Introduction
Recently, we compiled an initial list of angiosperms capable of germinating in less than 24 h (‘very fast germination’) and established that all are from high-stress habitats, while most have small to very small seeds, little or no endosperm or perisperm, and thin, membranous seed coats that imbibe water readily (Parsons, Reference Stevens2012). Here we add a further three families and 23 species to the five families and 28 species of the original list. We use this expanded database to refine the original findings and, especially, to explore the relationship between very fast germination (VFG) and the functional ecology of embryo size. Also, we plot the plant orders containing VFG species on a phylogenetic tree to explore how many times VFG has evolved throughout angiosperm history.
Methods
The nomenclature for families and genera follows Stevens (Reference Stevens2001). Authors for species names are given in the tables; those for species not in the tables are given in the text. The data on the Asteraceae family refer to the germination of cypselas; for brevity, these are treated as seeds here.
As in Parsons (2012), values for minimum time to germination were often gleaned from tables and figures in papers with a wide variety of main aims and germination conditions. Clearly, it would have been desirable for all the data to have been obtained under standard, defined conditions. It follows that our introductory conspectus needs following up by more detailed work.
Additional taxa
The original paper (Parsons, 2012) listed 28 species that can germinate in less than 24 h, 20 from the Amaranthaceae (all formerly treated as Chenopodiaceae) plus a few species from the Acanthaceae, Brassicaceae, Poaceae and Salicaceae. Here we add an additional 23 species, some from the families above (but now including Amaranthaceae sensu stricto, i.e. subfamily Amaranthoideae), whereas other species are found within Cactaceae, Asteraceae and Tamaricaceae (Table 1).
Table 1 Minimum time to germination, life form, natural range and habitat of flowering plants that can germinate in less than 24 h from imbibition
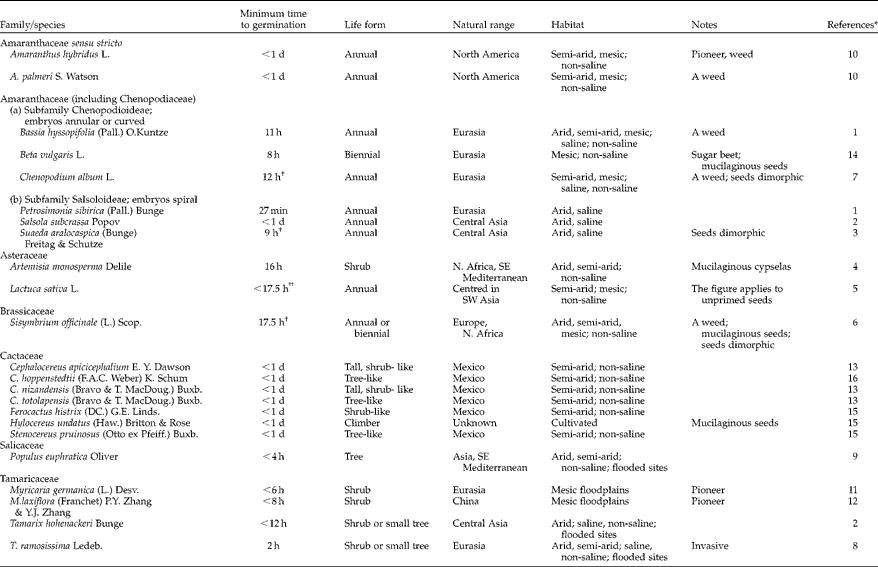
* References: 1, Liu et al. (Reference Liu, Zhang and Yin2013); 2, L. Wang (pers. comm., 2013); 3, Wang et al. (Reference Wang, Huang and Baskin2008); 4, Huang and Gutterman (Reference Huang and Gutterman1999); 5, Bradford et al. (Reference Bradford, Tarquis and Duran1993); 6, Iglesias-Fernandez et al. (Reference Iglesias-Fernandez, Matilla and Pulgar2007); 7, Yao et al. (Reference Yao, Chen and Zhao2010); 8, Yan et al. (Reference Yan, Wei and Yang2011); 9, Liu et al. (Reference Liu, Hao and Tan2011); 10, Steckel et al. (Reference Steckel, Sprague and Stoller2004); 11, Lener (Reference Lener2011); 12, Chen and Xie (Reference Chen and Xie2007); 13, Barcenas-Arguello et al. (Reference Barcenas-Arguello, Lopez-Mata and Terrazas2013); 14, Abts et al. (Reference Abts, Vissers, Vandenbussche and De Proft2013); 15, Seal et al. (Reference Seal, Flores, Ceroni Stuva, Davila Aranda, Leon-Lobos, Ortega-Baes, Galindez, Aparicio-Gonzalez, Castro Cepero and Daws2009); 16, Alvarez-Aguirre and Montana (Reference Alvarez-Aguirre and Montana1997).
