Introduction
The ability to predict the timing and magnitude of seedling emergence events would be most useful for optimizing control interventions, and to improve predictions of yield losses from weed competition with a crop in arable fields. It would then be possible to develop decision-making tools and design appropriate agronomic management approaches and understand the resilience of weed communities (Colbach et al., Reference Colbach, Biju-Duval, Gardarin, Granger, Guyot, Mézière, Munier-Jolain and Petit2014). From an evolutionary perspective, if seeds can remain viable without germinating, a species can retain its reproductive potential over time. Seasonal germination patterns would then match optimal climatic conditions and plants could overcome the impact of variation in growing conditions, either climatic or agricultural. In turn, weed species could then be selected to evolve in response to field management (Darmency et al., Reference Darmency, Colbach and Le Corre2017).
Physiological, morphological and physical dormancy mechanisms (and their combinations: Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006) account for the ability of seeds to remain viable without germinating. Previous research has described most patterns of seed dormancy, longevity and germination (Baskin and Baskin, Reference Baskin and Baskin2014). A few seed traits particularly influence seed behaviour (Gardarin and Colbach, Reference Gardarin and Colbach2014). For instance, seed size is generally negatively correlated with persistence in the seed bank (Thompson et al., Reference Thompson, Band and Hodgson1993; Bekker et al., Reference Bekker, Bakker, Grandin, Kalamees, Milberg, Poschold, Thompson and Willems1998), although some positive correlations have been documented (Moles and Westoby, Reference Moles and Westoby2006). Seed coat thickness is correlated with seed longevity in numerous species (Gardarin et al., Reference Gardarin, Dürr, Mannino, Busset and Colbach2010).
However, species show wide variation in seed morphology, and the common correlations between traits are generally understudied at the intraspecific level. Here we investigate the case of Raphanus raphanistrum L. (wild radish), a highly competitive weed in several crops showing widespread worldwide distribution (Warwick and Francis, Reference Warwick and Francis2005), adaptive capabilities (Sahli et al., Reference Sahli, Conner, Shaw, Howe and Lale2008), phenological adaptation (Ashworth et al., Reference Ashworth, Walsh, Flower, Vila-Aiub and Powles2016), numerous cases of herbicide resistance (Ashworth et al., Reference Ashworth, Walsh, Flower and Powles2014 and references therein), and even new habitat colonization (Ridley and Ellstrand, Reference Ridley and Ellstrand2009). Raphanus raphanistrum has wide variation in pod (or silique) size that has led to its division into subspecies, but this classification is useless as long as no reproductive or adaptive values were associated. Indeed, all Raphanus are fully cross-compatible with one another, including the cultivated radish, R. sativus L. (Stace, Reference Stace1975; Eber et al., Reference Eber, Boucherie, Broucqsault, Bouchet and Chèvre1998; Snow and Campbell, Reference Snow, Campbell and Gressel2005). Populations intermediate between so-called subspecies occur frequently (Chater, Reference Chater and Tutin1993).
Several studies of R. raphanistrum seed longevity in the soil seed bank found that the half-life is approximately 2 years (Roberts and Boddrell, Reference Roberts and Boddrell1983; Chancellor, Reference Chancellor1986; Code et al., Reference Code, Walsh and Reeves1987). One study estimated a shorter longevity, with only 0–5.4% viable seed recovered after 2 years (Roller and Albrecht, Reference Roller and Albrecht2006). Variability among experiments could be due to differences of ripening and germination conditions. For instance, Cheam (Reference Cheam1986) showed that seeds produced under cool conditions had deeper dormancy than those produced in warmer areas. Code et al. (Reference Code, Walsh and Reeves1987) showed that longevity increases with burial depth. Emergence occurs from spring to autumn, generally with two main peaks (Reeves et al., Reference Reeves, Code and Piggin1981; Roberts and Boddrell, Reference Roberts and Boddrell1983; Amor, Reference Amor1985; Chancellor, Reference Chancellor1986; Cheam, Reference Cheam1986; Panetta et al., Reference Panetta, Gilbey and D'Antuono1988; Warwick and Francis, Reference Warwick and Francis2005). This seasonality certainly corresponds with the rainfall pattern and soil cultivation practices before crop sowing, but also with the intensity of seed dormancy. Fresh seeds have deep primary dormancy (Roberts and Boddrell, Reference Roberts and Boddrell1983; Cheam, Reference Cheam1986; Malik et al., Reference Malik, Norsworthy, Riley and Bridges2010), with populations varying between 70 and 100% dormant seeds (Warwick and Francis, Reference Warwick and Francis2005). Per cent dormancy in a population decreases when sowing of the mother plant is delayed (20% less for a 4-month delay; Cheam, Reference Cheam1986). In subsequent months, dormancy is broken partially (Mekenian and Willemsen, Reference Mekenian and Willemsen1975; Cheam, Reference Cheam1986; Cheam and Code, Reference Cheam and Code1995). Thereafter, cold temperatures during late autumn induce secondary dormancy (Cheam, Reference Cheam1986). Regulation of dormancy in R. raphanistrum seeds has long been thought to be mediated by fruit wall integrity, both physically restricting water imbibition and germination (Cousens et al., Reference Cousens, Young and Tadayyon2010), and releasing a chemical inhibitor (Mekenian and Willemsen, Reference Mekenian and Willemsen1975). Young (Reference Young2001) has disputed the role of an inhibitor, noting the importance of seed coat integrity. Cousens et al. (Reference Cousens, Young and Tadayyon2010) gained extended knowledge on the role of both fruit persistence and seed coat on water uptake and desiccation.
Although dormancy, germination and emergence of R. raphanistrum have been studied in depth, it remains to be determined if the wide variability of dormancy and germination response is mostly due to the direct effect of environmental variations or if genetic factors are involved. We therefore set out to establish whether demographic and evolutionary models are valid when unique seed germination and emergence dynamics parameters are used, i.e. disregarding genetic variation. A simplified approach would be to use parameters presented in plant trait databases, whatever the population characteristics and the selection pressure prevailing in their habitat as reported above. In order to check whether seed longevity and dormancy are different or not according to putative genetic differences of populations, we have chosen two R. raphanistrum populations from contrasting climatic regions and habitats of origin and with different fruit wall thickness. The experiment started after the seeds were multiplied in our common garden to produce comparable seeds.
