Introduction
Seed germination is suppressed by multiple layers of molecular repression, which play an important role in seed dormancy (Nonogaki, Reference Nonogaki2008). Hormone levels in seeds are regulated by transcriptional control of hormone metabolism genes. Expression of genes encoding the rate-limiting enzymes for abscisic acid (ABA) and gibberellin (GA) biosynthesis and deactivation determines dormancy levels in seeds (Seo et al., Reference Seo, Nambara, Choi and Yamaguchi2009). In contrast, post-transcriptional and -translational regulation, such as mRNA destabilization by microRNA (Liu et al., Reference Liu, Montgomery, Fahlgren, Kasschau, Nonogaki and Carrington2007) and protein degradation or modification by the ubiquitin-26S proteasome pathway (Piskurewicz et al., Reference Piskurewicz, Jikumaru, Kinoshita, Nambara, Kamiya and Lopez-Molina2008; Lee et al., Reference Lee, Piskurewicz, Turečková, Strnad and Lopez-Molina2010), is important for hormone perception and signalling, which are also important determinants of seed dormancy and germination.
Suppression of embryo emergence from seeds is also controlled at the tissue level. The testa imposes seed dormancy through its chemical properties, such as proanthocyanidins (Debeaujon et al., Reference Debeaujon, Leon-Kloosterziel and Koornneef2000, Reference Debeaujon, Lepiniec, Pourcel, Routaboul, Bradford and Nonogaki2007). In hard seeds, physicochemical properties of the testa, i.e. impermeability of the testa to water, inhibits seed germination (Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013). The endosperm provides mechanical resistance to the embryo through its rigidity or elasticity (Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013). An extremely rigid and thick endosperm allows little expansion of the embryo contained in a seed, while a relatively thin and elastic endosperm allows embryo expansion during imbibition but still limits radicle emergence (Liu et al., Reference Liu, Koizuka, Homrichhausen, Hewitt, Martin and Nonogaki2005a, Reference Liu, Koizuka, Martin and Nonogakib).
In Arabidopsis seeds, distinct phases of seed germination are marked by testa and endosperm rupture. Testa rupture, which is not observed in dormant seeds, provides a visible sign of germination events happening in seeds, while endosperm rupture marks completion of germination (in a strict sense) (Liu et al., 2005 a, b). During these two phases, elongation of the endosperm together with the enclosed embryo is observed, suggesting that embryo growth potential is generated and increased and, more importantly, for the scope of this paper, that the micropylar region of endosperm (ME) is being weakened.
Recent work on tissue-specific gene expression in Arabidopsis seeds (Dekkers et al., Reference Dekkers, Pearce, Van Bolderen-Veldkamp, Marshall, Widera, Gilbert, Drost, Bassel, Muller, King, Wood, Grosse, Quint, Krasnogor, Leubner-Metzger, Holdsworth and Bentsink2013) presented a substantial amount of information about ME-enriched gene expression, which provided new insights into the mechanisms of embryo–endosperm interaction for regulation of ME-specific gene expression, an important research theme in seed biology. Interpretation of the new data and integration of the traditional and new concepts on the mechanisms of ME gene expression will be discussed in this research opinion paper.
ME weakening
It is now widely accepted that ME weakening is a prerequisite for completion of germination (Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013). Actual measurement of puncture forces necessary to penetrate ME provides strong evidence for reduction of mechanical resistance or weakening of ME during germination (Watkins and Cantliffe, Reference Watkins and Cantliffe1983; Groot and Karssen, Reference Groot and Karssen1987; Chen and Bradford, Reference Chen and Bradford2000; Muller et al., Reference Muller, Tintelnot and Leubner-Metzger2006; Lee et al., Reference Lee, Dekkers, Steinbrecher, Walsh, Bacic, Bentsink, Leubner-Metzger and Knox2012). The contention is also supported by the results of activity and localization assays and purification and characterization of cell wall enzymes and other proteins expressed in seeds (Nonogaki and Morohashi, Reference Nonogaki and Morohashi1996; Toorop et al., Reference Toorop, Bewley and Hilhorst1996; Sitrit et al., Reference Sitrit, Hadfield, Bennett, Bradford and Downie1999).