† Seeds dimorphic; this figure applies to the very fast germinating type.
†† The T 50 is 15.5 h.
As pointed out in Parsons (2012), the addition of Cactaceae is unsurprising, given that some species have very small seeds and thin seed coats and that the family lacks a true endosperm and is usually found in high-stress habitats. The addition of Asteraceae was also presaged by Parsons (2012), given the clear tendency in the family for fast germination, with some records as short as 2 d.
The Tamaricaceae family was overlooked by Parsons (2012). In many respects, it is strikingly similar to the very fast germinating Populus–Salix group, being dominated by shrub and tree species which are mainly found as pioneers on flooded sites. Seeds of both Populus and Salix, and selected Tamaricaceae species (Table 1) lack endosperm, have long hairs forming a coma at one end, are very small and are produced in large numbers, have short longevity and do not form a seed bank (Bill et al., Reference Bill, Spahn, Reich and Plachter1997; Gaskin, Reference Gaskin and Kubitzki2003; Chen and Xie, Reference Chen and Xie2007; Lener, Reference Lener2011; Yan et al., Reference Yan, Wei and Yang2011; Parsons, 2012). These similarities are underlined by the fact that an invasive species of Tamarix co-exists and competes with species of Populus and Salix (native species) on flooded sites along the Rio Grande in New Mexico (Hultine and Dudley, Reference Hultine, Dudley, Sher and Quigley2013). It may turn out that both the Populus–Salix lineage and the Tamaricaceae have most, or all, of their species adapted for VFG; certainly no exceptions have yet been found.
When orders containing VFG species are plotted on a phylogenetic tree, it is clear that evolution towards VFG took place many times throughout angiosperm history (Fig. 1). Within orders, VFG is restricted to single families, except for the Caryophyllales. This order is characterized by VFG species in three different, distantly related families (Amaranthaceae, Cactaceae and Tamaricaceae), indicating that within the Caryophyllales the VFG trait has originated at least three times.