Materials and methods
Seed production
A population with small pods was collected in Lechâtelet, Burgundy (France, 47° 03′ 41″ N, 5° 08′ 42″ E), a region with continental climate, under an artificial poplar grove in acidic soil. It was typified as R. raphanistrum subsp. raphanistrum according to Chater (Reference Chater and Tutin1993). A population with large pods was collected in an arable field near Rennes, Brittany (France, 48° 06′ 53″ N, 1° 40′ 46″ W), 500 km westward from Lechâtelet, a region with oceanic climate. Based on characteristics noted in Pistrick (Reference Pistrick1987), it could be tentatively identified as R. raphanistrum subsp. landra (Moretti ex DC.) Bonnier & Layens, but the sampling location was on acidic and not on basic soil, and far from its typical seashore habitat. Thus, this population did not fit the ecological criteria of the subspecies so we identified it simply as R. raphanistrum. In fact, all Raphanus spp. or subsp. are the same biological species as all intercross freely (Stace, Reference Stace1975; Eber et al., Reference Eber, Boucherie, Broucqsault, Bouchet and Chèvre1998; Snow and Campbell, Reference Snow, Campbell and Gressel2005) and there are no clear subspecific boundaries because of widespread introductions and hybridizations as noted by Chater (Reference Chater and Tutin1993), so that morphologically mediated taxonomical ranking is of poor value for that genus.
Seeds were multiplied in 2008 in separate cages in the experimental garden at INRA in Dijon (47° 19′ 18″ N, 5° 02′ 29″ E, 35 km north of Lechâtelet) in order to control for differences in maternal environment that are known to influence seed characteristics (as extensively reviewed in Baskin and Baskin, Reference Baskin and Baskin2014). For each population, 35 plants were grown in a cage that excluded insect predators, and with houseflies (Musca domestica L) as pollinators. About 0.5 litre of larvae (more than 500 maggots) was placed in each cage weekly to ensure continuous presence of houseflies. Mature pods (silique) were collected at shedding by gently shaking the plant, and periodically intact pods were taken at random to form a separate batch of 100 pods to serve for measurements. Intact pods show a seedless beak-like tip at the extremity of one to 10 seeded segments separated by prominent constrictions. The fruit length and diameter were measured, and segments were counted and weighed. The segments were then carefully cracked using electrician's pliers, the number of naked seeds counted, and 100-seed lots weighed. Other pods were disarticulated by hand-rubbing to obtain single segments, and they were stored for 4 months at 20°C under room humidity conditions while waiting for appropriate weather and soil conditions for burial.
Seed burial
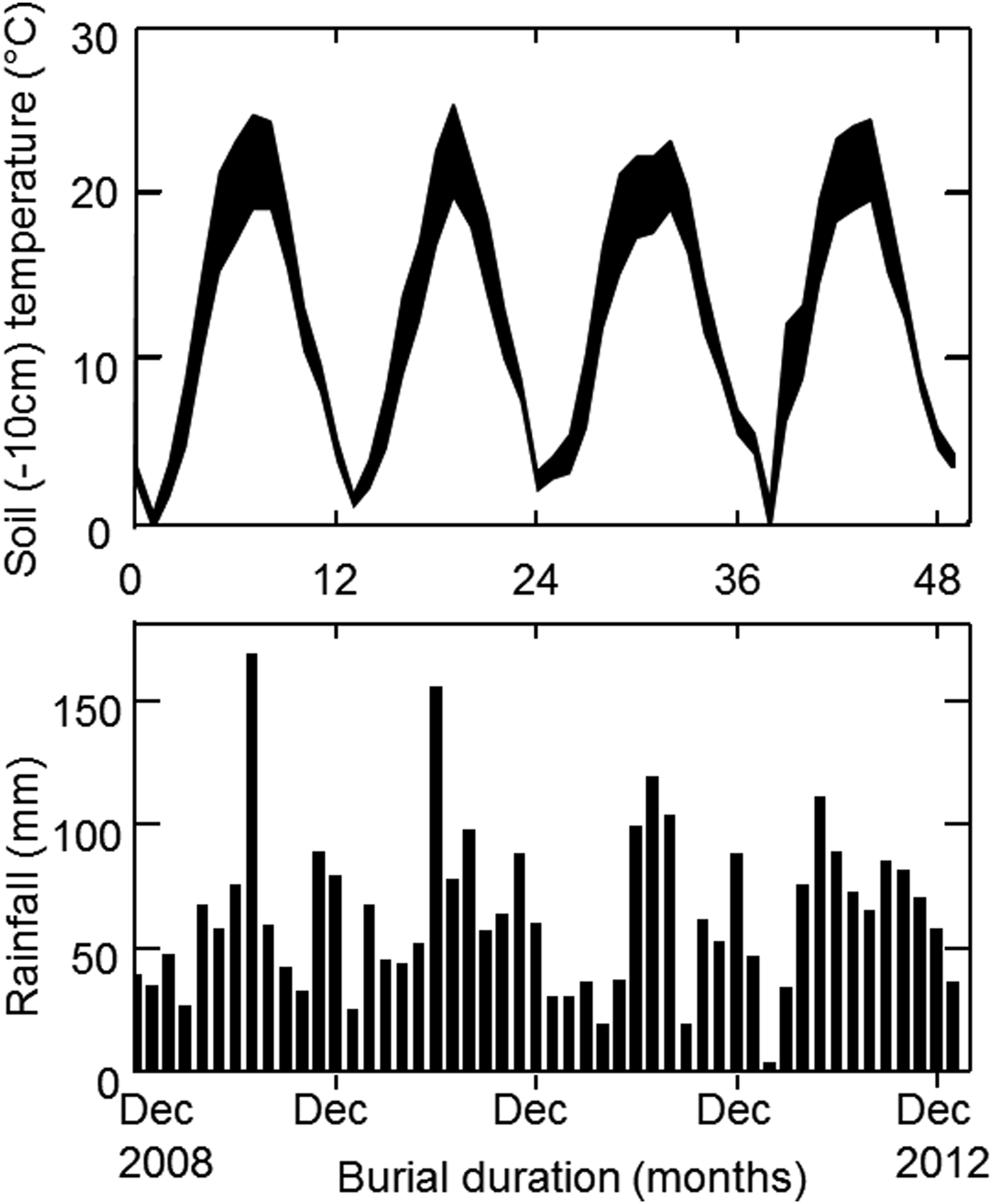
For the field experiment, we buried seed bags and then excavated them each month over 4 years to record the number of surviving seeds and the proportion of non-dormant seeds. Samples of 110 segments (to ensure at least 100 filled segments) were mixed with 100 g sieved dry soil from the excavation site and put into woven nylon (Tergal) bags (mesh size 400 μm). Three bags of each population were kept for immediate testing; the others were put at the bottom of open mesh plastic baskets (diameter 30 cm), with three bags from each population in each basket. The resulting 39 baskets were buried in a 30-cm deep trench (a depth at which we assumed few seeds germinate, thus allowing us to study longevity kinetics) in a silty clay loamy soil on 17 December 2008 in Dijon. The trench was refilled with soil and then left without disturbance for 4 years. About every month over the next 4 years, six bags (i.e. one basket) were randomly excavated for germination tests: the last basket was excavated on 11 January 2013. Temperature 10 cm below the surface and rainfall were recorded daily (Fig. 1); both daily and seasonal temperature fluctuations would be slightly further buffered at 30 cm below the soil surface.
