Introduction
Although many species of flowering plants produce fruits and seeds that vary continuously in size, mass and various other traits, individuals of a few hundred species produce two or more kinds of diaspores that exhibit distinct differences in morphology as well as in ecology (Harper, Reference Harper1977; Mandák, Reference Mandák1997; Imbert, Reference Imbert2002). This latter phenomenon is known as fruit/seed heteromorphism, and it is considered to be an adaptation of species to the spatio-temporal variability (unpredictability) of stressful habitats (Venable and Lawlor, Reference Venable and Lawlor1980; Venable, Reference Venable1985). That is, if dispersal units from the same plant differ in how they respond to the environment, there is an increased chance that some of them will survive, germinate and produce new plants.
Baskin and Baskin (Reference Baskin and Baskin2014) recognized two general categories of fruit/seed heteromorphism: heterodiaspory and amphicarpy. In heterodiaspory, two or more morphs are produced above ground, and in amphicarpy one or more morph(s) are produced above ground and one or more below ground. Heterodiaspory was divided further into four subcategories: heterocarpy, two or more types of fruits (sometimes with accessory parts, such as perianth, bracteoles or phyllary); heteroarthrocarpy, one type of fruit with distinct segments (i.e. proximal versus distal), each bearing a distinct seed morph; heterospermy, one or more types of fruits containing seeds that differ within and/or between fruits; and amphi-basicarpy, flowers and fruits produced at or near ground level and on aerial parts of the stem.
The ecology of heteromorphic fruits and seeds has been studied extensively in heterocarpous (e.g. Baker and O'Dowd, Reference Baker and O'Dowd1982; Venable and Levin, Reference Venable and Levin1985a, Reference Venable and Levinb; Venable et al., Reference Venable, Burquez, Corral, Morales and Espinosa1987; Imbert et al., Reference Imbert, Escarré and Lepart1996, Reference Imbert, Escarré and Lepart1999; Brändel, Reference Brändel2007), heteroarthrocarpous (e.g. Payne and Maun, Reference Payne and Maun1981; Maun and Payne, Reference Maun and Payne1989; Zhang and Maun, Reference Zhang and Maun1992; Donohue, Reference Donohue1998; Cordazzo, Reference Cordazzo2006), heterospermous (e.g. Cronin, Reference Cronin1965; Wang et al., Reference Wang, Huang, Baskin and Baskin2008; Lu et al., Reference Lu, Tan, Baskin and Baskin2010; Yao et al., Reference Yao, Lan and Zhang2010) and amphicarpous (e.g. Weiss, Reference Weiss1980; Cheplick and Quinn, Reference Cheplick and Quinn1982; Cheplick, Reference Cheplick1994; Ruiz de Clavijo and Jiménez, Reference Ruiz de Clavijo and Jiménez1998; Sadeh et al., Reference Sadeh, Guterman, Gersani and Ovadia2009) species. However, the only amphi-basicarpic species that we are aware of, for which the ecology of its heterodiaspores has been compared is the chenopod species Ceratocarpus arenarius L. (Amaranthaceae). In this heteromorphic species, the aerial morphs have high dispersal ability and a low degree of dormancy, and the basal morphs have low dispersal ability and a high degree of dormancy (Gao et al., Reference Gao, Wei and Yan2008; Zhou, Reference Zhou2009; Lu et al., Reference Lu, Tan, Baskin and Baskin2013). That is, the aerial morphs exhibit a high-risk strategy for dispersal and dormancy and the basicarp morph a low-risk strategy (Baskin et al., Reference Baskin, Lu, Baskin, Tan and Wang2014).
Several studies have compared the ability of the different morphs of heteromorphic species to form a soil seed bank. Most of these studies have been on dimorphic species, and in the majority of cases one morph forms a transient seed bank and the other a persistent seed bank (Robocker et al., Reference Robocker, Williams, Evans and Torell1969; Philipupillai and Ungar, Reference Philipupillai and Ungar1984; Ungar, Reference Ungar, Tiedemann, McArthur, Durant, Stutz, Stevens and Johnson1984; Venable and Levin, Reference Venable and Levin1985b; Wertis and Ungar, Reference Wertis and Ungar1986; Carter and Ungar, Reference Carter and Ungar2003; Cao et al., Reference Cao, Baskin, Baskin, Yong and Huang2012). In the trimorphic species Atriplex sagittata, one morph forms a transient seed bank and the other two a persistent seed bank (Mandák and Pyšek, Reference Mandák and Pyšek2001). However, a few studies have reported that both morphs formed a transient (Venable et al., Reference Venable, Burquez, Corral, Morales and Espinosa1987) or a persistent (Callihan et al., Reference Callihan, Prather and Northam1993; Joley et al., Reference Joley, Maddox, Schoenig and Mackey2003) soil seed bank.