ME-specific genes are regulated by plant hormones. GA induces ME-specific expression of MAN2, an endo-β-mannanase gene (Nonogaki et al., Reference Nonogaki, Gee and Bradford2000), as well as germination in the GA-deficient gib-1 tomato mutant, which does not germinate otherwise (Groot and Karssen, Reference Groot and Karssen1987). Other ME cell wall genes, such as xyloglucan endotransglycosylases (XTHs) and expansins (EXPs) are also dependent on GA (Chen and Bradford, Reference Chen and Bradford2000; Chen et al., Reference Chen, Dahal and Bradford2001). Recent studies have revealed the importance of ethylene biosynthesis and signalling in ME gene induction: ethylene promotes ME weakening and rupture in Lepidium sativum seeds (Linkies et al., Reference Linkies, Muller, Morris, Tureckova, Wenk, Cadman, Corbineau, Strnad, Lynn, Finch-Savage and Leubner-Metzger2009). The intersection between GA and ethylene signalling in ME gene expression is not clear yet (discussed below).
ME gene expression
Dekkers et al. (Reference Dekkers, Pearce, Van Bolderen-Veldkamp, Marshall, Widera, Gilbert, Drost, Bassel, Muller, King, Wood, Grosse, Quint, Krasnogor, Leubner-Metzger, Holdsworth and Bentsink2013) performed a comprehensive and dynamic transcriptomic analysis for the two major seed compartments – the embryo and the endosperm in Arabidopsis thaliana. They also divided the embryo into radicle/hypocotyl (RAD) and cotyledon (COT) regions and the endosperm into the micropylar plus charazal endosperm (MCE) and the rest of the endosperm (PE: peripheral endosperm). Furthermore, they divided the imbibition time course into 11 time points, including stages prior to and following testa rupture (TR) or endosperm rupture (ER). The high-resolution data set from this genome-wide gene expression analysis provided detailed information about temporal and spatial expression of seed germination-associated genes, which highlighted the importance of ME-specific gene expression. The data indicated that TR was marked by a large number of differentially expressed genes, compared to non-ruptured seeds, the majority of which were genes up-regulated in MCE, suggesting ME-specific activation of gene expression. Thirty out of the 104 genes up-regulated (>fivefold) in MCE were related to cell wall function (Dekkers et al., Reference Dekkers, Pearce, Van Bolderen-Veldkamp, Marshall, Widera, Gilbert, Drost, Bassel, Muller, King, Wood, Grosse, Quint, Krasnogor, Leubner-Metzger, Holdsworth and Bentsink2013), which supports the idea of germination induction through ME weakening by cell wall degradation. Comparison of genes expressed 31 h after the start of imbibition showed that 200 genes were differentially expressed (>threefold), with the majority of these (95%) being up-regulated in MCE compared to PE (Dekkers et al., Reference Dekkers, Pearce, Van Bolderen-Veldkamp, Marshall, Widera, Gilbert, Drost, Bassel, Muller, King, Wood, Grosse, Quint, Krasnogor, Leubner-Metzger, Holdsworth and Bentsink2013). These results suggest the significance of ME gene expression for seed germination control.
It is possible that the changes of MCE gene expression profiles before and after TR are caused by environmental factors, such as light and oxygen, because testa rupture exposes ME to these environmental factors. However, their data sets did not support this hypothesis (Dekkers et al., Reference Dekkers, Pearce, Van Bolderen-Veldkamp, Marshall, Widera, Gilbert, Drost, Bassel, Muller, King, Wood, Grosse, Quint, Krasnogor, Leubner-Metzger, Holdsworth and Bentsink2013). Therefore, the large change in gene expression profiles in MCE is not caused by TR. Most likely, TR (and ER) is rather a consequence of gene expression in ME during this specific period of time of germination.