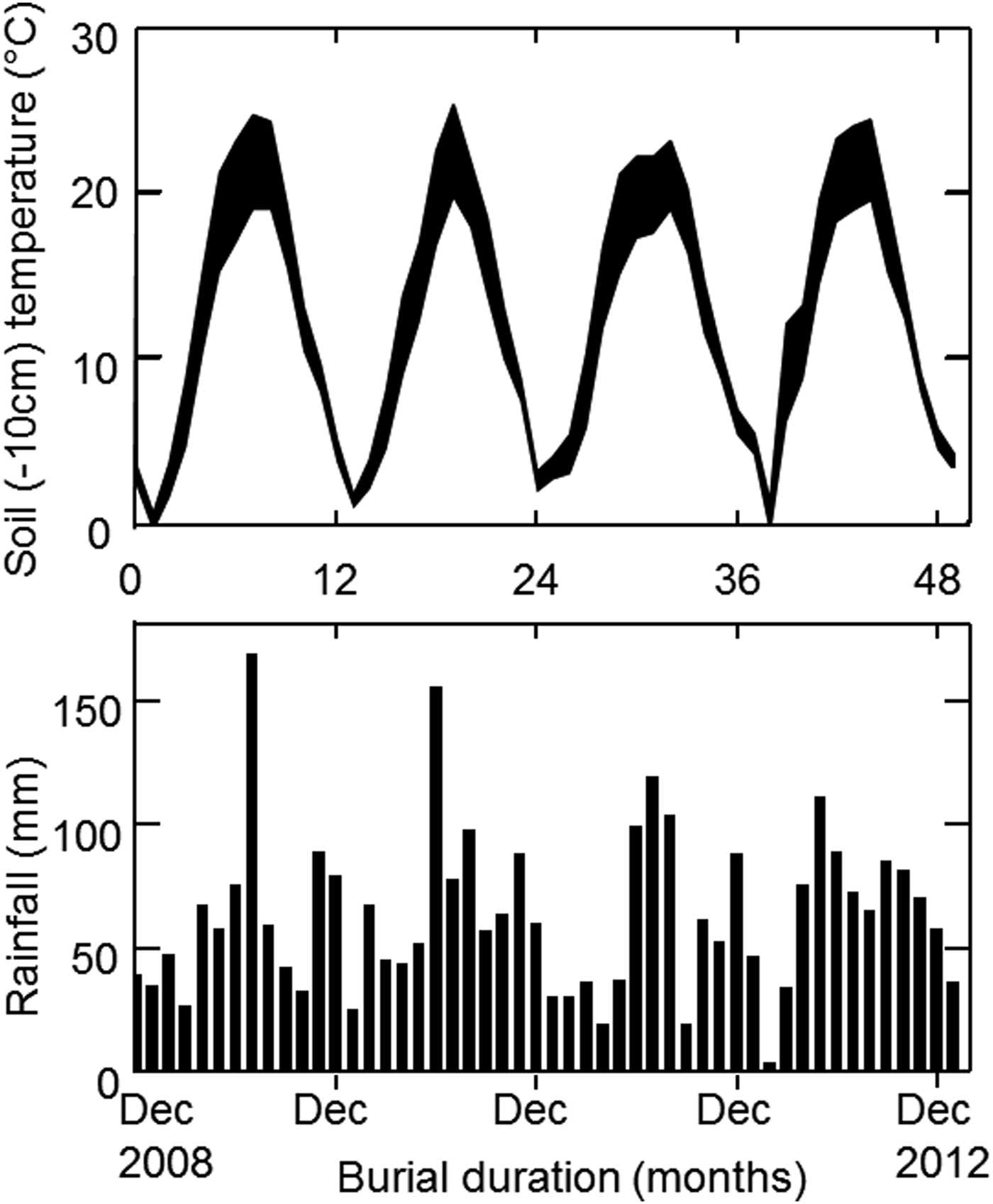
Figure 1. Variation of the lowest and highest mean daily temperatures at 10 cm below the soil surface and rainfall, throughout the seed burial experiment.
The segments were extracted by hand from the soil and counted as intact (I) or non-persistent (NP), whatever the cause (in situ germination, predation or rotting); therefore, NP = 110 – I). Segments were briefly washed with water, then deposited on folded Whatman grade 3014 seed test paper (Whatman GmbH, Dassel, Germany) in Caubère crystal polystyrene boxes with pure water to obtain non-limiting water conditions. This method is best adapted to germination from fruit, as in Sester et al. (Reference Sester, Dürr, Darmency and Colbach2006) for beet seed clusters. Boxes were incubated at 25/15°C with a 12-h light period (six fluorescent lighting tubes; Osram, L 18 W/77, 20 μmol m−2 s−1). Germinated seeds were assessed weekly over 3 weeks, and then gibberellic acid (0.3 g l−1 GA3) was added to test whether physiological embryo dormancy was involved. A final count was carried out 1 week later, then the remaining segments were dissected under a binocular magnifier to determine the viability of the remaining seeds: if the embryo of a dissected seed was translucent and firm, the seed was considered viable; if the embryo was brown and soft or if the segment was empty, the seed was considered unviable. For each sample bag, the number of surviving seeds (SS) was calculated as the sum of germinated seeds before (GS) and after (GA3S) hormonal priming and ungerminated viable seeds after dissection (VS); hence, SS = GS + GA3S + VS. The number of dead seeds (NSS for ‘not surviving seeds’) among intact segments was simply estimated as NSS = I – SS. Finally, the number of dormant seeds (DS) was estimated as DS = GA3S + VS.