Heretofore, the relative ability of aerial and basal diaspore morphs of amphi-basicarpic species to form a seed bank has not been reported. The purpose of our study was to compare the seed-bank dynamics of aerial and basicarpic diaspores of the amphi-basicarpic annual C. arenarius. Based on the high-risk strategy of the aerial morphs of this species for dispersal and dormancy (see below) and the low-risk strategy for the basicarps (see below), we hypothesized that the basicarp morph had a greater ability to form a persistent seed bank than the aerial morphs. To test this hypothesis, we compared seed-bank dynamics, germination phenology and retention of seed viability during burial of aerial and basicarpic morphs of C. arenarius.
Materials and methods
Study species, field site and dispersal unit collection
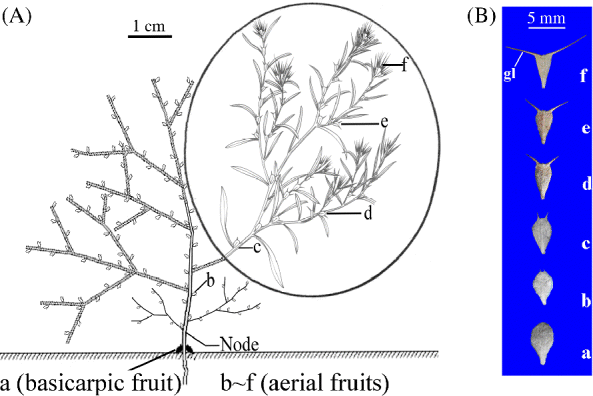
Ceratocarpus arenarius is a summer annual species that occurs in middle and central Asia (including north-west China) (Mao, Reference Mao1994). In China, the species is found only in the cold deserts of northern Xinjiang Province. The fruit, with two permanently attached bracteoles, is the dispersal and germination unit of this species. Plants produce two basicarps (dispersal unit type a) near the soil surface and a gradient of aerial dispersal types (b–f) in different canopy positions (Fig. 1A). The total number of aerial dispersal units was 35–740, depending on the plant size. Thus, C. arenarius is an amphi-basicarpic species (sensu Barker, Reference Barker2005). When mature, some aerial dispersal units are dispersed by wind before the stem breaks near the base, and others become detached as plants tumble across the landscape (Zhou, Reference Zhou2009; Lu et al., Reference Lu, Tan, Baskin and Baskin2013). The two dispersal units at the base of the plant remain attached to the rooted basal part of the dead plant after the stem breaks and are not dispersed. Flowering of C. arenarius begins in late April and ends in September, dispersal/germination units (fruits+bracteoles) mature from late July to early October and seeds germinate in late March to mid-April.

Figure 1 (colour online) (A) Spatial position of basicarpic (a) and aerial (b–f) dispersal units of Ceratocarpus arenarius on an individual plant, and (B) morphology of the six types of dispersal units of C. arenarius. The stem of this tumbleweed breaks at the node that is labelled. Circled material from (www.chinabaike.com). gl, glochid.
The study on seed-bank dynamics was done in three natural populations of C. arenarius, each consisting of several thousand plants growing on sand dunes in the vicinity of Fukang City on the southern edge of the Junggar Basin of Xinjiang Province, China (44°09′N, 87°58′E, 438 m above sea level). The sand does not exhibit soil profile development, and it is low in organic matter. Mean annual temperature is 8.3°C, and the mean temperatures of the coldest (January) and hottest (July) months are − 15.6°C and 26.0°C, respectively. Average annual precipitation (including rain and snow) is 222 mm, about two-thirds of which falls in spring and summer, and the snow that falls in winter begins to melt in March or April (data from Fukang weather station, 2001–2010). Annual potential evaporation is >2000 mm (Wei et al., Reference Wei, He, Liu and Gao2003).
Among other species, the flora/vegetation of sand dunes in the southern part of the Junggar Basin consists of the dwarf tree Haloxylon persicum (Amaranthaceae); the shrub Calligonum leucocladum (Polygonaceae); the dwarf subshrub Artemisia arenaria (Asteraceae); the perennial herbs Allium and Astragalus species; various species of summer annual chenopods (Amaranthaceae), e.g. Agriophyllum squarrosum and C. arenarius; winter annual/spring ephemeral (ephemeretum) species of Asteraceae, Boraginaceae, Brassicaceae, Fabaceae, Geraniaceae, Poaceae and Papaveraceae; and the perennial ephemerals (ephemeroids) Carex psychodes (Cyperaceae), Eremurus anisopteris (Liliaceae) and Tragopogan sabulosus (Asteraceae) (Wang et al., Reference Wang, Jiang, Lei, Zhang and Qian2003).
