Introduction
In temperate regions, environmental conditions, especially temperature, during the winter or summer prior to germination in spring and autumn, respectively, either break dormancy (i.e. physiological or morphophysiological dormancy) or make seeds sensitive to dormancy break (i.e. physical or combinational dormancy). If dormancy break or induction of sensitivity to dormancy break occurs in summer, seeds can germinate in autumn, whereas if they occur in winter, seeds can germinate in spring. Furthermore, after seeds become nondormant (or sensitive to dormancy break), germination depends on the soil being moist and on temperatures in the habitat overlapping with those required for germination (or dormancy break of sensitive seeds with physical or combinational dormancy). Also, some seeds require light and others darkness to germinate (Baskin and Baskin, Reference Baskin and Baskin2014).
Seeds exposed to natural temperature regimes in the temperate region of eastern North America have different germination seasons. Seeds of winter annuals (WA) and summer annuals (SA) germinate in autumn and in spring/summer, respectively, while those of monocarpic perennials (MP) and polycarpic (herbaceous) perennials (PP) germinate in autumn or in spring/summer, depending on the species (Baskin and Baskin, Reference Baskin and Baskin1988). In considering the ecological significance of seeds of herbaceous species living in the same geographical region having different germination seasons, a logical question is: what is the range of temperatures over which seeds germinate?
The temperature range for germination can have important consequences on the ecological niche of species (Donohue et al., Reference Donohue, Rubio de Casas, Burghardt, Kovach and Willis2010). A narrow temperature range means a short period of time during which seeds could germinate in the field and probably only a small number of seedling cohorts. On the other hand, a wide temperature range could result in many seedling cohorts that emerged when environmental conditions vary greatly. If the timing of germination is changed, the resulting plants will grow in different post-germination environments (Donohue, Reference Donohue2002; Donohue et al., Reference Donohue, Rubio de Casas, Burghardt, Kovach and Willis2010), which may affect plant fitness (Lu et al., Reference Lu, Tan, Baskin and Baskin2014). Also, if mother plants produce seeds under different environmental conditions, new variations of traits may occur that are subjected to selection by the environment (Donohue et al., Reference Donohue, Rubio de Casas, Burghardt, Kovach and Willis2010; Burghardt et al., Reference Burghardt, Edwards and Donohue2016).
Also, it is assumed that species whose seeds can germinate over a wide range of temperatures would have a wider geographical distribution than those with a narrow temperature range for germination (Donohue et al., Reference Donohue, Rubio de Casas, Burghardt, Kovach and Willis2010). However, Thompson and Ceriani (Reference Thompson and Ceriani2003) found no relationship between the range of temperatures at which seeds can germinate and the size of the geographical range of species in the UK. On the other hand, Rosbakh and Poschlod (Reference Rosbakh and Poschlod2015) showed that for species with a broad geographical range, the initial temperature at which seeds would germinate increased with a decrease in mean annual temperature in the habitat.
Aleman (Reference Aleman2014) concluded from her studies in southern Australia that ‘ … annuals may germinate under a greater range of conditions than perennials’. The reason for this difference could be related to how annual and perennial species persist (without immigration) at sites where conditions are unpredictable for seed set each year. Annual species without a seed bank must produce seeds each year; thus, a relatively long germination season or ability to germinate over a wide range of temperatures may result in more than one chance for germination/seedling establishment (e.g. Baskin and Baskin, Reference Baskin and Baskin1972). In contrast, perennials can persist in unpredictable sites without producing seeds each year or without forming a persistent seed bank because individual plants remain alive for some number of years. In which case, a relatively short germination season or a narrow range of germination temperatures for perennials could restrict germination to the time that is most favourable for germination and seedling establishment (Donohue et al., Reference Donohue, Rubio de Casas, Burghardt, Kovach and Willis2010). In view of the potential differences in requirements to persist in unpredictable sites, we predicted that annuals have a wider range of germination temperatures than perennials.
Over a 37-year period, we conducted germination phenology studies on 350 species (SA, 63; WA, 83; MP, 28; and PP, 176) in 59 families in a non-temperature-controlled glasshouse in Kentucky (USA), with continuous temperature monitoring. Using the data from these germination phenology studies, we tested the prediction that annuals have a wider temperature range for germination than perennials.
The timing of germination is relevant to seedling survival and, in the case of annuals, seed production by the surviving plants. Thus, germination early in spring (or autumn) would give seedlings the maximum period of time to grow and become established before the onset of summer drought (or winter cold). In a literature survey of 58 species, Baskin and Baskin (Reference Baskin and Baskin2014, see their Table 12.6) found that the first seedling cohort (vs the intermediate or last cohort) had the highest percentage of seedlings surviving for 31 species and the highest seed production per surviving plant for 14 species. In 16 species, the early seedling cohorts had the lowest survival but the highest seed production per plant, and in seven species, the last cohort had the lowest survival but the highest seed production per plant. Our prediction that annuals have a wider temperature range for germination than perennials leads us to predict that annuals begin to germinate at lower temperatures than perennials in spring and that the reverse is true for autumn-germinating annuals and perennials. Furthermore, we predicted that the temperature during the week with the highest germination percentage is higher for annuals than for perennials. To test these two predictions, temperatures were compared for the week when seeds of WA, SA, MP and PP first being to germinate and the week with the highest germination.