Data analysis
Seed survival was modelled as a binomial variable [dead seeds (NSS) or living seeds (SS)]. We fitted a generalized linear mixed model (with binomial errors and a logit link function) to the monthly data recorded during burial. This consisted of burial time and its interaction with population as the two explanatory variables. It takes the form:
where %SS is the proportion of living seeds, Time is the burial time and intercept, α and β are model parameters to be estimated. Given that seed bags from the two populations were always excavated from the same basket, basket identity (ID) was treated as a random factor. We made the random effect affecting the slope of the relationship between seed survival and burial time. Hence, we kept the same intercept for both populations (see Results). We performed deletion tests in order to remove any non-significant explanatory variable from the initial model, and retained the minimal adequate model (Crawley, Reference Crawley2013). Comparison of alternative models was based on analysis of deviance with chi-squared tests.
The proportion of non-persistent pod segments was modelled as a binomial variable [non-persistent (NP) or not (I)]. The same generalized linear mixed model as for the modelling of seed survival was fitted to the monthly data recorded over the whole burial experiment. Population was treated either as an interacting factor or as a separated factor. The minimal adequate model was selected based on model comparison (analysis of deviance with chi-squared tests).
Seed dormancy was measured as the proportion of all living seeds that were dormant [DS/(GS + DS)]. A generalized linear model was fitted to the monthly data with quasi-binomial errors to account for overdispersion. This model included a linear trend over burial time and an interaction term where burial time moderates a cyclic seasonal function (Crawley, Reference Crawley2013). It takes the form of a logit function:
 $$\eqalign{\ln \left[ {\displaystyle{{\% DS} \over {1 - \% DS}}} \right] =& \; intercept + \; \alpha. \; Time \cr & + \; Time.\left[ {\beta. \cos \left( {2.\pi. Z} \right) + \; \gamma. \sin \left( {2.\pi. Z} \right)} \right]}$$
$$\eqalign{\ln \left[ {\displaystyle{{\% DS} \over {1 - \% DS}}} \right] =& \; intercept + \; \alpha. \; Time \cr & + \; Time.\left[ {\beta. \cos \left( {2.\pi. Z} \right) + \; \gamma. \sin \left( {2.\pi. Z} \right)} \right]}$$
where %DS is the proportion of dormant seeds (binomial variable), Time is the burial time, Z is a scaling index accounting for the cyclic behavior (e.g. annual periodicity) and intercept, α, β and γ are model parameters to be estimated. This model makes it possible to account for both stationary (cyclic behaviour with constant amplitude) and non-stationary processes (e.g. cyclic behaviour with damped or amplified oscillations). As above, we performed deletion tests, using analysis of deviance (with F-tests) to compare models and retained the minimal adequate model (Crawley, Reference Crawley2013).
For all analyses we used the ‘lme4'package in R 3.2.1 (R Core Team, 2015), under Rstudio (version 0.99.467).
Results
Pod and seed characteristics
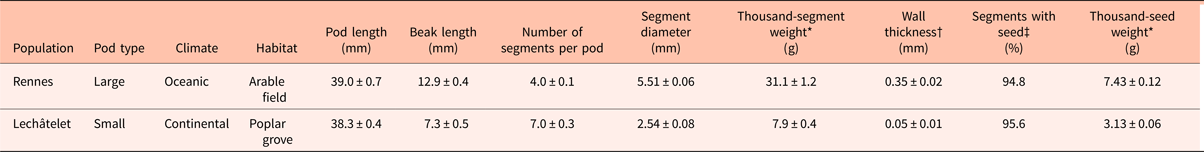
Both populations showed a high rate of seed fill (~95%) during the multiplication year in the cages in Dijon, showing efficient pollination by domestic flies. They displayed similar pod length, but the Rennes population displayed longer beaks and fewer segments than the Lechâtelet population (Table 1). The large pods from the Rennes population were characterized by an average segment diameter twice that of Lechâtelet and fruit wall thickness seven times that of the small pods from Lechâtelet population (Fig. 2), resulting in a much heavier segment weight. Seeds of the Rennes population were more than twice as heavy as seeds from the Lechâtelet population, resulting in larger holes inside the fruit. The cage with small-seeded plants produced 30% more pod segments than the one with large seeds (24,000 versus 18,100).

Figure 2. Cross-section of single cracked segments of the two populations (upper: Rennes, large pod; lower: Lechâtelet, small pod).
Table 1. Pod and seed characteristics of the two populations of Raphanus raphanistrum after multiplication in 2008

Data from 100 pods; values are means ± SEM. *Average value calculated from three to seven lots of 100 segments or seeds; †average value of the thinnest part of 12 segments; ‡unique count for the 100 pods.