Freshly matured fruits with khaki-coloured persistent bracteoles were collected on 6 October 2010 in the above-described area, and dispersal unit morphs from individual plants were separated into: (1) those at soil surface; and (2) each of five aerial fruit types in the gradient of dispersal units above the soil surface (Fig. 1B). Dispersal unit types a, c and f were bulked separately and used in the germination phenology study.
Soil seed bank
Soil samples were collected from three populations of C. arenarius before the beginning of germination (1 March 2012), at the end of the germination season but before the beginning of dispersal (15 May 2012) and after many of the aerial dispersal units had been dispersed (15 October 2012). Preliminary observations (by randomly sampling 8-cm-deep soil cores) showed that dispersal units were not buried deeper than 5 cm. Thus, in each population three 20-m transects were laid out, and at 1-m intervals along each transect ten 1 m × 1 m quadrats were established. A 20 cm × 20 cm sample to a depth of 0–5 cm was taken in the centre of each 1 m × 1 m quadrat. Then, each soil core was divided into depth-sections of 0–2 and 2–5 cm (three populations × three 20-m transects × ten 1 m × 1 m quadrats × one 20 × 20 cm sample × two soil depth layers = 180 soil samples). Using a trowel, we removed the top 2 cm of sand and then the next 3 cm. It was difficult to distinguish among dispersal units b–f in the soil samples since glochids on the bracteoles of many of these dispersal units had been lost in whole or in part. Thus, they were combined into ‘aerial dispersal units’. Soil sieves were used to separate all dispersal unit morphs from the sand. Dispersal unit a (i.e. basicarps) could be distinguished from the aerial dispersal units b–f by its differences in morphology (Fig. 1B) and density of trichomes (Lu et al., Reference Lu, Tan, Baskin and Baskin2013).
Germination phenology and retention of seed viability
The purpose of this experiment was to determine when dispersal units a, c and f germinate under field (experimental garden) conditions. Three replicates of 200 dispersal units of each of the three morphs collected on 6 October 2010 were sown to a depth of c. 0.5 cm in plastic pots (18 cm deep and 21 cm in diameter with drainage holes at the bottom) filled with sand from the natural habitat of C. arenarius on 10 October 2010 (i.e. 3 replicates × 3 dispersal unit morphs = 9 pots). The pots were buried in soil with the top even with the soil surface. After the germination trials were completed (i.e. 28 April 2013), the non-germinated dispersal units were tested for viability. Seeds were dissected out of the dispersal units, cut open and their embryos observed. Seeds with white, firm embryos were counted as viable.
The experiment was carried out in the experimental garden on the campus of Xinjiang Agricultural University, Urümqi, China, located at the southern edge of the Junggar Desert. The soil (sand) received water only via natural rainfall and snowmelt. Germination (seedlings) was monitored at 7-d intervals from the time of sowing to April 2013, and seedlings were removed from the pots. Information on temperature, rainfall and snowfall at the study site was obtained from the Meteorological Bureau of Xinjiang Uyghur Autonomous Region.
Data analysis
All data were analysed for normality and homogeneity of variance prior to analysis, to fulfil requirements of one-way, two-way and four-way ANOVAs. Normal and homogeneous data were subjected to further analysis. If data were not normally distributed, or if variances were not homogeneous, they were arcsine (square-root %) transformed before analysis to ensure homogeneity of variance. Non-transformed data appear in all tables and figures. When variances of non-transformed or transformed data were homogeneous, a one-way ANOVA was used to determine differences in percentage of germination, viability and loss of viability of dispersal units among the three morphs. A four-way ANOVA was used to test for significance of main effects (sampling period, population, burial depth and dispersal unit type) and their interactions on number of dispersal units per square metre in the soil seed bank. When the variance of logarithmically transformed data was not homogeneous, differences among dispersal units in these characteristics were determined by the Kruskal–Wallis non-parametric test. Tukey's HSD test was performed for multiple comparisons to determine significant (P< 0.05) differences among the three morphs and treatments. Statistical tests were conducted at P= 0.05 (Sokal & Rohlf, Reference Sokal and Rohlf1995). All data analyses were performed with SPSS 13.0 software (SPSS Inc., Chicago, Illinois, USA).