In the first, second and third germination seasons after burial, exhumed seeds of the SA Ambrosia artemisiifolia germinated to 70, 40 and 3%, respectively, at 15/6°C (Baskin and Baskin, Reference Baskin and Baskin1980), and those of the WA Lamium purpureum germinated to 68, 14 and 0%, respectively, at 35/20°C (Baskin and Baskin, Reference Baskin and Baskin1984). On the other hand, seeds of the PP Carex stricta exhumed in the first, second and third germination seasons after burial germinated to 95, 63 and 63%, respectively, at 15/6°C (Baskin et al., Reference Baskin, Baskin and Chester1996). These data lead us to predict that the width of the temperature range for germination of seeds of a particular cohort that fail to germinate the first year is reduced in subsequent years and that the reduction is greater for annuals than for perennials. To test this prediction, we selected only species that germinated for three consecutive years in the phenology studies.
For species that sexually reproduce and maintain stable populations in a temperate region, it is reasonable to assume that the temperature range for germination is wide enough for seeds to germinate in their normal spring or autumn germination season each year. Thus, we predict that the width of the temperature range for germination of annuals and perennials is as wide as the expected variability in temperatures for each month during the germination season. To test this prediction, the mean daily maximum and mean daily minimum monthly temperatures for each month of the years 1970–2006 and the variation in temperature for each month during the 37-year period were determined, and the results compared to the temperature range for germination for each life cycle type.
Although various germination phenology studies have been done (e.g. Kondo et al., Reference Kondo, Sato, Baskin and Baskin2006; Vandelook and Van Assche, Reference Vandelook and Van Assche2008; Porceddu et al., Reference Porceddu, Mattana, Pritchard and Bacchetta2013), we do not have a comprehensive overview of the range of temperatures over which seeds of WA, SA, MP and PP germinate when exposed to natural temperature regimes. This kind of information would provide new insight on how species with different kinds of life cycles respond to temperature variations during the normal germination season and thus how species with different kinds of life cycles are adapted to the same region.
Materials and methods
Between 1969 and 2006, germination phenology studies were conducted for 350 species. Freshly matured seeds collected from 25 or more individuals per species in various kinds of habitats in Kentucky and Tennessee (USA) were sown on soil that was kept in a non-temperature-controlled glasshouse in Lexington, KY (38.0406°N, 84.5037°W). There was no heating or cooling in this glasshouse, and the windows were kept open all year, with the temperatures being similar to those outdoors (Baskin and Baskin, Reference Baskin and Baskin1985). Seeds were sown on the surface of soil (3:1 v/v mixture of limestone-derived topsoil and sand) in 20 × 30 cm metal flats. For seeds collected from 1969 to 1972, two replicates of 200 seeds each were sown, but for seeds collected in 1973 and thereafter three replicates of 300 seeds each were sown. To approximate natural seasonal soil moisture conditions in the region, the soil was watered to field capacity once each week during summer (1 May–31 August), and during the remainder of each year, it was watered daily, except on days in winter when it was frozen.
All flats were examined at 7-d intervals, and if seeds with an emerged radicle (or seedlings in the cotyledon stage) were present, they were counted as germinated and discarded. Seeds of some species germinated in only 1 year, while those of others germinated in several years. Regardless of how many years seeds in a planting germinated, the soil was kept in the greenhouse for 1 year after the last seeds germinated. Seeds of 226 species were collected and sown one time, while those of 124 species were collected in different years and thus sown more than once. The number of families, species and datasets for each type of life cycle are presented in Table 1. The number of years seeds germinated and the final germination percentage for each life form are shown in Fig. 1. Each planting of each species is referred to as a dataset; the total number of datasets is 541. Species whose seeds germinated in July–December were classified as autumn-germinating and those germinating in January–June as spring-germinating. For seeds that germinated throughout the growing season (e.g. some species with physical dormancy, PY), the species was classified as autumn- or spring-germinating depending on when the peak of germination occurred. In laboratory studies, we confirmed via comparison of imbibition of intact and mechanically scarified seeds that seeds of Anacardiaceae, Convolvulaceae, Fabaceae, Geraniaceae and Malvaceae had water-impermeable seed/fruit coats and thus PY. Seeds with PY were sown on soil in the glasshouse shortly after collection, and they were not given any dormancy-breaking pre-treatments.
