 and S2).
$ \hat{\updelta\ } $) was used. For its interpretation, the conventions d = 0.2 small, d = 0.5 medium and d = 0.8 large were used. With respect to the direction, the positive sign in both statistics and in the effect sizes indicated, depending on the analysis: More memory in the condition without interference, more memory in the group without disability or more memory in the group of young adults. Moreover, the original data of the intergroup and intragroup analyses can be consulted in the supplementary material file (Tables S1 and S2).
Also, the presence of cognitive impairment was evaluated in all participants, estimating its presence if the score was lower than the CAMCOG cut-off point, a GDS > 2 score and, if the participant expressed subjective memory complaints -QSM questionnaire> 2-. No participant met the criteria established for the presence of cognitive impairment, therefore, all of them were part of the analyzed sample. Likewise, there were no missing values either in the cognitive evaluation or in the experimental task.
Results
Cognitive Assessment
After performing an intergroup analysis (see Table 1), significant differences were observed in CAMCOG, the number of omission errors of the Go/No-Go Test, in the Yesavage Test and in the FAQ. However, in the analysis by age, no statistically significant differences were found between young adults and older adults.
Table 1. Cognitive Assessment by Group: Mean (Standard Deviation)

Note. - = not administered; DS = Down syndrome; TD = typical development.
Experimental Task
Status of the mnesic cognitive inhibition processes. Participants' performance was evaluated by comparing the mean correct answers in the recognition phase of each experimental task - see Table 2 and Figure 3- (Solesio-Jofre et al., Reference Solesio-Jofre, Lorenzo, Gutiérrez, López-Frutos, Ruiz-Vargas and Maestú2012).
Table 2. Experimental Task by Group: Mean (Standard Deviation)

Note. DS = Down syndrome; TD = typical development.

Figure 3. Effectiveness of CMI According to the Group of Participants.
To observe the relationship between the variables age, population and level of interference, a repeated measures ANOVA was carried out with a 2 x 2 x 2 (Population x Age x Interference level) design. The results indicated: a) A main effect of the population variable, F(1, 68) = 159.09, p < .001,  $ {\upeta}_{\mathrm{p}}^2 $ = .70; the performance of people with DS, Mean = 12.97, Standard deviation (SD) = 2.24, was lower than that of people with TD (Mean = 18.14, SD = 1.13) in the CMI task; and b) a main effect of the interference variable, F(1, 68) = 11.26, p = .001,
$ {\upeta}_{\mathrm{p}}^2 $ = .70; the performance of people with DS, Mean = 12.97, Standard deviation (SD) = 2.24, was lower than that of people with TD (Mean = 18.14, SD = 1.13) in the CMI task; and b) a main effect of the interference variable, F(1, 68) = 11.26, p = .001,  $ {\upeta}_{\mathrm{p}}^2 $ = .14, delayed recognition decreased due to the presence of interference (interruption) in the maintenance phase (Mean = 15.56, SD = 3.14) with respect to the condition without interference (Mean = 16.47, SD = 2.44). Likewise, a statistically significant interaction was found between population and interference level, F(1, 68) = 12.67, p = .001,
$ {\upeta}_{\mathrm{p}}^2 $ = .14, delayed recognition decreased due to the presence of interference (interruption) in the maintenance phase (Mean = 15.56, SD = 3.14) with respect to the condition without interference (Mean = 16.47, SD = 2.44). Likewise, a statistically significant interaction was found between population and interference level, F(1, 68) = 12.67, p = .001,  $ {\upeta}_{\mathrm{p}}^2 $ = .16. However, no age-related effects were found, F(1, 68) = 0.70, p = .406,
$ {\upeta}_{\mathrm{p}}^2 $ = .16. However, no age-related effects were found, F(1, 68) = 0.70, p = .406,  $ {\upeta}_{\mathrm{p}}^2 $ = .01, Power = .13, nor with the interaction between the variables age and level of interference, F(1, 68) = 0.00, p = 1,
$ {\upeta}_{\mathrm{p}}^2 $ = .01, Power = .13, nor with the interaction between the variables age and level of interference, F(1, 68) = 0.00, p = 1,  $ {\upeta}_{\mathrm{p}}^2 $ = .00, Power = .05, nor in the interaction between population, age and interference, F(1, 68) = 0.04, p = .839,
$ {\upeta}_{\mathrm{p}}^2 $ = .00, Power = .05, nor in the interaction between population, age and interference, F(1, 68) = 0.04, p = .839,  $ {\upeta}_{\mathrm{p}}^2 $ = .00, Power = .06.