Results
Soil seed bank
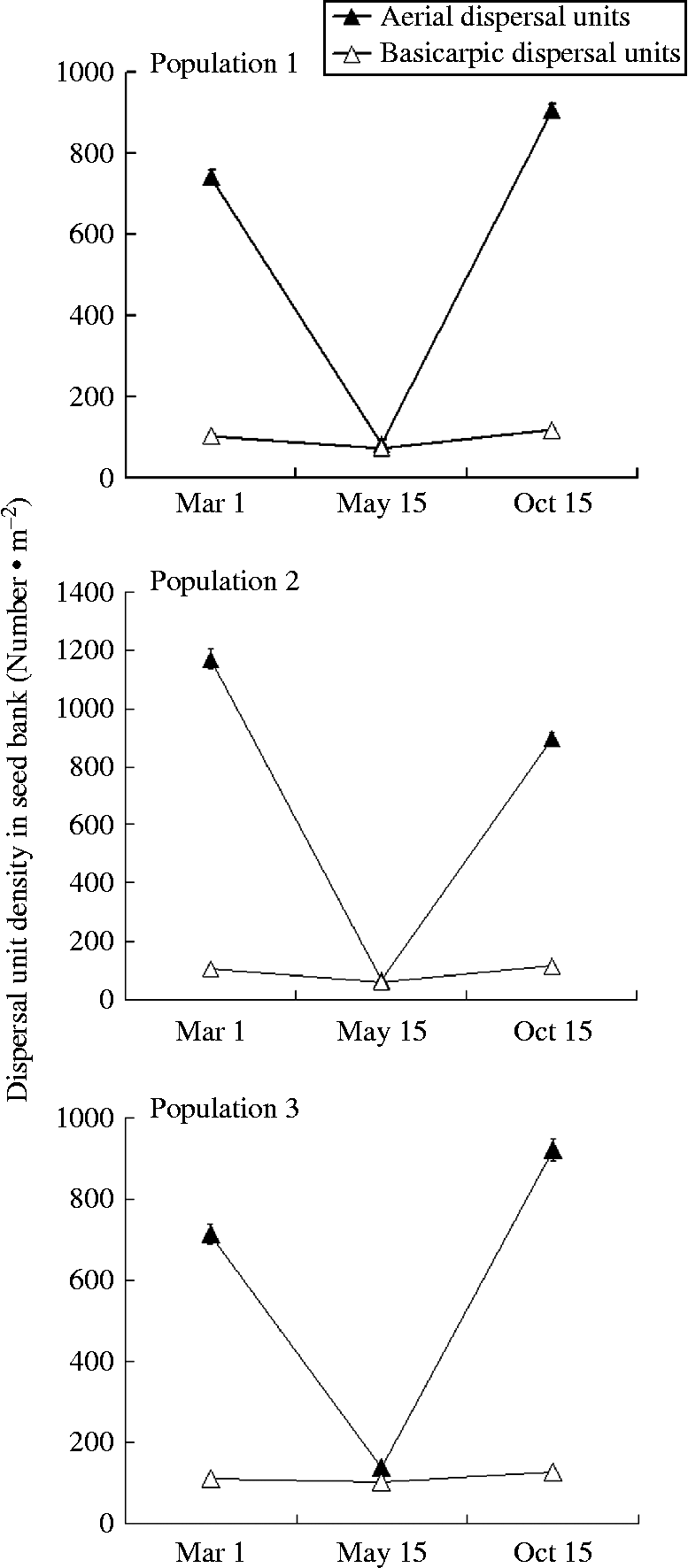
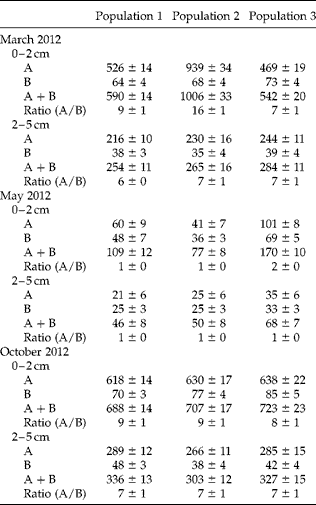
A four-way ANOVA showed that the number of dispersal units was significantly affected by sampling period (P< 0.05), population (P< 0.05), depth of sample (P< 0.05), type of dispersal unit (P< 0.05) and their interactions (P< 0.05) (Table 1). Many fewer dispersal units were found in the three populations in May (after germination but before the beginning of dispersal) than in March (before the beginning of spring germination) and October (after many of the aerial dispersal units had been released from the plants). The ratio of aerial/basicarpic dispersal unit morphs was high in March, decreased to about unity in May and then increased back to a high number in October (Fig. 2, Table 2). Thus, a high percentage of the aerial dispersal units in each of the three populations germinated, or were otherwise lost from the seed bank, between March and May, whereas a high percentage of the basicarpic dispersal units remained in the seed bank throughout summer and autumn.