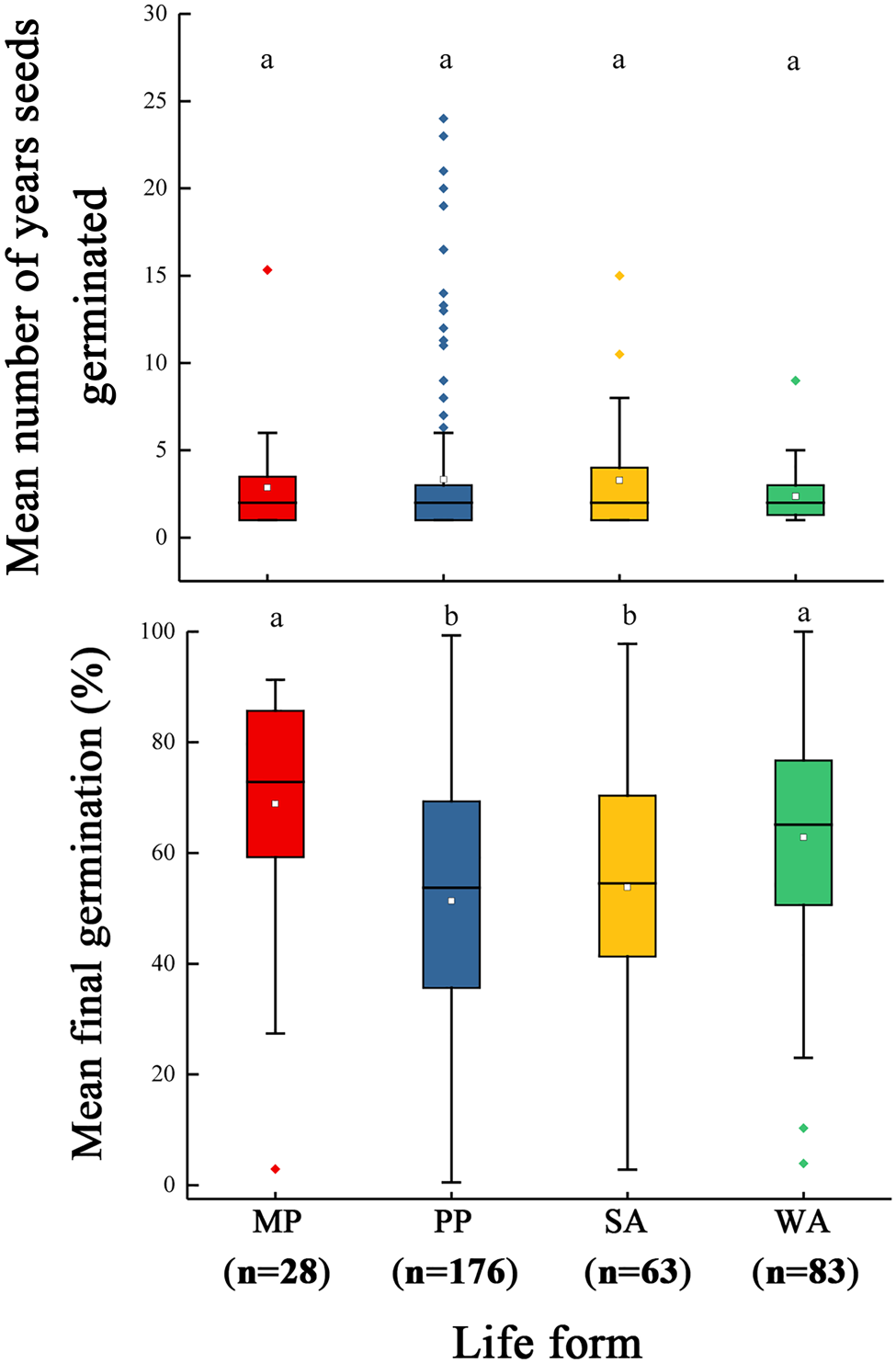
Fig. 1. Box plots for the number of years seeds germinated (top) and the final germination percentage (bottom) for monocarpic perennials (MP), polycarpic perennials (PP), summer annuals (SA) and winter annuals (WA). The mean is indicated by an open square inside the box and the 25th, 50th and 75th percentiles by solid horizontal lines. Solid horizontal lines outside the box are minimum and maximum values. Different lowercase letters indicate significant difference at P < 0.05.
Table 1. Number of families, species and datasets for monocarpic perennials, polycarpic perennials, summer annuals and winter annuals included in the germination phenology study conducted in a nonheated greenhouse in Lexington, KY, USA

Each planting is a dataset.
Air temperatures in the non-temperature-controlled glasshouse were recorded continuously with an electric thermograph located in a standard weather station house. The maximum and minimum temperatures for each day were determined from the thermograph records and recorded on data paper. Using the daily maximum and minimum temperatures, the mean weekly temperature was calculated for the week of first, highest (peak) and last germination for each dataset, and for the 143 species (200 datasets) in which seeds germinated in three consecutive years, these values were calculated for years 1, 2 and 3 after sowing. The germination temperature range (GTR) for each species is the difference in temperature between the week with the highest and the week with the lowest temperature during the time when germination occurred. Also, the mean monthly daily maximum and minimum temperatures were calculated for each month from 1970 to 2006.