$ {\upeta}_{\mathrm{p}}^2 $ = .00, Power = .06.
Regarding the effects of the interaction between population and interference level variables, in both conditions significant differences were observed. Thus, in the condition without interference, the group with TD (Mean = 18.08, SD = 1.05) recognized more food than the group with DS, Mean = 14.86, SD = 2.38, Z(72) = 6.05, p < .001,  $ {\upeta}_{\mathrm{p}}^2 $ = .44. In the same way, the DS group (Mean = 12.97, SD = 2.24) had a lower performance than the TD group (Mean = 18.14, SD = 1.13) in the recognition task in the condition in which there was a concurrent task during the maintenance phase, Z(72) = 7.11, p < .001,
$ {\upeta}_{\mathrm{p}}^2 $ = .44. In the same way, the DS group (Mean = 12.97, SD = 2.24) had a lower performance than the TD group (Mean = 18.14, SD = 1.13) in the recognition task in the condition in which there was a concurrent task during the maintenance phase, Z(72) = 7.11, p < .001,  $ {\upeta}_{\mathrm{p}}^2 $ = .69. The mean scores of each group reflect greater recall in the population with typical development both in the condition without interference and in the condition with interference -interruption- (see Figure 3).
$ {\upeta}_{\mathrm{p}}^2 $ = .69. The mean scores of each group reflect greater recall in the population with typical development both in the condition without interference and in the condition with interference -interruption- (see Figure 3).
The analysis of the effects of the level of interference in the group of people with DS indicated that the presence of interference -interruption- (Mean = 12.97, SD = 2.24) significantly reduced recognition regarding the condition without interference- without interruption- (Mean = 14.86, SD = 2.38) during the maintenance phase, Z(36) = 3.3, p = .001,  $ \hat{\updelta} = $ 0.80. On the contrary, in the group of people with TD, both in the condition without interference (Mean = 18.08, SD = 1.05) and in the condition with interference -interruption- (Mean = 18.14, SD = 1.13) there were no significant differences, Z(36) = –0.22, p = .823,
$ \hat{\updelta} = $ 0.80. On the contrary, in the group of people with TD, both in the condition without interference (Mean = 18.08, SD = 1.05) and in the condition with interference -interruption- (Mean = 18.14, SD = 1.13) there were no significant differences, Z(36) = –0.22, p = .823,  $ \hat{\updelta\ } $= –0.05, Power = .26.
$ \hat{\updelta\ } $= –0.05, Power = .26.
Effect of age. The second objective was to observe the effects of age on the mechanisms of resolution of interference in people with and without DS. Although no significant effects were found on the levels of the age variable, nor on its interaction with other variables, a set of analyses were carried out in order to analyze in greater depth its possible effects.
First, we analyzed how the presence of a secondary task with its consequent interference (interruption) during the maintenance phase affected performance in both populations according to different age groups (see Table 3).
Table 3. Experimental Task by Age: Mean (Standard Deviation)

Note. DS = Down syndrome; TD = typical development.