Table 1 Four-way ANOVA of effects of sampling period (S), population (P), sample depth (D), dispersal unit type (T) and their interactions on number of dispersal units per square metre of C. arenarius in the soil seed bank
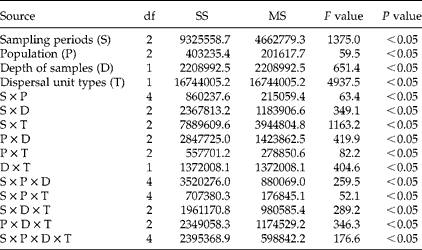
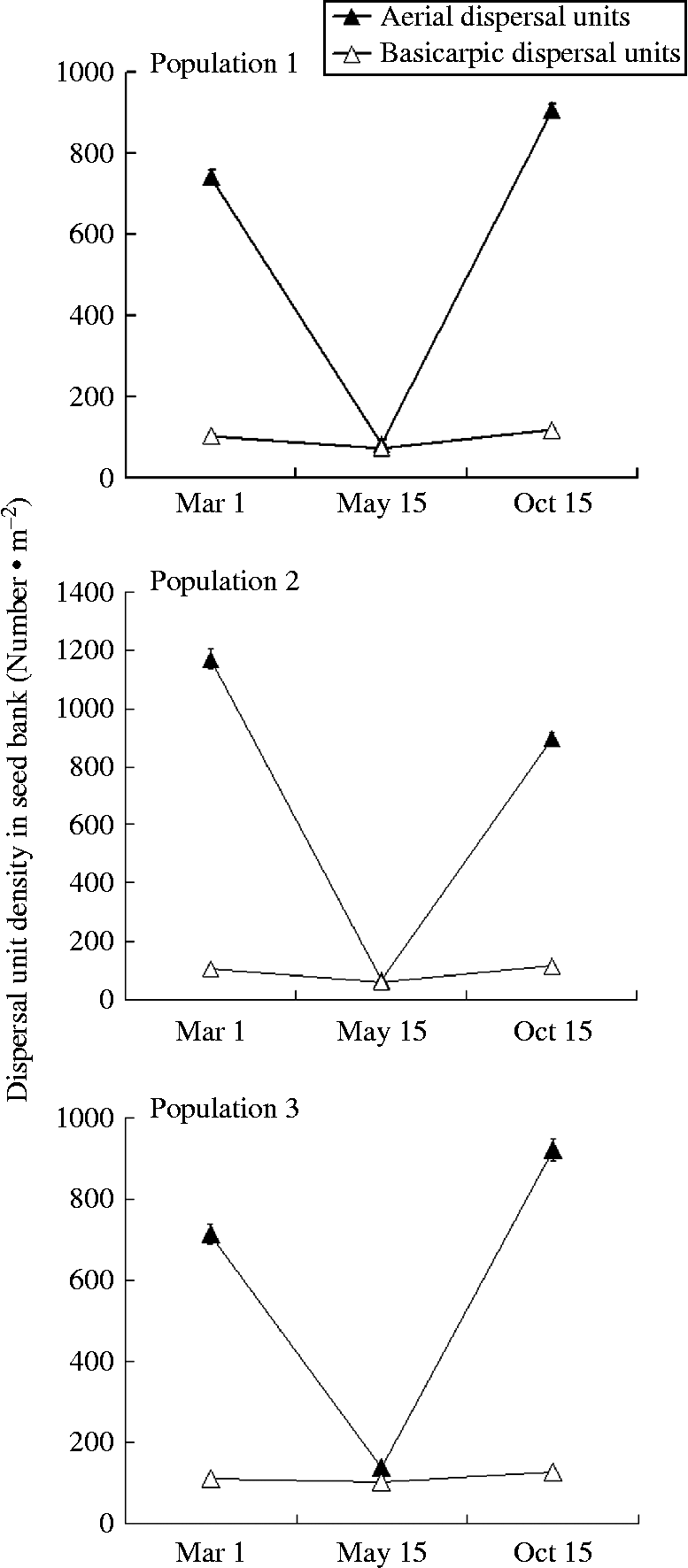
Figure 2 Seed-bank dynamics from before the beginning of the germination season until after dispersal for three populations of C. arenarius. Seeds from 30 plots to a depth of 5 cm were collected from each population on 1 March, 15 May and 15 October of 2012.