Non-persistent pod segments
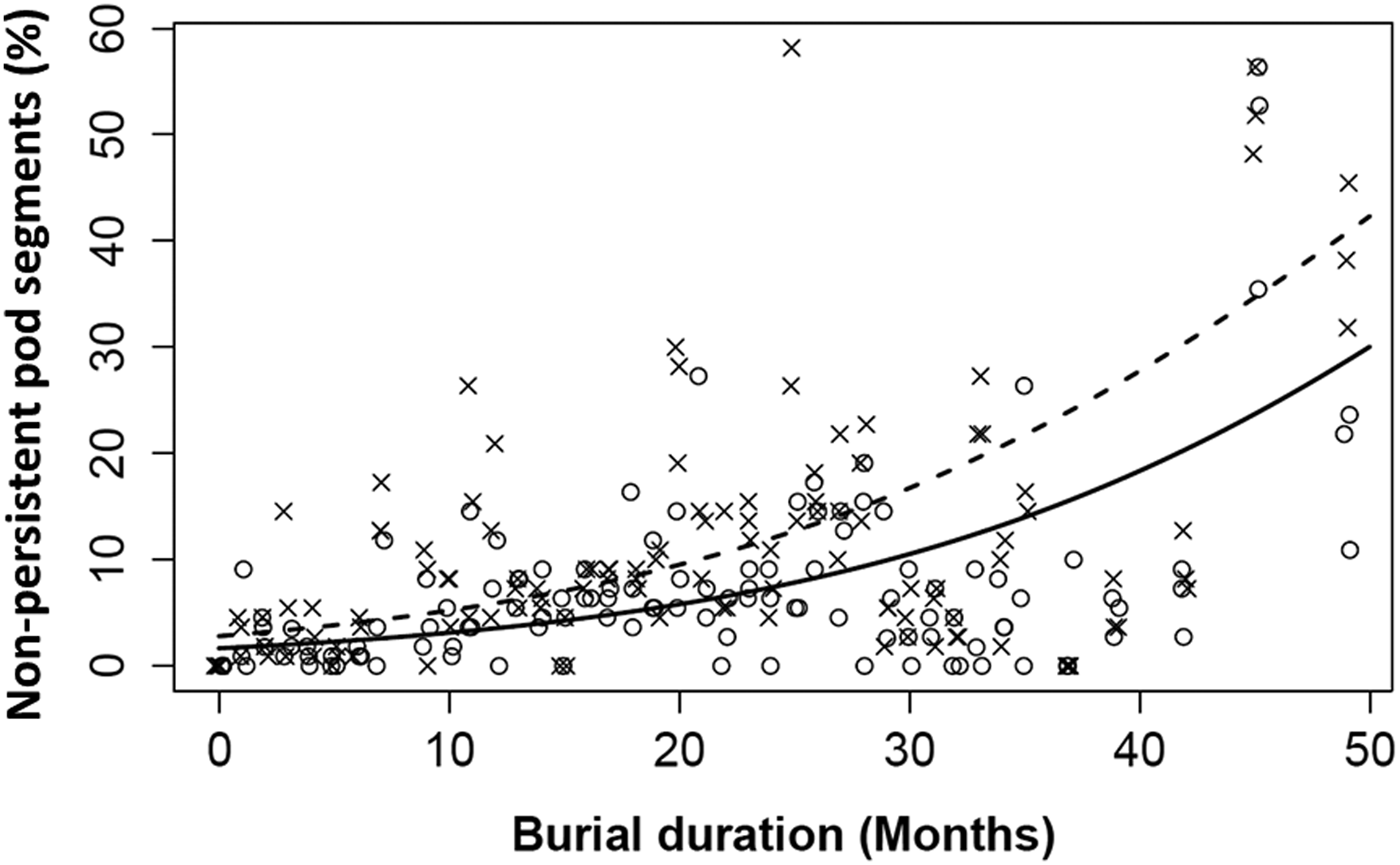
The initial proportion of non-persistent segments (NP) predicted for the Lechâtelet population was slightly higher than for the Rennes population (2.8 vs 1.7%). The proportion of NP increased with burial time for both populations, but at a faster rate for Lechâtelet (Fig. 3). The best model predicted a burial time effect and a distinct effect of populations rather than an interaction between population and burial time. There was great variability among seed samples, indicating a large effect of micro-heterogeneity of soil conditions.

Figure 3. Proportion of non-persistent pod segments (NP) over 4 years of seed burial (2009–2012): data points and model lines for the Rennes (open circles and continuous line; large pod) and Lechâtelet (crosses and dashed line; small pod) populations. Model lines are predictions (back-transformed to percentages) from the same model [see Eqn (1)] including burial time and population effects without interaction (see text). Parameter estimates (SEM) are: intercept = –4.08 (0.13), α = 0.065 (0.011), β = 0.536 (0.046).
Seed survival
The initial percentage of viable seeds (SS) at the testing immediately before burial in December was 91.8 ± 2.0% for the Rennes population and 93.9 ± 1.6% for the Lechâtelet population. Hence, the experiment actually started with an average of 102 viable seeds per bag.
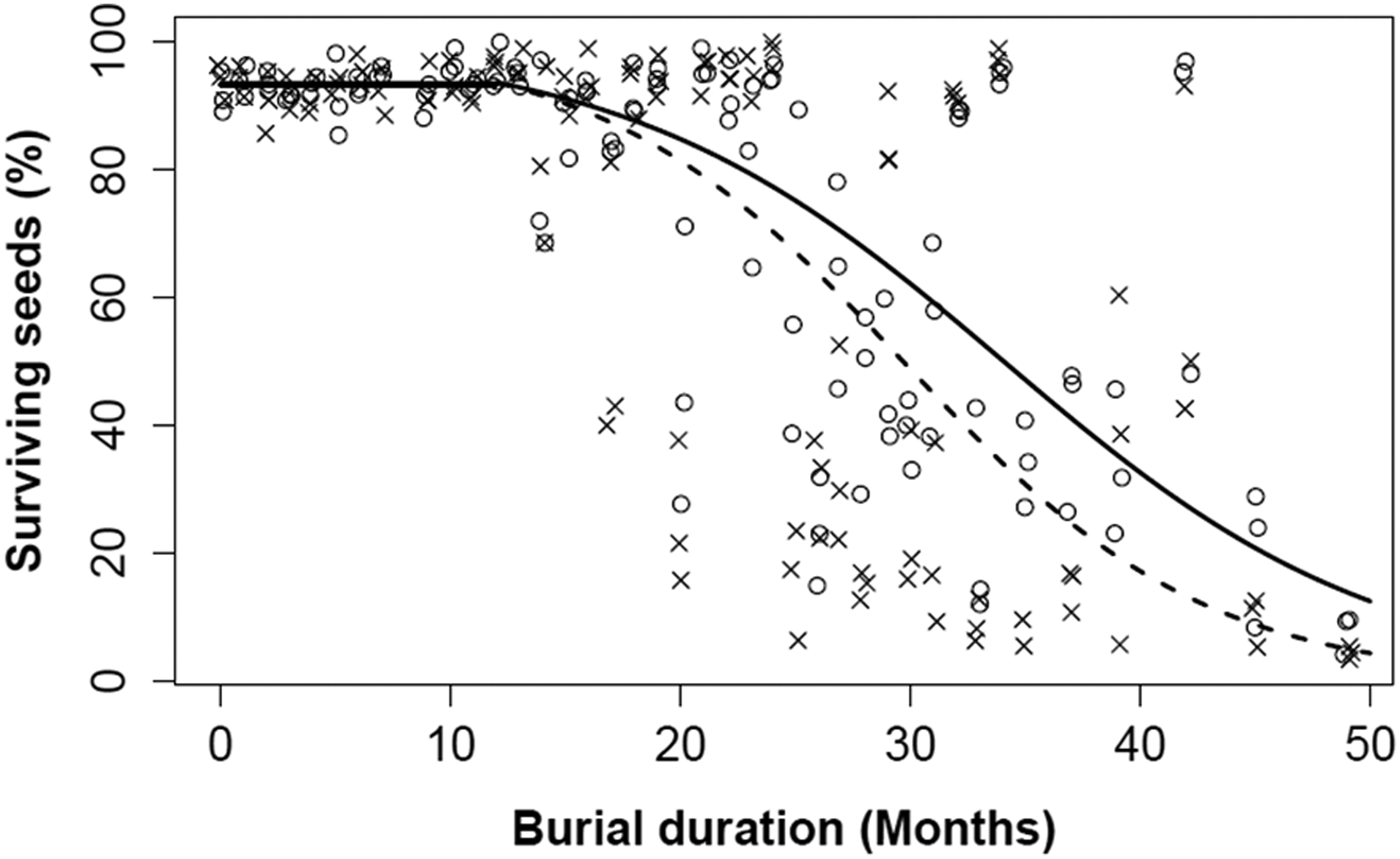
The percentage of surviving seeds roughly remained constant over the first year of burial for both populations (Fig. 3). Hence, separate models were fitted to data in the first year of burial and after the first year. The first year model predicted no significant effects of burial time or population and the estimated SS was 93.1%. After 1 year of burial, mortality increased with burial time for both populations, but to a greater extent for Lechâtelet (Fig. 4). Hence, there was a significant interaction between seed survival and population. However, there was considerable variation between seed samples after 2 years, indicating a large effect of micro-heterogeneity of soil conditions even for a given basket and population: this is supported by the high correlation (r = 0.83) of survival rates between the two populations for samples extracted from the same basket. Only a few seeds were still alive after 4 years of burial. From the two model fits (first year and later), correcting for the initial mortality before burial, seed half-life in the soil can be estimated around 35.3 months for Rennes and 30.7 months for Lechâtelet.