To do this, a U test was performed for independent samples where the group of young adults with DS was compared with the group of young adults with TD. In the condition with interference (interruption), significant differences were found, z(36) = 4.99, p < .001,  $ {\upeta}_{\mathrm{p}}^2 $= .69, among young adults with DS (Mean = 13.06, SD = 2.29) and young people with TD (Mean = 18.33, SD = 1.19). In addition, statistically significant differences were found, z(36) = 4.52, p < .001,
$ {\upeta}_{\mathrm{p}}^2 $= .69, among young adults with DS (Mean = 13.06, SD = 2.29) and young people with TD (Mean = 18.33, SD = 1.19). In addition, statistically significant differences were found, z(36) = 4.52, p < .001,  $ {\upeta}_{\mathrm{p}}^2 $= .56, in the condition without interference between young adults with DS (Mean = 15, SD = 1.82) and young adults with TD (Mean = 18.22, SD = 1). A difference of 0.13 was observed between the effect sizes. As for the older adult groups, in both conditions, significant differences were found. Thus, the older adults with DS in the condition with interference (Mean = 12.89, SD = 2.25) had a significantly worse performance, z(36) = 5.02, p < .001,
$ {\upeta}_{\mathrm{p}}^2 $= .56, in the condition without interference between young adults with DS (Mean = 15, SD = 1.82) and young adults with TD (Mean = 18.22, SD = 1). A difference of 0.13 was observed between the effect sizes. As for the older adult groups, in both conditions, significant differences were found. Thus, the older adults with DS in the condition with interference (Mean = 12.89, SD = 2.25) had a significantly worse performance, z(36) = 5.02, p < .001,  $ {\upeta}_{\mathrm{p}}^2 $= .69, with respect to the older adult controls (Mean = 17.94, SD = 1.06). The same happened in the condition without interference, where the older adults with DS (Mean = 14.72, SD = 2.89) had a significantly worse performance, z(36) = 4.22, p < .001,
$ {\upeta}_{\mathrm{p}}^2 $= .69, with respect to the older adult controls (Mean = 17.94, SD = 1.06). The same happened in the condition without interference, where the older adults with DS (Mean = 14.72, SD = 2.89) had a significantly worse performance, z(36) = 4.22, p < .001,  $ {\upeta}_{\mathrm{p}}^2 $= .37, than the older adult controls (Mean = 17.94, SD = 1.11) (see Figure 4). A difference of 0.32 was observed between the effect sizes.
$ {\upeta}_{\mathrm{p}}^2 $= .37, than the older adult controls (Mean = 17.94, SD = 1.11) (see Figure 4). A difference of 0.32 was observed between the effect sizes.

Figure 4. Effectiveness of CMI According to Age Group.
Specifically, in young adults with DS, the appearance of interference caused a decrease in memory. Being the difference between the memory in the interference condition (Mean = 13.06, SD = 2.29) and the condition without interference (Mean = 15, SD = 1.82) statistically significant, z(18) = 2.45, p = .014,  $ \hat{\updelta\ } $= 0.88. Older adults with DS suffered a decrease in the execution of the task when there was interference in the maintenance phase (Mean = 12.89, SD = 2.25) compared to when there was no interference (Mean = 14.72, SD = 2.89), t(17) = 2.52, p = .022,
$ \hat{\updelta\ } $= 0.88. Older adults with DS suffered a decrease in the execution of the task when there was interference in the maintenance phase (Mean = 12.89, SD = 2.25) compared to when there was no interference (Mean = 14.72, SD = 2.89), t(17) = 2.52, p = .022,  $ \hat{\updelta\ } $= 0.67 (see Figure 4).
$ \hat{\updelta\ } $= 0.67 (see Figure 4).
In the group of young adults with TD, the appearance of interference did not affect their memory. The differences in the performance of the task with and without interference were not significant, t(17) = –0.33, p = .742,  $ \hat{\updelta\ } $= –0.1, Power = .26, specifically, in the condition without interference (Mean = 18.22, SD = 1) and in the condition with interference (Mean = 18.33, SD = 1.19). In addition, the presence of interference did not sufficiently affect older adults with TD so as to observe statistically significant differences between both conditions, t(17) = 0, p = 1,
$ \hat{\updelta\ } $= –0.1, Power = .26, specifically, in the condition without interference (Mean = 18.22, SD = 1) and in the condition with interference (Mean = 18.33, SD = 1.19). In addition, the presence of interference did not sufficiently affect older adults with TD so as to observe statistically significant differences between both conditions, t(17) = 0, p = 1,  $ \hat{\updelta\ } $= 0, Power = .26 (see Figure 5). The means in both conditions were exactly the same (Mean = 17.94), with a SD = 1.11 in the condition without interference and SD = 1.06 in the condition with interference.