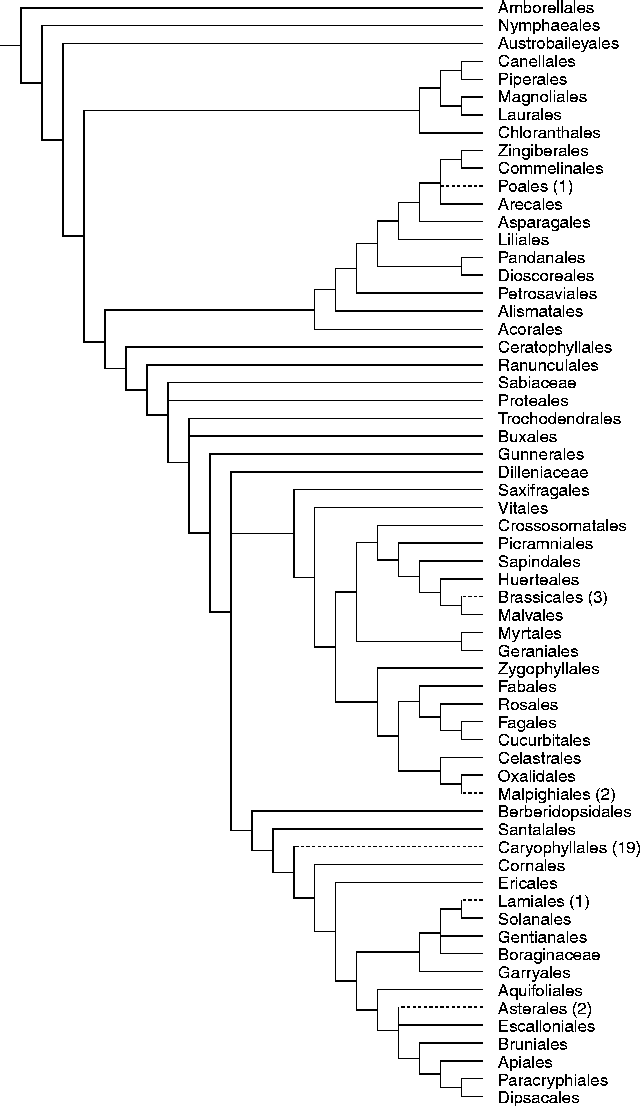
Figure 1 Orders containing very fast germinating species (dashed branches) are plotted on to a phylogenetic tree derived from APG III (Angiosperm Phylogeny Group III, 2009). Numbers between parentheses indicate the number of genera with VFG species.
Evolution of VFG in relation to relative embryo size
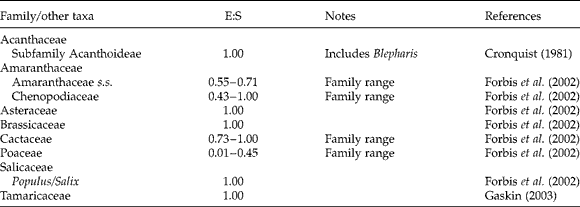
It is generally accepted that small embryos embedded in copious endosperm, represent the ancestral state of angiosperm seeds (Stebbins, Reference Stebbins1974; Forbis et al., Reference Forbis, Floyd and De Queiroz2002). Such embryos require a long time to grow before they can germinate; prolonged seed imbibition is required to allow post-dispersal embryo growth (Vandelook et al., Reference Vandelook, Janssens and Probert2012, and references therein). Such a growth requirement means that fast germination was physically impossible in early angiosperms. In agreement with this hypothesis, species with VFG are mainly restricted to advanced clades such as the Asteraceae and Brassicaceae (Fig. 1), which almost completely lack extra-embryonic reserve tissue (Table 2). Note that Schismus arabicus Nees, the only monocot we know with VFG, just like all other Poaceae, very likely does have a copious endosperm. However, we should remark that the E:S values analysed by Forbis et al. (Reference Forbis, Floyd and De Queiroz2002) are embryo to seed surface (not length!) ratios and that post-dispersal embryo growth is not required in any of the VFG species.
Table 2 Embryo to seed ratio (E:S) for taxa that include species capable of very fast germination
Concluding discussion
The main additional VFG families reported here fit neatly into the main VFG ecological groups described by Parsons (2012), the Cactaceae into the aridity-adapted group and the Tamaricaceae into the floodplain-adapted group. Combining all taxonomic information, it appears that VFG is a derived trait that evolved independently many times during angiosperm evolution. This evolution towards VFG is associated with substantial changes in seed morphology, such as the evolution of soft, thin seed coats (Parsons, 2012) and increased nutrient storage in the embryo relative to non-embryonic tissues.
Acknowledgements
We owe a particular debt to Dr Joel Flores, who very kindly provided expert advice on the germination literature concerning the family Cactaceae.
Conflicts of interest
None.