A similar transcriptomic analysis has been performed for four different tissues – EC (endosperm cap, similar to ME), LE (lateral endosperm, similar to PE), R (radicle/hypocotyl, similar to RAD), C (cotyledons, similar to COT) of germinating tomato seeds. In this study also, cell wall-associated genes were enriched in ME (Martinez-Andujar et al., Reference Martinez-Andujar, Pluskota, Bassel, Asahina, Pupel, Nguyen, Takeda-Kamiya, Toubiana, Bai, Gorecki, Fait, Yamaguchi and Nonogaki2012). In this tomato seed transcriptome, ME-enriched expression of TERF1 (Tomato Ethylene Response Factor 1), a transcription factor involved in ethylene signalling, and its direct targets NP24, P23 and PR5-like, which are pathogenesis-related (PR) and wound-induced genes, was detected. It is possible that these genes are expressed as a pre-programmed defence mechanism against possible attacks by microorganisms to ME after its rupture by the radicle tip, which exposes the nutritious tissue to the outer environment. However, another possibility is that TERF1 and the downstream targets are induced as a wounding response, in which ethylene signalling is typically involved (Johnson and Ecker, Reference Johnson and Ecker1998). The increase in embryo growth potential probably raises pressure inside a seed, with the radicle tip pressing down on to ME, which mimics a wounding response. The latter contention invokes an idea that ME-specific gene expression may be a consequence of ‘mechanosensing’, in which ME senses the mechanical forces generated by the embryo (Martinez-Andujar et al., Reference Martinez-Andujar, Pluskota, Bassel, Asahina, Pupel, Nguyen, Takeda-Kamiya, Toubiana, Bai, Gorecki, Fait, Yamaguchi and Nonogaki2012) (Fig. 1).

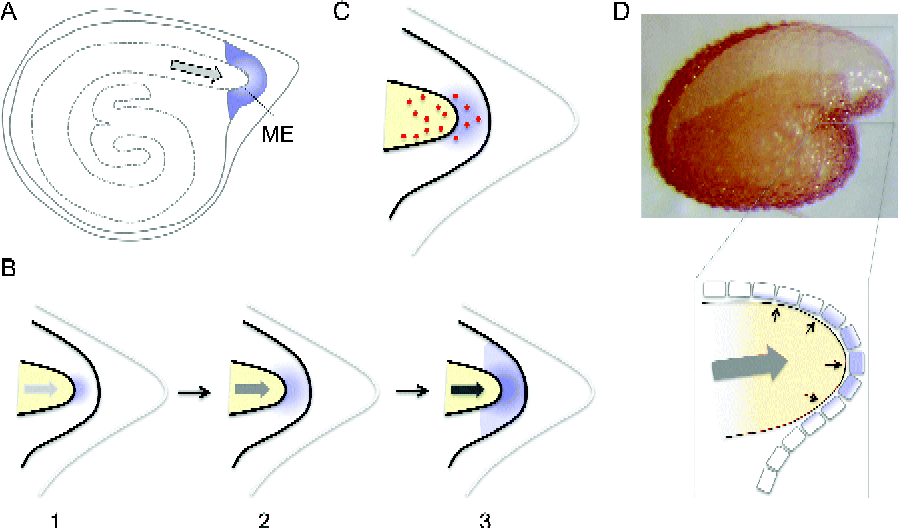
Figure 1 (colour online) Schematic representation of possible mechanisms of embryo–endosperm interaction for gene induction in the micropylar endosperm. (A) Schematic representation of tomato seed with the micropylar endosperm (ME) highlighted by shading and embryo growth potential by a grey arrow. (B) Three progressive stages (1–3) of tomato ME illustrating the mechano- or touch-sensing hypothesis. In this hypothesis, embryo growth potential (grey arrow) increases during imbibition (indicated by increased intensities), which causes the radicle tip to press (‘touch’) on to ME. This pressure is perceived by ME as a wounding response, which triggers ethylene signalling and ME gene induction (diffuse shading). (C) An alternative hypothesis for ME gene induction, in which gibberellins or insoluble secondary messengers (dots) are transferred from the embryo to ME. See text for details. (D) Photograph of Arabidopsis seeds with testa rupture and endosperm elongation (top). The scheme (bottom) shows that mechano- or touch-sensing (shading) described for tomato seeds may also be occurring in the single-cell layer ME in Arabidopsis seeds. Small black arrows indicate pressure.