$ \hat{\updelta\ } $= 0, Power = .26 (see Figure 5). The means in both conditions were exactly the same (Mean = 17.94), with a SD = 1.11 in the condition without interference and SD = 1.06 in the condition with interference.

Figure 5. Efficacy of CMI as a Function of Age within Each Group of Participants.
In order to establish the existence of some degree of relationship between the variables of age and interference, the Pearson correlation index was calculated. No significant correlations were found. Not finding differences based on age, we analyzed the possible grouping of participants based on the resolution of the interference. To do so, three clusters were calculated: The first with the entire group of participants, the second with participants with DS, and the third with participants with TD (see Figure 6). The selected analysis was the classification by two hierarchical clusters. When analyzing the cluster between age and interference, a coherent pattern was not found to justify a change in the age cut-off points of the groups.

Figure 6. Hierarchical Clusters as a Function of the Efficiency in the Resolution of the CMI.
As several studies have observed early premature aging in the population with ID from the age of 35 (Das & Mishra, Reference Das and Mishra1995; Hawkins et al., Reference Hawkins, Eklund, James and Foose2003) and a greater probability of manifesting more prominent functioning difficulties after 40 years of age (Esbensen et al., Reference Esbensen, Seltzer and Krauss2008; Ghezzo et al., Reference Ghezzo, Salvioli, Solimando, Palmieri, Chiostergi, Scurti and Franceschi2014; Pearlson et al., Reference Pearlson, Warren, Starkstein, Aylward, Kumar, Chase and Folstein1990), it was decided to analyze whether there were differences in the CMI according to age between adults over 40 years and younger adults. Seven people were over 40 years of age, so the seven youngest people in the sample were selected to do this analysis. Finally, the differences between groups were not statistically significant, t(12) = –0.10, p = .92,  $ \hat{\updelta\ } $= –0.05, Power = .26. Specifically, in the group of younger adults, the mean hits were 3.29 (SD = 2.36) and in the group of older participants the mean hits were 3.43 (SD = 2.82).
$ \hat{\updelta\ } $= –0.05, Power = .26. Specifically, in the group of younger adults, the mean hits were 3.29 (SD = 2.36) and in the group of older participants the mean hits were 3.43 (SD = 2.82).
Discussion
The first objective was to establish the functioning of cognitive inhibition processes in memory tasks in the adult population with DS. The general results indicate that the participants of the group with DS had a worse execution in CMI tasks of delayed visual recognition compared to their controls. These differences are interpreted as large by having an effect size of 0.70. Specifically, the execution of the group with DS was worse than that of the control group, both in the condition without interference and in the interference -interruption- condition. Therefore, the differences between both groups were obvious, with or without interruption. In addition, when it was required to start the inhibition processes to control the interference generated by a concurrent task, the differences between the groups increased (0.44 in the condition without interference and 0.69 in the condition with interference). This result is in line with previous work on CMI in people with ID (Baker et al., Reference Baker, Hooper, Skinner, Hatton, Schaaf, Ornstein and Bailey2011; Belacchi et al., Reference Belacchi, Passolunghi, Brentan, Dante, Persi and Cornoldi2014; Brega et al., Reference Brega, Goodrich, Bennett, Hessl, Engle, Leehey and Grigsby2008; Danielsson et al., Reference Danielsson, Henry, Rönnberg and Nilsson2010; Lanfranchi et al., Reference Lanfranchi, Cornoldi and Vianello2004; Merril & Taube, Reference Merrill and Taube1996; O'Dekirk & Merrill, Reference O'Dekirk and Merrill2006; Sampaio et al., Reference Sampaio, Sousa, Férnandez, Henriques and Gonçalves2008). Specifically, the results obtained follow the line of studies that have analyzed CMI processes in other etiologies, such as Fragile X syndrome (Baker et al., Reference Baker, Hooper, Skinner, Hatton, Schaaf, Ornstein and Bailey2011; Brega et al., Reference Brega, Goodrich, Bennett, Hessl, Engle, Leehey and Grigsby2008), where a joint effect size of 0.56 - confidence interval between 0.25 and 0.87- between people with TD and with FXS was observed. Likewise, these data are similar to those found in resolution of mnesic interference in people with unspecified ID (Danielsson et al., Reference Danielsson, Henry, Rönnberg and Nilsson2010; Merril & Taube, Reference Merrill and Taube1996; O’Dekirk & Merrill, Reference O'Dekirk and Merrill2006), where the associated joint effect size was 0.54 -confidence interval between 0.30 and 0.78-.