Figure 4. Percentage of surviving seeds (SS) over 4 years of seed burial (2009–2012): data points and model lines for the Rennes (open circles and continuous line; large pod) and Lechâtelet (crosses and dashed line; small pod) populations. Model lines are predictions (back-transformed to percentages) from the same hypothetical model [see Eqn (1)] including a burial time effect and an interaction effect between burial time and population. The model was fitted separately to the data recorded before (horizontal line) and after (sigmoid curves) 12 months of burial. For the first year of burial, the model simplified to the null model with intercept = 2.60 (SEM = 0.06). For the subsequent years of burial, both explanatory variables were significant with parameter estimates (SEM): intercept = 2.70 (0.20), α = –0.122 (0.037), β = –0.030 (0.002).
Seasonal dormancy of seeds
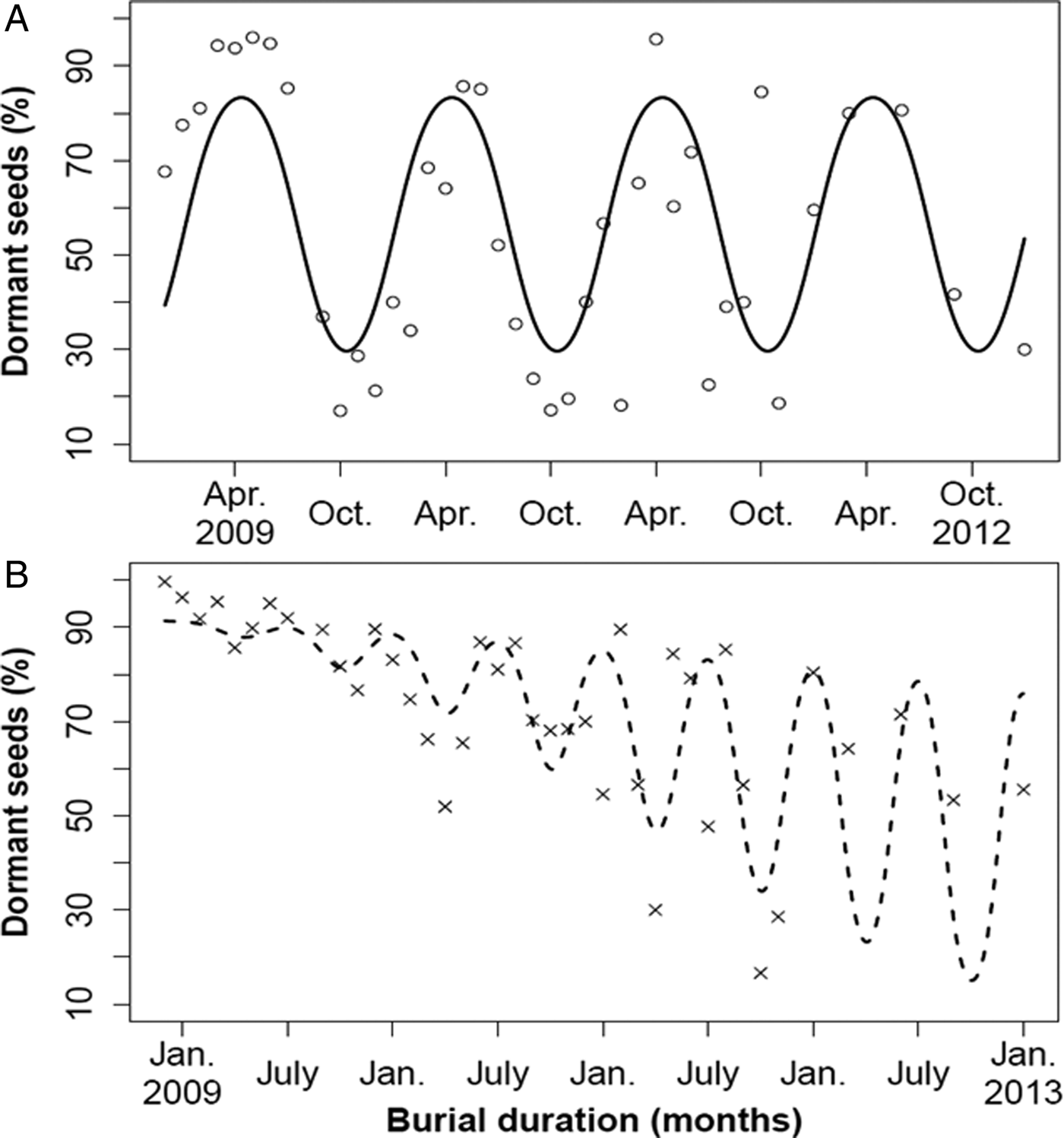
At the immediate testing of the bags before burial, the percentage of seeds that germinated was 32.3 ± 6.2% (mean ± SEM) for Rennes but 0% for Lechâtelet. After GA3 addition, the extra germination rate was 6.7 ± 3.0% for Rennes and 31.4 ± 12.6% for Lechâtelet. Further on, the seeds of the Rennes population exhibited cyclic seasonal dormancy without any significant interaction with burial time, i.e. the process is stationary [model comparisons with (d.f. = 34) and without (d.f. = 37) interaction: F = 2.14, P = 0.113]. The seasonal pattern was annual with maximal and minimal proportions of 83% dormant seeds in April and 30% in October, and an overall annual mean of 58% dormant seeds. However, the fitted model underestimated extreme recorded values (Fig. 5A).