Table 2 Number (mean ±1 SE) per square metre of aerial and basicarpic dispersal units of C. arenarius in soil at two depths in March, May and October 2012 in the three study populations. No dispersal units were found below 5 cm depth. A, aerial dispersal units (b–f); B, basicarpic dispersal unit (a). All numbers have been rounded to the nearest whole number

Germination phenology and retention of seed viability
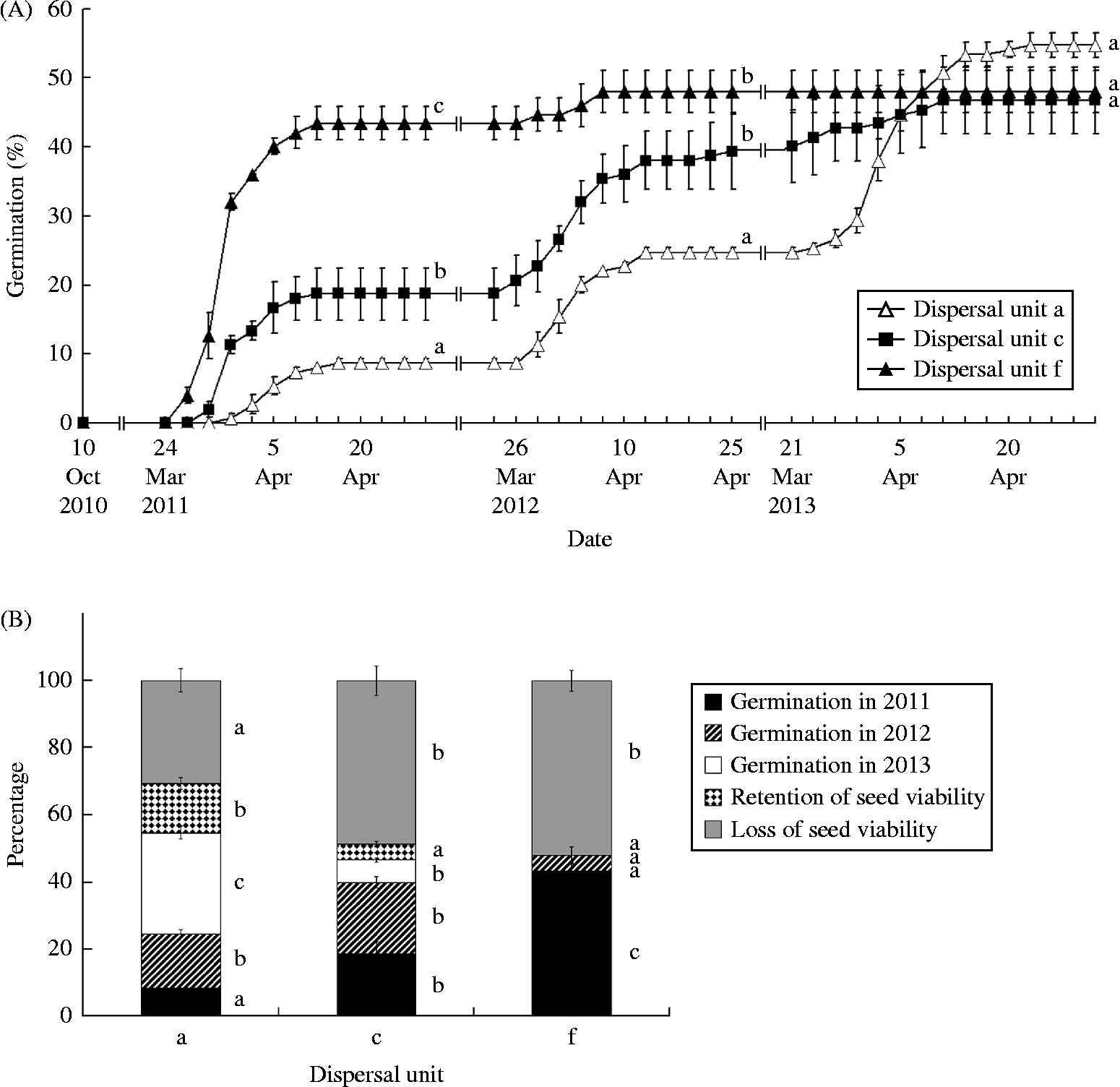
Rainfall during October and November was 62.0, 30.5 and 76.5 mm in 2010, 2011 and 2012, respectively, and mean daily maximum/minimum temperatures 19.9/–15.3, 7.6/0.2 and 14.3/–7.4°C, respectively, but no dispersal units germinated in autumn. In spring 2011, seedlings from dispersal unit f emerged 4 and 7 d earlier, respectively, than those from dispersal units c and a. In 2012, dispersal unit c emerged 2 d earlier than those of dispersal units a and f, and in 2013 dispersal unit a emerged 3 d earlier than those of dispersal unit c; no dispersal unit f morphs emerged in 2013 (Fig. 3A). The greatest number of seedlings emerged between 26 March and 8 April 2011, 29 March–13 April 2012 and 21 March–11 April 2013, when mean daily maximum/minimum air temperatures were 7.2/–5.3, 13.5/–0.45 and 17.9/1.0°C, respectively. In 2011, 2012 and 2013, seedlings emerged from 8.7, 16.0 and 30.0% of dispersal unit a, respectively; 18.7, 21.3 and 6.7 of dispersal unit c, respectively; and 43.3, 4.7 and 0.0 of dispersal unit f, respectively (Fig. 3B). After the first and second spring germination seasons, emergence of dispersal unit a was significantly lower than that of dispersal units c and f, which did not differ significantly from each other (Fig. 3A). Emergence of unit c was significantly lower than that of unit f in the first but not in the second spring. After three spring germination seasons, there was no significant difference in cumulative emergence percentages among the three dispersal unit morphs (P>0.05).