The results obtained (interference significantly affected the group with DS, but not the group with TD) can be assessed by the processing load involved in the tasks. A medium or low processing load only affected the group with ID, whereas the people in the control group were able to keep in their short-term memory the information they had to remember after a few seconds, while performing a concurrent task. In addition, even with small processing loads, performance was affected in the participants in the group with DS, not being able to keep the memory trace activated while performing a concurrent task. According to Hasher, Lustig and Zacks (Reference Hasher, Lustig and Zacks2007), in the presence of interference -interruption-, inhibition processes are started, which fulfill three functions: (a) Allow access to information stored in working memory, (b) discard information that is not relevant at that time, (c) restrict the presence of distractors. In this sense, we interpret that the people with DS evaluated showed difficulties in at least one of these three functions. Likewise, several authors relate the malfunction of any of these three functions with problems in the explicit recovery of relevant information and, therefore, with forgetting (Anderson, Reference Anderson2003; Hasher, Lustig, & Zacks, Reference Hasher, Lustig and Zacks2007).
In the case of participants with TD, the CMI processes worked optimally, the person retrieved the requested information and inhibited the distracting information, which will be more difficult to access in the future. On the other hand, if CMI processes failed, they could have decreased accessibility to relevant information (Anderson & Levy, Reference Anderson, Levy and Benjamin2011). Along the same lines, Friedman and Miyake (Reference Friedman and Miyake2004) understood that inefficiency in the resolution of interference could be related to the inhibition of automatic or preponderant responses, resisting interference from distractors and resisting proactive interference.
Problems of effectiveness in resolving interference have also been observed in other populations, such as in people with mild cognitive impairment. Aurtenetxe et al. (Reference Aurtenetxe, García-Pacios, del Río, López, Pineda-Pardo, Marcos and Maestú2016), applying a task similar to that used in the present study, with people with mild cognitive impairment, found that, in the presence of interference -interruption-, there was a greater number of forgotten information. These results are consistent with other inhibition studies with populations with Alzheimer's dementia (Collette, Schmidt, Scherrer, Adam, & Salmon, Reference Collette, Schmidt, Scherrer, Adam and Salmon2009).