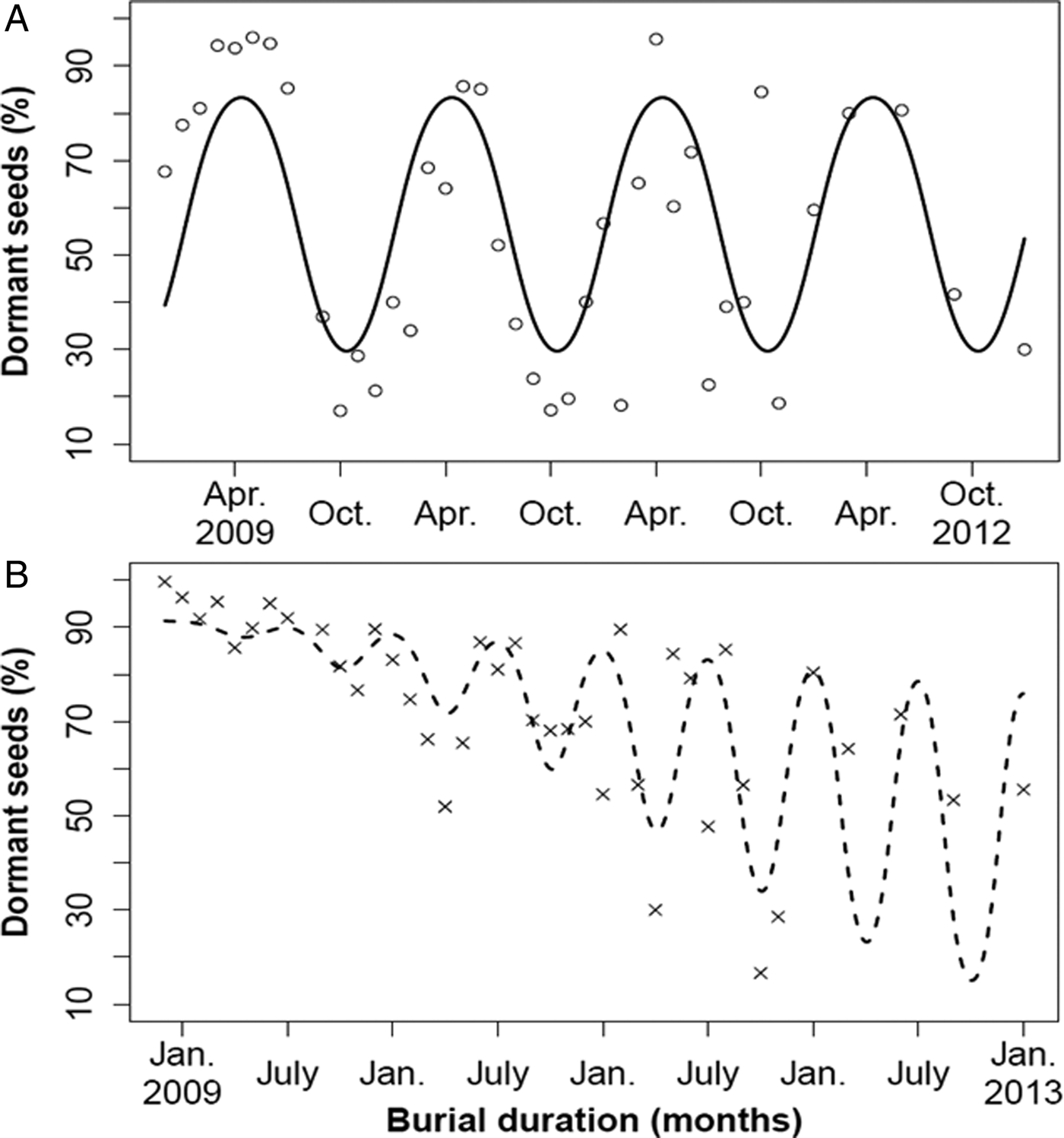
Figure 5. Mean monthly percentage of dormant seed (DS) over 4 years of seed burial (2009–2012, three replicates each dot) for the Rennes (A, large pod) and Lechâtelet (B, small pod) populations. Model lines are predictions (back-transformed to percentages) from the same hypothetical model [see Eqn (2)] including a burial time effect and an interaction effect between burial time and a cyclic seasonal model. For the Rennes population, the model simplified to a 12-month cycle (A, stationary process) with parameter estimates (SEM): intercept = 0.37 (0.15), β = 0.94 (0.22), γ = –0.81 (0.21). For the Lechâtelet population, a decreasing time trend and a 6-month cycle interacting with burial time were retained (B, non-stationary process) with parameter estimates (SEM): intercept = 2.36 (0.22), α = –0.057 (0.010), β = 0.028 (0.008), γ = 0.016 (0.007).
The seeds of the Lechâtelet population exhibited cyclic seasonal dormancy in interaction with a significant decreasing trend over burial time, i.e. the process is non-stationary [model comparisons with (d.f. = 36) and without (d.f. = 37) interaction: F = 38.7, P = 0.3 × 10–6]. The best model predicted 6-month dormancy cycles, with the proportion of dormant seeds lowest in April and October, and peak dormancy in January and July, but with large discrepancies as in January and July 2011 (Fig. 5B). This model accounted for very little of the total variation and was a poor fit for underground temperature cycling. There was no correlation between dormancy values of the Lechâtelet and Rennes populations in the same basket (r = 0.06), indicating that the same temperature and osmotic conditions experienced in any given basket had different effects on the two populations. Climatic data (Fig. 1) could not help interpreting variations around the models.