Since seeds were collected and planted immediately after they matured, dormancy break and germination occurred under the natural conditions appropriate for each species. For example, seeds of WA mature in spring, and after planting in the glasshouse, they were subjected to high summer temperatures, which promote the breaking of dormancy (Baskin and Baskin, Reference Baskin and Baskin1986). On the other hand, seeds of SA mature in autumn, and after planting, they were subjected to (moist) low autumn–winter temperatures, which break dormancy (Baskin and Baskin, Reference Baskin and Baskin1987). Seeds with PY also received the natural sequence of seasonal temperature changes that promote dormancy break. For example, seeds of some species with PY become sensitive to dormancy break during dry, hot weather and become water-permeable and germinate after a summer rainfall event (Jayasuriya et al., Reference Jayasuriya, Baskin, Geneve and Baskin2009). Thus, the design of this study ensured that all seeds would be exposed to the natural conditions required to break dormancy.
Data analysis
To test the response of the seven studied traits (number of years seeds germinated, germination temperature for the first week, germination temperature for the last week, duration of germination season, final germination percentage, germination temperature range GTR) to the type of life cycle, we used generalized linear mixed-effects models in the nlme package implemented in R version 3.2.1 (R. Core Team, 2015).
Accounting for phylogenetic relationships
The V.PhyloMaker R package (Jin and Qian, Reference Jin and Qian2019) was used to construct a hypothetical phylogenetic tree incorporating all species in the present study, with the latest GBOTB tree including 74,533 species and all extant vascular plant families as the backbone.
To account for phylogenetic relationships, we tested for a phylogenetic signal in all studied traits (listed above) by obtaining two frequently used metrics, Pagel's λ (Pagel, Reference Pagel1999) and Blomberg's K (Blomberg et al., Reference Blomberg, Garland and Ives2003). Both Pagel's λ and Blomberg's K assess the degree to which a trait exhibits a phylogenetic signal according to a Brownian motion model of evolution. The phylogenetic signal was estimated using the ‘phylosig’ function in the R package phytools (Revell, Reference Revell2012; R. Core Team, 2015). We used a randomization test consisting of 1000 simulations to test for the statistical significance of the phylogenetic signal. Pagel's λ can vary from 0 (no phylogenetic signal) to 1 (strong phylogenetic signal) (Pagel, Reference Pagel1999; Freckleton et al., Reference Freckleton, Harvey and Pagel2002; Orme et al., Reference Orme, Freckleton, Thomas, Petzoldt, Fritz, Isaac and Pearse2011). We tested for the significance of phylogenetic signal against the assumption of no signal (λ = 0) and complete signal (λ = 1) using a likelihood ratio test. Values of K close to 0 indicate random evolution of traits, values close to 1 correspond to a Brownian motion-type evolution and values of <1 indicate a strong phylogenetic signal and trait conservatism.
Results
Phylogenetic signal
Based on Pagel's λ, there was a significant phylogenetic signal in number of years seeds germinated, germination temperature for the first week, peak and last weeks, and duration of germination season, but there was no significant phylogenetic signal for the final germination percentage or the GTR. Based on Blomberg's K, there was a significant phylogenetic signal for number of years seeds germinated, germination temperature for the first week and duration of germination season. However, the values for Blomberg's K and Pagel's λ were small in all cases (Table 2; Supplementary Fig. S1).
Table 2. Phylogenetic signal (Pagel's λ and Blomberg's K and their associated P-values from a randomization test) for a mean number of years seeds germinated (NYSG), mean final germination percentage (FGP), mean weekly temperature for the week of first (GTFW), highest (GTHW) and last germination (GTLW), germination temperature range (GTR) and duration of germination season (DGS)

n, number of species. Bold values represent a significant phylogenetic signal.
GTR in the first germination season
When combined data for each type of life cycle are considered (and the germination season is not considered), temperature during the first week of germination was significantly higher for WA than for MP, PP or SA, which did not differ from each other (Fig. 2). Temperatures during the week of the highest germination percentage ranged from 14.7°C (MP) to 20.6°C (WA) and were significantly different from each other and from PP and SA; MP and PP did not differ significantly. Temperature during the week of the last germination ranged from 11.5°C (WA) to 19.8°C (SA), and the temperature for SA was significantly higher than that for WA, MP and PP; MP and PP did not differ significantly. The GTR was the widest (11.8°C) for WA, which was significantly wider than the GTRs for SA (8.8°C) and PP (9.2°C) but not significantly different from the GTR for MP (10.4°C). The duration of the germination season did not differ significantly for MP, PP, PP and WA.