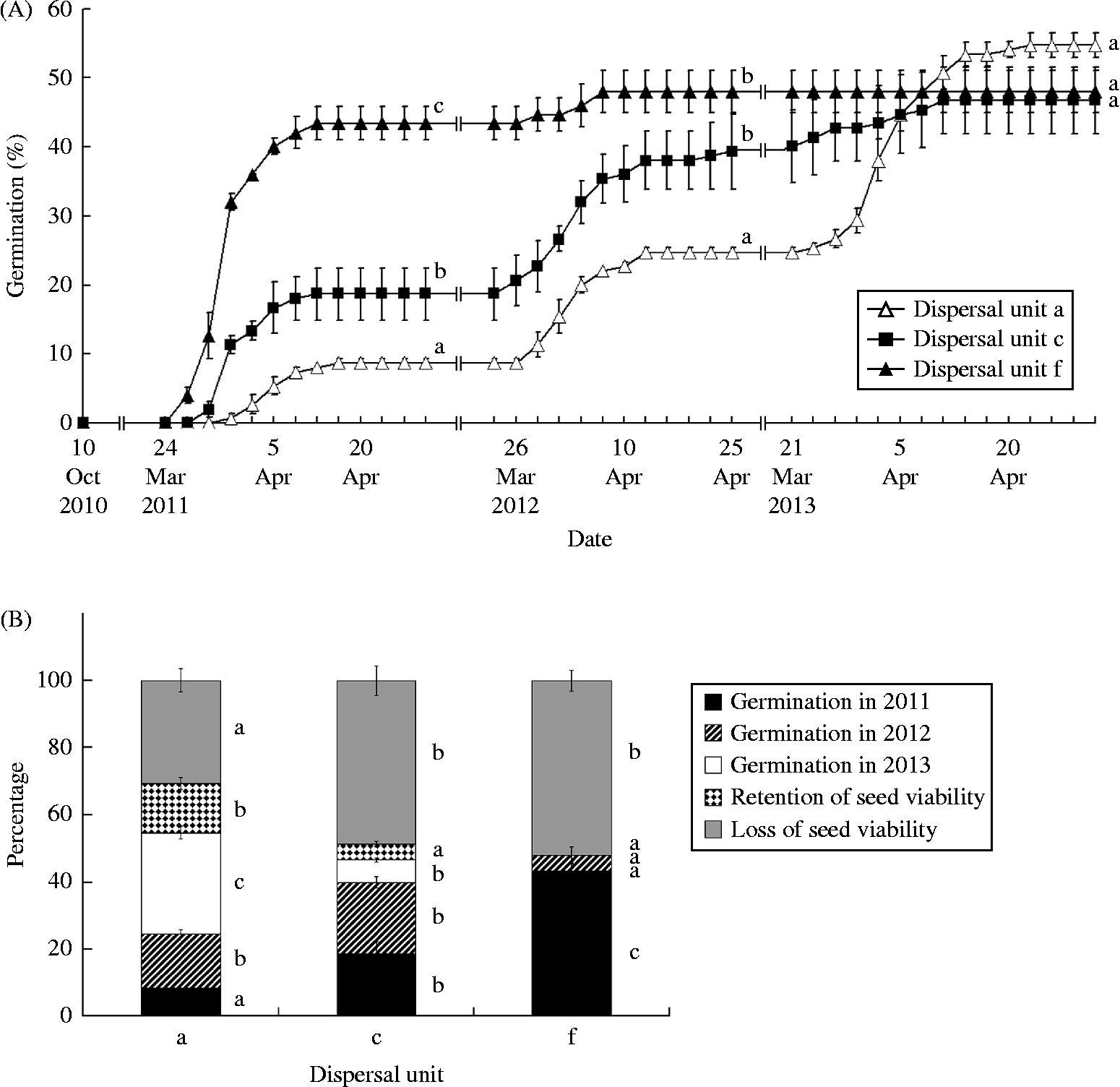
Figure 3 (A) Germination percentages (mean ± 1 SE) in spring 2011, 2012 and 2013 of dispersal units a, c and f of C. arenarius sown in pots in the experimental garden in autumn 2010. (B) Percentages (mean ± 1 SE) of germination, retention of seed viability and loss of seed viability of dispersal units a, c and f after 2.5 years. Significant differences among dispersal units at the end of each spring are indicated by different lower-case letters (Tukey's HSD, P< 0.05). In (A), statistical comparisons are for 28 April 2011, 28 April 2012 and 28 April 2013. No seeds of dispersal unit f germinated in 2013, and none had retained viability. Vertical bars represent standard errors.