Discussion
The relatively rapid decline of surviving R. raphanistrum seeds, with a half-life around 2.5–3 years for both populations, confirms earlier findings (Roberts and Boddrell, Reference Roberts and Boddrell1983; Chancellor, Reference Chancellor1986; Code et al., Reference Code, Walsh and Reeves1987). However, many samples retained high viability, even after 2 years, which could allow the species to persist in the soil seed bank for long periods of time if buried deep enough. Roberts and Boddrell (Reference Roberts and Boddrell1983) observed an average of 18% living seeds after 5 years, and Chancellor (Reference Chancellor1986) calculated a 33% yearly decrease in viable seeds, with depletion to 1% within 20 years. Code et al. (Reference Code, Walsh and Reeves1987) found 43% seed viability after 4 years buried at 10 cm depth. Seed persistence in the Rennes population decreased slowly from 90 to 10% of the initial seed stock between the twentieth and fiftieth month of burial. In contrast, the decrease was more rapid for the Lechâtelet population with some bags showing 40–80% intermediate viability (Fig. 3). Such a rapid and dramatic change in seed viability has rarely been described, perhaps because most seed longevity studies used a more spaced sampling protocol with reduced power to detect this type of change. For instance, monthly sampling made it possible to detect the yearly step-by-step decrease in weedy beet seed viability, which otherwise would have been interpreted as a continuous decrease (Sester et al., Reference Sester, Dürr, Darmency and Colbach2006). Among many potential genetic differences between the two populations, a thinner fruit wall may possibly be a direct cause of the higher proportion of wasted seeds observed for the Lechâtelet population compared with that of Rennes, as thinner pods could physically restrict germination less efficiently, as observed in another study (Eslami et al., Reference Eslami, Gill, Bellotti, McDonald, Preston, Watts and Crossman2006), or because of more rapid fruit wall degradation, which could result in more suicide germination of seeds buried deep underground. Mekenian and Willemsen (Reference Mekenian and Willemsen1975) observed that 10% of the seeds were free of the pod after only one overwintering in the field.
A second interesting finding from our study was the complete primary dormancy of the Lechâtelet seeds, which was partially broken by GA3 addition. The Rennes population initially had half non-dormant seeds, but responded slightly to GA3. These results fall in between the range observed in previous studies (Roberts and Boddrell, Reference Roberts and Boddrell1983; Cheam, Reference Cheam1986; Warwick and Francis, Reference Warwick and Francis2005; Malik et al., Reference Malik, Norsworthy, Riley and Bridges2010). Again, pod structure could be involved. Given the thinnest structure of the fruit wall of the Lechâtelet population, it is possible that such a primary dormancy could be an efficient defence mechanism against germination under unfavourable conditions. In turn, the increased protection provided by large pods probably does not drive genetic selection for complete dormancy in the Rennes population. Here, physiological germination traits support morphological features in their ability to schedule germination, and in this way maximize the fitness of offspring and enhance soil seed bank persistence. However, not all the differences observed here are necessarily due to fruit wall thickness or to the seed size inside the pod: genetic differences between the two populations certainly include many other factors that influence germination. A breeding plan to select for plants having thick versus thin fruit wall in the same genetic background, and a study using more populations of different origins with different pod shapes, should be undertaken to study more in depth the impact of the fruit wall on the difference of germination and dormancy observed here.
Finally, we observed a striking difference in the dormancy cycling between the two populations. Although the seeds were obtained under the same conditions, the Rennes population displayed cyclic dormancy from the beginning of the experiment. A thick fruit wall provides mechanical protection, but temperature-induced phenomena override mechanical effects to maintain dormancy or to trigger germination. This corresponds well with the emergence time recorded in most regions (Reeves et al., Reference Reeves, Code and Piggin1981; Roberts and Boddrell, Reference Roberts and Boddrell1983; Amor, Reference Amor1985; Chancellor, Reference Chancellor1986; Cheam, Reference Cheam1986; Panetta et al., Reference Panetta, Gilbey and D'Antuono1988; Warwick and Francis, Reference Warwick and Francis2005). Cyclic germination is a common phenomenon for winter weeds (see references in Baskin and Baskin, Reference Baskin and Baskin2014). It allows two peaks of germination and emergence, as observed for R. raphanistrum. A joint experiment with another winter weed, Cyanus segetum, showed the same cyclic dormancy as the Rennes population (Guillemin et al., Reference Guillemin, Bellanger, Reibel and Darmency2017). In contrast, the Lechâtelet population displayed strong initial dormancy that decreased slowly over time, thus suggesting that the population has a different dormancy mechanism. It is noteworthy that the Lechâtelet population did not respond to the seasonal temperature fluctuation pattern of its own climatic region, in contrast to the C. cyanus from the same region when tested at the same time in the same trench (Guillemin et al., Reference Guillemin, Bellanger, Reibel and Darmency2017). Perhaps deeper and constant dormancy is a greater requirement in the continental region to prevent too many seeds from germinating in the autumn when R. raphanistrum may not survive the continental winter climate. For the Rennes population, the oceanic climate offers staggered opportunities for seedlings to establish through the year, and persist through the mild winter. Geo-climatic conditions have also been found to structure the population diversity of R. raphanistrum in Western Australia (Bhatti et al., Reference Bhatti, Cocks, Bennett and Malik2016). It is also possible that the variability seen mainly in the Rennes population was caused, in part, by evolutionary pressures from the arable environment: seed-to-seed variability in dormancy loss is beneficial in frequently disturbed environments (Donohue et al., Reference Donohue, Rubio de Casas, Burghardt, Kovach and Willis2010; Gremer and Venable, Reference Gremer and Venable2014). The Lechâtelet population had a more stable habitat, without periodic cultivation, thus having less selection pressure linked to the crop rotation and date of sowing.
On the whole, whatever the role of the fruit wall thickness, field selection can take different paths and mechanisms for protecting seed longevity and seed persistence in arable soils. Deciphering such variations in seed behaviour is important to determine the emergence dynamics of wild radish and its potential to propose models as accurate as possible and to adapt to cropping practices such as soil cultivation and herbicide application. Modelling life cycles of weeds should therefore pay attention to the use of appropriate values for the considered populations instead of using fixed parameters from databases.
Acknowledgements
Y.T. thanks the French Ministry of Education and Research (ANR) for granting a fellowship.
Financial support
This work was supported by a grant from the French Ministry of Education and Research, ANR-07-POGM-001-01.
Conflicts of interest
None.