Fig. 2. Box plots for germination temperatures for weeks of first, highest and last germination, germination temperature range and duration of germination season for monocarpic perennials (MP), polycarpic perennials (PP), summer annuals (SA) and winter annuals (WA) during the first germination season. The mean is indicated by an open square inside the box and the 25th, 50th and 75th percentiles by solid horizontal lines. Solid horizontal lines outside the box are minimum and maximum values. Different lowercase letters indicate significant difference at P < 0.05.
For the spring-germinating species (MP, PP and SA), the mean temperature during the first week of germination ranged from 9.7°C (MP) to 12.7°C (SA) (Fig. 3). The GTR ranged from 8.1°C (PP without PY) to 9.6°C (MP), and PP with and without PY differed significantly. The duration of the germination season ranged from 70.8 d (PP without PY) to 196.9 d (SA with PY). PP with and without PY differed significantly, and SA with and without PY differed significantly. For autumn-germinating species (MP, PP and WA), temperatures during the week of the first germination ranged from 19.7°C (MP) to 22.6°C (WA) and differed significantly, but neither WA nor MP differed significantly from PP (Fig. 4). The GTR ranged from 11.8°C (WA) to 12.8°C (MP) and did not differ significantly. The duration of the germination season ranged from 73.9 d (PP) to 108.2 d (MP), and MP and PP differed significantly but neither differed from WA.

Fig. 3. Box plots for germination temperatures for weeks of first, highest and last germination, germination temperature range and duration of germination season for monocarpic perennials (MP), polycarpic perennials (PP) and summer annuals (SA) during spring of the first germination season. The mean is indicated by an open square inside the box and the 25th, 50th and 75th percentiles by solid horizontal lines. Solid horizontal lines outside the box are minimum and maximum values. Different lowercase letters indicate significant difference at P < 0.05. PY, physical dormancy; +PY, with physical dormancy; −PY, without physical dormancy.

Fig. 4. Box plots for germination temperatures for weeks of first, highest and last germination, germination temperature range and duration of germination season for monocarpic perennials (MP), polycarpic perennials (PP) and winter annuals (WA) during autumn of the first germination season. The mean is indicated by an open square inside the box and the 25th, 50th and 75th percentiles by solid horizontal lines. Solid horizontal lines outside the box are minimum and maximum values. Different lowercase letters indicate significant difference at P < 0.05.
Comparison of the GTR for first, second and third germination seasons
For MP, PP, SA and WA, 12, 57, 26 and 48 species, respectively, germinated in three consecutive years after planting. For combined data for each type of life cycle, the width of the GTR decreased significantly between the first and third years for MP, PP and WA but did not change significantly for SA (Fig. 5). A decrease in the duration of the germination season was significant for WA, marginally significant for MP and SA was and not significant for PP.

Fig. 5. Mean (±SE) germination temperatures for weeks of first, highest and last germination, germination temperature range and duration of germination season for monocarpic perennials (MP), polycarpic perennials (PP) summer annuals (SA) and winter annuals (WA) that germinated in three consecutive years. Different lowercase letters within each life form indicate significant difference at P < 0.05 among years.
For spring-germinating species (MP, PP and SA), the width of the GTR was significantly narrower in the third than in the first year for all life cycle types, except PP and SA with PY (Fig. 6). The duration of the germination season did not differ significantly between the first and third years for MP, PP and SA, but it was significantly shorter in the third than in the first year for PP and SA with and without PY. For autumn-germinating species, the GTR for MP, PP and WA was significantly narrower in the third than in the first year, and the duration of the germination season was significantly shorter in the third than in the first year for MP and WA but not for PP (Fig. 7).