Retention of viability of dispersal unit a was higher than that of dispersal unit c, which did not differ significantly from that of dispersal unit f (Fig. 3B). Approximately 31, 49 and 52% of dispersal units a, c and f, respectively, lost viability during 2.5 years of burial.
Discussion
Our results clearly showed that a basicarp morph of C. arenarius is much more likely to become part of a persistent seed bank than an aerial morph. Thus, the seed bank formed by the aerial morphs fits the concept of a Thompson and Grime (Reference Thompson and Grime1979) type III seed bank, in which only a small proportion of the seeds in the seed bank persists from one germination season to the next, and the basal morph fits the concept of a type IV seed bank, in which a large proportion of seeds persists in the seed bank from one germination season to the next. However, whereas in Thompson and Grime's types III and IV models seeds germinate primarily in autumn, soon after dispersal in late summer and autumn, both basal and aerial seeds of C. arenarius germinate only in spring, following maturity and dispersal (only aerial morphs dispersed) in autumn, or following more than 1 year of persistence in the seed bank. Thus, aerial seeds of C. arenarius form a modified type III seed bank and basal seeds a modified type IV seed bank. Further, a significantly higher proportion of the basicarp morphs than of the aerial morphs: (1) remained ungerminated after both 1 and 2 years in the soil in the germination phenology study; and (2) retained viability during 2.5 years of burial in sand. Thus, our hypothesis that the basicarp morph has a greater potential to form a persistent seed bank than the aerial morphs was supported, and an obvious reason for this is that morph a is more dormant and has a higher potential to retain viability during burial in the soil seed bank than morphs b–f (Fig. 3B; Gao et al., Reference Gao, Wei and Yan2008; Lu et al., Reference Lu, Tan, Baskin and Baskin2013).
Although several studies have compared the relative ability of the seed/fruit morphs of heteromorphic species to form a seed bank (see Introduction), this study is the first one to do so for an amphi-basicarpic species. Our results are similar to those in the other studies, in that different morphs of C. arenarius differ in potential to form a persistent soil seed bank. Most previous studies compared the two morphs of aerial dimorphic species, whereas our study compared the distinct basal diaspore with the gradient of aerial diaspores combined (Fig. 1). In addition to showing that the basal diaspore a has a higher capability to form a persistent seed bank than the aerial diaspores, it also shows that this capability varies among the aerial diaspores. Thus, based on number of diaspores that germinated during the 2.5-year germination phenology study, most of those of dispersal unit f did so the first spring after dispersal, whereas only about 40% those of dispersal unit c germinated the first spring and about 60% the second and third springs. Furthermore, retention of viability was higher in morph c than in morph f, but not significantly so.
In an extensive study of dispersal and dormancy in C. arenarius, Lu et al. (Reference Lu, Tan, Baskin and Baskin2013) compared morphology, dispersal ability and degree of dormancy in dispersal units a, c and f. They presented results that supported a conceptual model for a linear-inverse relationship between dispersal ability and degree of dormancy in these three dispersal units. Thus, with regard to dispersal ability and degree of dormancy a < c < f and a>c>f, respectively. The results of the present study show that the relative ability of the diaspores of C. arenarius to form a persistent seed bank is in full agreement with the degree of dormancy in that model, i.e. a>c>f. Thus, our seed-bank study offers strong additional support that dispersal unit a of C. arenarius represents a low-risk strategy and the aerial morphs a variable high-risk strategy, i.e. c>f. Further, the study strongly suggests that diaspore heteromorphism in C. arenarius is a bet-hedging strategy.
Financial support
This work was supported in part by the National Natural Science Foundation of China (31160093, U1130301), the Key Project of Chinese Ministry of Education (No. 213038A) and the Specimen Platform of China, Teaching Specimens Sub-platform (2005DKA21403-JK, http://mnh.scu.edu.cn/).
Conflicts of interest
None.