The second objective focused on the analysis of the functioning of CMI in adults with DS in relation to their age. Some studies indicate that in the population with DS, there is a greater probability of premature aging and that the onset of this aging usually appears in this population around the age of 35 (Das & Mishra, Reference Das and Mishra1995; Hawkins et al., Reference Hawkins, Eklund, James and Foose2003). In addition, in this population, there is a greater probability of manifesting more prominent functioning difficulties from the age of 40 (Esbensen et al., Reference Esbensen, Seltzer and Krauss2008; Ghezzo et al., Reference Ghezzo, Salvioli, Solimando, Palmieri, Chiostergi, Scurti and Franceschi2014; Pearlson et al., Reference Pearlson, Warren, Starkstein, Aylward, Kumar, Chase and Folstein1990). This objective was relevant because no previous research had studied CMI processes in people with DS in relation to their age. In the present study, the mean age of the younger adult group was 27 years and the mean age of the older adult group was 40 years. In general, no main effect of the age variable was found, nor were there any effects of the interaction between this variable and the interference. Within each group, when comparing young and older adults with DS, we found higher mean scores in younger adults compared to older adults in all experimental conditions, but these differences were not statistically significant. Likewise, we observed similar results in the population with TD. A possible explanation of the results could be found in the fact that difficulties in CMI appear in the population with DS from an early age (Belacchi et al., Reference Belacchi, Passolunghi, Brentan, Dante, Persi and Cornoldi2014; Lanfranchi et al., Reference Lanfranchi, Cornoldi and Vianello2004; Merrill & Taube, Reference Merrill and Taube1996; O'Dekirk & Merrill, Reference O'Dekirk and Merrill2006). This would be similar to the affectation that is observed in other cognitive processes. Thus, the group of younger adults with DS had a significantly lower functioning in the resolution of interference compared to younger adults with TD. In addition, the involvement of CMI processes in younger adults with DS could not be explained by a general cognitive impairment, as, based on the results obtained with CAMCOG, GDS and the QSM questionnaire, no participant presented previous general cognitive impairment.
More exhaustive analyses, at the intragroup level, showed that, in younger adults with DS, the appearance of interference caused a significant decrease in memory in the working memory ( $ \hat{\updelta\ } $= 0.88). These data are consistent with previous research, where interference control has been studied in working memory tasks with word lists in young people with DS (Belacchi et al., Reference Belacchi, Passolunghi, Brentan, Dante, Persi and Cornoldi2014; Lanfranchi et al., Reference Lanfranchi, Cornoldi and Vianello2004). In the present study, this same pattern of results was observed also in the older population with DS, that is, in the presence of interference, caused by a concurrent task, memory efficiency decreased significantly (
$ \hat{\updelta\ } $= 0.88). These data are consistent with previous research, where interference control has been studied in working memory tasks with word lists in young people with DS (Belacchi et al., Reference Belacchi, Passolunghi, Brentan, Dante, Persi and Cornoldi2014; Lanfranchi et al., Reference Lanfranchi, Cornoldi and Vianello2004). In the present study, this same pattern of results was observed also in the older population with DS, that is, in the presence of interference, caused by a concurrent task, memory efficiency decreased significantly ( $ \hat{\updelta\ } $= 0.67). Although there are no previous studies that relate both variables directly, Danielsson et al. (Reference Danielsson, Henry, Rönnberg and Nilsson2010) observed similar data when they studied the resistance to interference in people with disabilities without a specified etiology. On the other hand, in the younger population with TD, the appearance of interference did not cause a decrease in the recall of information from the working memory system. In the older population with TD, the interference did not influence memory either. It could be possible that the characteristics of the experimental task could explain this pattern in people with DS and not in people with TD. On the one hand, the processing load necessary to perform the task correctly could be low and, on the other, the interference -interruption- situation generated by the concurrent task may not have enough impact on the maintenance of the memory trace for the performance of the population with TD to be diminished. However, since there is no ceiling effect for any of the groups in any of the experimental conditions, we cannot attribute the results to the difficulty of the task. It could be attributed to an alteration of the cognitive inhibition processes. This interpretation is related to the data obtained in different studies, where they state that the processing load reduces the amount of resources available to perform a secondary task (Naveh-Benjamin, Craik, Guez, & Kreuger, Reference Naveh-Benjamin, Craik, Guez and Kreuger2005). In this study, the processing load of the task did not make it difficult for the participants with TD to actively keep the information to be remembered later. This proposal was widely developed by Hasher and Zacks (Reference Hasher and Zacks1988), who stated that those interference tasks that do not pose a high demand in working memory would not cause disadvantages in young adults or in adults with typical development. On the other hand, in people with DS -regardless of their age- the presence of interference is sufficient to cause them to forget the relevant information.