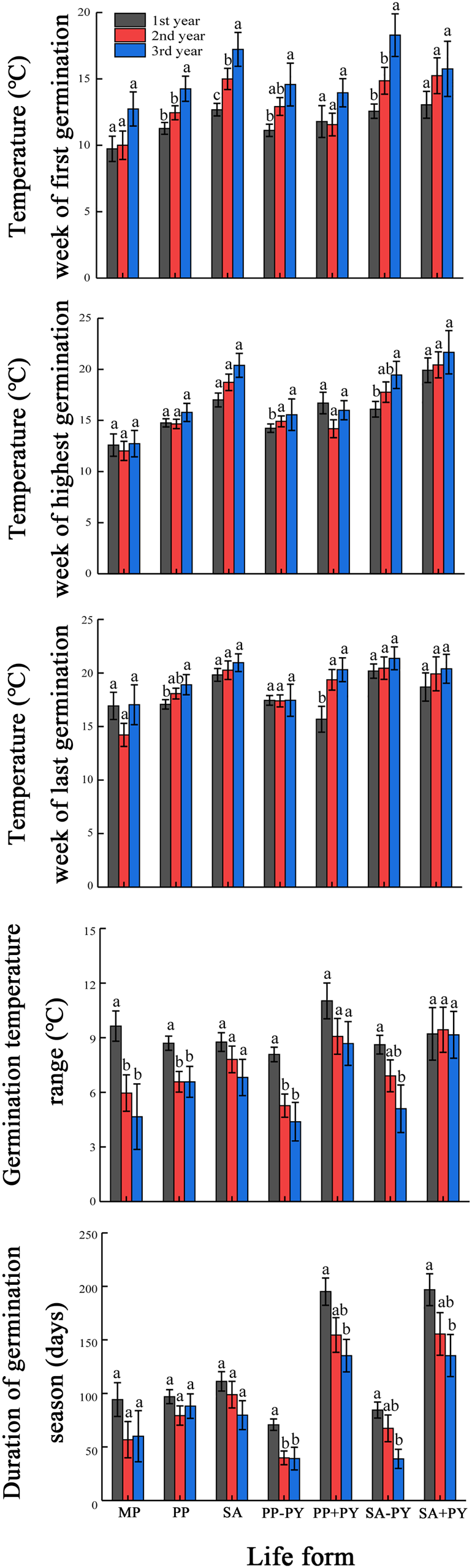
Fig. 6. Mean (±SE) germination temperatures for weeks of first, highest and last germination, germination temperature range and duration of germination season for monocarpic perennials (MP), polycarpic perennials (PP) and summer annuals (SA) that germinated in spring in three consecutive years. Different lowercase letters within each life form indicate significant difference at P < 0.05 among years. PY, physical dormancy; +PY, with physical dormancy; −PY, without physical dormancy.
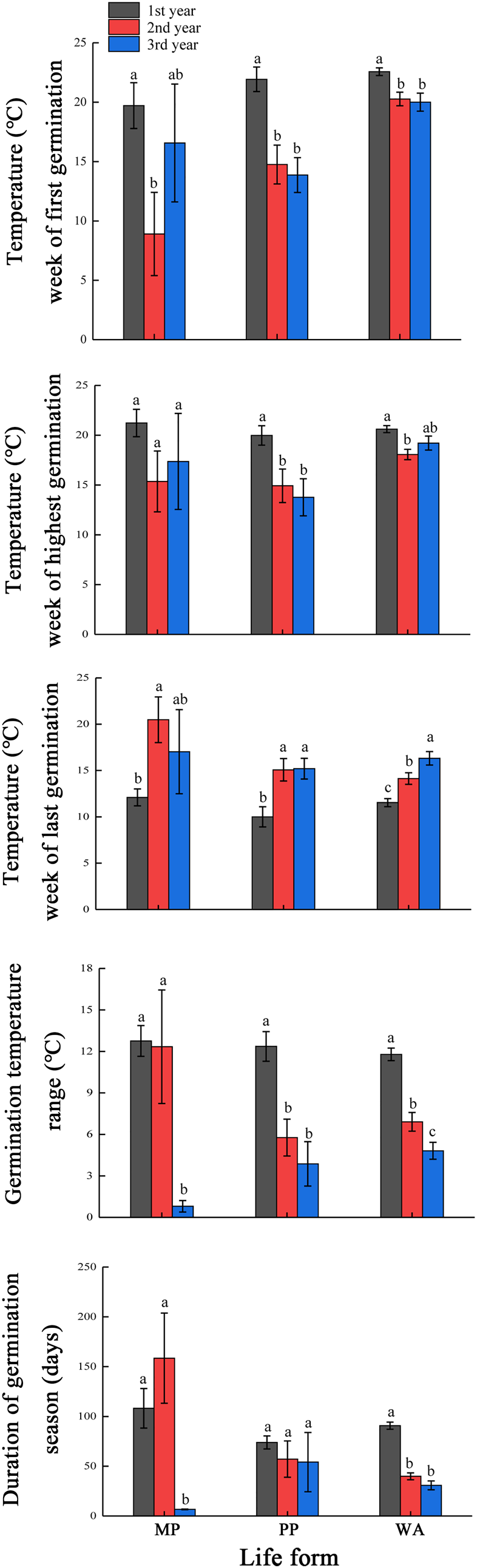
Fig. 7. Mean (±SE) germination temperatures for weeks of first, highest and last germination, germination temperature range and duration of germination season for monocarpic perennials (MP), polycarpic perennials (PP) and winter annuals (WA) that germinated in autumn in three consecutive years. Different lowercase letters within each group indicate significant difference at P < 0.05 among years.
Width of the GTR and temperature variation
The month of the beginning of germination varied with the type of life cycle and season of the year: MP, February and September; PP, January and August; SA, February; and WA, July (Table 3). However, the main period of spring germination was in March and April and that of autumn germination was in September and October. The difference between the highest and lowest mean maximum monthly temperatures for March, April, September and October over the 37-year period is 14.5, 10.3, 7.1 and 7.1°C, respectively, and the difference between the mean minimum monthly temperatures is 10.3, 9.3, 6.7 and 6.5°C, respectively (Table 4). The GTR for spring-germinating species was MP, 9.6 ± 0.8°C; PP, 8.7 ± 0.4°C; and SA, 8.8 ± 0.5°C, and the GTR of autumn-germinating species was MP, 12.8 ± 1.1°C; PP, 12.4 ± 1.1°C; and WA, 11.8 ± 0.5°C (Figs 3 and 4).
Table 3. Proportion of species with each life form that began to germinate in each month of the year.

MP, monocarpic perennials; PP, polycarpic perennials; SA, summer annuals; WA, winter annuals.
Table 4. Average of the mean daily maximum and minimum monthly temperatures (°C) from 1970 to 2006 in the non-temperature-controlled glasshouse in Lexington, KY, USA

For maximum, difference = highest minus lowest mean maximum monthly temperature for each month during the 37-year period. For minimum, difference = highest minus lowest mean minimum monthly temperature for each month during the 37-year period
Discussion
Phylogeny plays a key role in regulating seed traits such as seed mass (Lord et al., Reference Lord, Westoby and Leishman1995; Moles et al., Reference Moles, Ackerly, Webb, Tweddle, Dickie, Pitman and Westoby2005) and class of dormancy (Willis et al., Reference Willis, Baskin, Baskin, Auld, Venable and Cavender-Bares2014; Dayrell et al., Reference Dayrell, Garcia, Negreiros, Baskin, Baskin and Silveira2017), and the seed germination response to temperature can be phylogenetically conserved (Grime et al., Reference Grime, Mason, Curtis, Rodman, Band, Mowforth and Shaw1981; Arène et al., Reference Arène, Affre, Doxa and Saatkamp2017). Although the phylogenetic signal for number of years seeds germinated; germination temperature for first, peak and last weeks; and duration of the germination season is significant, Blomberg's K value was much less than 1. Thus, the phylogenetic signal is lower than expected under Brownian motion, and accordingly, there is substantial evolutionary lability in germination temperature requirement, even between closely related species. Rosbakh and Poschlod (Reference Rosbakh and Poschlod2015) showed that the initial germination temperature is not phylogenetically constrained.
For the first germination season after planting and for all data combined, the GTR for WA was greater than that for MP and PP. This result supports our prediction that seeds of annuals are able to germinate over a wider range of temperatures than those of perennials. However, when autumn- and spring-germinating species are considered separately, annuals did not have a wider GTR than perennials in either germination season.
Among the spring-germinating PP and SA, the presence (or not) of PY influenced the width of the GTR. Seeds of PP and of SA with PY generally had a germination season that was more than twice as long as that of seeds without PY, perhaps due to periods of summer drought making seeds sensitive to dormancy break (Jayasuriya et al., Reference Jayasuriya, Baskin, Geneve and Baskin2009). Furthermore, the GTR for PP and for SA with PY was wider than that for PP and for SA without PY. Seeds of many species of PP and of SA with PY can become permeable and germinate throughout much of the growing season, whereas germination of PP and SA seeds without PY usually occurs only in spring and/or early summer.
Our prediction that annuals begin to germinate at lower temperatures in spring and at higher temperatures in autumn than perennials was not well supported. In spring, MP germinated at a significantly lower temperature and thus earlier than SA, but PP did not differ significantly from SA or MP. In autumn, WA germinated at a significantly higher temperature and thus earlier than MP but not PP. Our prediction that the temperature during the week of the highest germination is higher for annuals than perennials was not supported.
Overall, the germination season has a stronger effect on the temperature at which seeds begin to germinate than does annual versus perennial life cycle. Regardless of life cycle type, seeds started to germinate in spring when mean weekly temperatures were 10–13°C and stopped germinating when temperatures were 17–20°C. In autumn, seeds for all types of life cycles began to germinate when temperatures were 20–23°C and stopped when temperatures decreased to 10–12°C. That is, seeds of spring-germinating species germinate as temperatures increase, and those of autumn-germinating species germinate as temperatures decrease, with the overall width of the GTR for autumn-germinating species (11.8 ± 0.5 to 12.8 ± 1.1°C) being wider than that of spring-germinating species (8.7 to 9.6°C).
Our prediction that the width of the GTR and the duration of the germination season for seeds in a particular cohort that fail to germinate the first year are reduced in subsequent years and that the reduction is greater for annuals than perennials was only partly supported. For all data combined, the GTR decreased significantly from the first to the third year for MP, PP and WA but not for SA. Thus, annuals per se did not exhibit a greater reduction in the GTR than perennials. Instead, the reduction was greater for WA than SA and greater for MP than PP. SA and PP exhibited less reduction in the duration of the germination season than MP and WA, probably because 11 and 26 species of SA and PP, respectively, had seeds with PY, which can have an extended germination season.