$ \hat{\updelta\ } $= 0.67). Although there are no previous studies that relate both variables directly, Danielsson et al. (Reference Danielsson, Henry, Rönnberg and Nilsson2010) observed similar data when they studied the resistance to interference in people with disabilities without a specified etiology. On the other hand, in the younger population with TD, the appearance of interference did not cause a decrease in the recall of information from the working memory system. In the older population with TD, the interference did not influence memory either. It could be possible that the characteristics of the experimental task could explain this pattern in people with DS and not in people with TD. On the one hand, the processing load necessary to perform the task correctly could be low and, on the other, the interference -interruption- situation generated by the concurrent task may not have enough impact on the maintenance of the memory trace for the performance of the population with TD to be diminished. However, since there is no ceiling effect for any of the groups in any of the experimental conditions, we cannot attribute the results to the difficulty of the task. It could be attributed to an alteration of the cognitive inhibition processes. This interpretation is related to the data obtained in different studies, where they state that the processing load reduces the amount of resources available to perform a secondary task (Naveh-Benjamin, Craik, Guez, & Kreuger, Reference Naveh-Benjamin, Craik, Guez and Kreuger2005). In this study, the processing load of the task did not make it difficult for the participants with TD to actively keep the information to be remembered later. This proposal was widely developed by Hasher and Zacks (Reference Hasher and Zacks1988), who stated that those interference tasks that do not pose a high demand in working memory would not cause disadvantages in young adults or in adults with typical development. On the other hand, in people with DS -regardless of their age- the presence of interference is sufficient to cause them to forget the relevant information.
Finally, an interpretation of the results obtained regarding the effects of age on the processes of inhibition in memory tasks could lie in the choice of the age range of the groups. To contrast this possibility, different analyses were performed. It was found that, in people with DS, there does not appear to be a direct association between age and resistance to interference, as indicated by other studies on other cognitive processes. Nor were differences found in the organization of the groups according to their age. An analysis comparing young people with DS and those over 40 with DS also revealed no significant differences in the effects of interruption in short-term visual recognition tasks.
Moreover, it was observed that the differences between the population with DS and TD increased as the chronological line progressed. In this way, if we subtract the effect sizes in the resolution of the task between the younger population with DS and the younger population with TD, we observe a difference of 0.13. Whereas, if we subtract the effect sizes between the older population with DS and the older population with TD, the difference is 0.32. Therefore, there is a greater difference in the resolution of CMI between the older populations than among the younger populations of DS and TD. At the intergroup level, this pattern could be reflecting differences between the group with DS and the group with TD in how aging affects the CMI processes. So that within the CMI processes, being over 35 years affects people with DS more than people with TD. Therefore, it seems that CMI processes are affected years before in people with DS compared to people with TD. This data is consistent with results of cognitive processes collected longitudinally, where the decline associated with age occurs earlier in people with DS than in people with TD (Hawkins et al., Reference Hawkins, Eklund, James and Foose2003). This is, in our opinion, one of the most interesting and novel findings of the present study, as it reflects that as the participants progress in chronological age, the intergroup difference in the CMI processes is greater, which speaks of an early and more pronounced aging in populations with DS compared to populations with TD.
The results obtained indicate a worse starting performance in the group with ID. Likewise, the statistically significant effect of the interference condition on the delayed recognition of the stimuli is confirmed. A novel result of the present study is that the affectation of CMI observed differentially in the group with DS is detected in situations of interference -interruption- with an experimental paradigm that had not previously been applied with people with ID. This allows us to affirm that the group of people with DS evaluated shows lower levels of efficacy in cognitive inhibition processes in interruption tasks. In addition, when analyzing the effects of age, we observe that, in terms of effectiveness in the processing of CMI, being over 35 years of age affects people with DS more than people with TD. Finally, it should be noted that the difficulties encountered in resolving interference in people with DS are especially important in their daily lives because CMI processes have the function of controlling information that remains activated in memory (Anderson, Reference Anderson2003; Hasher & Zacks, Reference Hasher and Zacks1988). Therefore, from the data obtained on CMI processes in people with DS, it seems necessary to develop intervention programs on these processes to improve the quality of life of adults with DS.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/SJP.2020.4.