Although the GTR of SA did not change from the first to the third year, the temperature of the first week of germination and that of the week of the highest germination increased. This shift to germinating at increased temperatures with the increased age of seeds cannot be directly correlated to the temperatures in the year of germination because year 3 for one species may have been year 1 for another species. As the seed bank for a species is depleted, an increase in temperature when germination begins in spring would help ensure that germination occurs after the threat of cold periods in late spring has passed. It should be noted that seeds of some SA that exhibit annual dormancy cycles when buried under natural temperature conditions have a higher percentage of seeds that germinate, especially in the dark, in the second and/or third than in the first germination season (e.g. Baskin and Baskin, Reference Baskin and Baskin1980; Baskin et al., Reference Baskin, Baskin and Chester1995).
A comparison between the first and third years for autumn-germinating species shows a decrease in temperature the first week of germination, a decrease in the week of the highest germination and an increase in the week of the last germination, resulting in a decrease of the GTR and in the duration of the germination season for MP, PP and WA. This narrowing of the width of the GTR for autumn-germinating seeds would help restrict germination to a period in autumn when the soil moisture level is high but before temperatures become too low for successful seedling establishment.
Our prediction that the GTR of MP, PP, SA and WA is as wide as the expected variability in temperatures for each month of the germination season is generally supported. For each month of the year for the 37-year period of our study, large year-to-year variations in mean maximum (ca. 7–15°C) and minimum (−4 to 11°C) monthly temperatures occurred. The magnitude of year-to-year fluctuations in maximum monthly temperatures for the main germination periods in March, April, September and October is 14.5, 10.3, 7.1 and 7.1°C, respectively, and that in minimum monthly temperatures is 10.3, 9.3, 6.7 and 6.6°C, respectively. Thus, since the mean width of the GTR for spring-germinating species ranges from 8.7 ± 0.4°C (PP) to 9.6 ± 0.8°C (MP) and that for autumn-germinating species from 11.8 ± 0.5°C (PP) to 12.8 ± 1.1°C (MP), the year-to-year fluctuation in spring and autumn temperatures would not prevent them from germinating.
In view of the width of the GTR of MP, PP, SA and WA included in our study, it would seem that global warming would have little effect on the germination of herbaceous species in temperate eastern North America. However, global warming needs to the considered from a dormancy-breaking and timing of germination perspective (Walck et al., Reference Walck, Hidayati, Dixon, Thompson and Poschlod2011). In species whose seeds mature in spring and undergo dormancy break in summer and germinate in autumn, dormancy break may be facilitated by increased summer temperatures. Increased temperatures in early autumn could potentially delay germination, especially if autumn rains also are delayed (Kimball et al., Reference Kimball, Angert, Huxman and Venable2010). In species whose seeds mature in autumn, undergo dormancy break in winter and germinate in spring, increased winter temperatures could inhibit dormancy break (e.g. Mondoni et al., Reference Mondoni, Ross, Orsenigo and Probert2012; Orrù et al., Reference Orrù, Mattana, Pritchard and Bacchetta2012). Thus, shifts in the timing of dormancy break and germination of individual species may occur, resulting in changes in the environmental conditions encountered by the offspring.
In conclusion, annuals do not have a wider GTR than perennials in either the spring or autumn germination season. Within a germination season, there are relatively small differences in the GTR between types of life cycles, but between germination seasons (spring vs autumn), there are relatively large differences in GTR regardless of the type of life cycle. In spring, the GTR is the same for MP, PP and SA, and in autumn it is the same for MP, PP and WA. However, the GTR is wider for autumn- than for spring-germinating species. Based on the temperature for the week of the first germination, MP are the first to germinate in spring and WA are the first to germinate in autumn. The overall width of the GTR for herbaceous species in temperate eastern North America in the first season after seed dispersal is sufficient to allow seeds to germinate in their normal spring or autumn germination season, regardless of whether temperatures in early spring or early autumn are above or below normal. Although one life cycle type may begin to germinate a little sooner than another, within a germination season all life cycle types have overlapping times of germination. Consequently, there is no clear separation of the timing of germination according to life cycle within a germination season, and seedlings of species with different kinds of life cycles could be competing for resources during the seedling establishment phase of the life cycle. Thus, selection for early germination (in either spring or in autumn) has resulted in only a little separation in the timing of germination of species with different types of life cycles that germinate in each season. Regardless of the evolutionary/ecological forces involved in shaping differences in the germination of seeds with different kinds of life cycles in temperate eastern North America, they have not resulted in great differences between the width of the GTR for the different types of life cycles.
Supplementary material
To view supplementary material for this article, please visit: https://doi.org/10.1017/S0960258522000010.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgements
This study was supported in part by HATCH Project No. 1013862 and by the (China) National Natural Science Fund (Nos 31672473 and 31001030).
Author contributions
C.C.B. and J.M.B. conducted the germination phenology studies; X.W.H. analysed the data; C.H.Z. did the phylogenetic analysis and C.C.B., J.M.B., X.W.H. and C.H.Z. wrote the manuscript.
Conflicts of interest
No conflicts of interest have been declared.