Interestingly, the Arabidopsis seed transcriptome mentioned above demonstrated a more than eightfold increase of expression of TOUCH3 and TOUCH4 in MCE after TR (Dekkers et al., Reference Dekkers, Pearce, Van Bolderen-Veldkamp, Marshall, Widera, Gilbert, Drost, Bassel, Muller, King, Wood, Grosse, Quint, Krasnogor, Leubner-Metzger, Holdsworth and Bentsink2013), which are known to be expressed rapidly in response to touch or thigmotropism (Braam, Reference Braam2005). This result provides evidence to support the idea of mechano- or touch-sensing as a possible mechanism for ME-specific gene induction. Their finding was further strengthened by the comparison of the set of MCE TR up-regulated genes in Arabidopsis seeds with genes up-regulated upon touch in the aerial part of Arabidopsis plants (Lee et al., Reference Lee, Polisensky and Braam2005), which showed a 30% overlap between the two sets of gene expression data. The overlap was even more striking when GO (gene ontology) was considered for analysis; touch-induced signalling resulted in relatively higher abundance of genes associated with cell wall, calcium-binding, disease resistance, kinase and transcription factor (Lee et al., Reference Lee, Polisensky and Braam2005), which matched well with the GO classes of the MCE TR genes (Dekkers et al., Reference Dekkers, Pearce, Van Bolderen-Veldkamp, Marshall, Widera, Gilbert, Drost, Bassel, Muller, King, Wood, Grosse, Quint, Krasnogor, Leubner-Metzger, Holdsworth and Bentsink2013). Thus, both the Arabidopsis and tomato seed research suggest mechano- or touch-sensing as a possible mechanism of ME gene induction.
Reconsideration of embryo–endosperm interaction in ME gene induction
The traditional hypothesis about the mechanisms of ME gene expression is that genes expressed in ME are induced by GA, which is secreted from the embryo (Groot and Karssen, Reference Groot and Karssen1987). This hypothesis is supported by the fact that ME-specific gene expression is induced in gib-1 tomato seeds by exogenous GA (Chen and Bradford, Reference Chen and Bradford2000; Nonogaki et al., Reference Nonogaki, Gee and Bradford2000; Chen et al., Reference Chen, Dahal and Bradford2001). MAN expression is induced in isolated tomato ME by co-incubating it with the embryonic axes, which can be replaced by exogenous GA (Martinez-Andujar et al., Reference Martinez-Andujar, Pluskota, Bassel, Asahina, Pupel, Nguyen, Takeda-Kamiya, Toubiana, Bai, Gorecki, Fait, Yamaguchi and Nonogaki2012). This result suggests that the embryo has the capacity to secrete GA, which also supports the traditional view. However, it is not explained why such a diffusible signal like GA does not stimulate gene expression in the rest of the endosperm (LE or PE). Isolated (embryo-less) tomato LE responds to GA and induces MAN in this tissue (Martinez-Andujar et al., Reference Martinez-Andujar, Pluskota, Bassel, Asahina, Pupel, Nguyen, Takeda-Kamiya, Toubiana, Bai, Gorecki, Fait, Yamaguchi and Nonogaki2012). Therefore, it is unlikely that GA receptors localize exclusively in ME. Non-diffusible secondary messengers, such as peptide ligands, could be produced by the embryo and transferred to ME (Fig. 1). However, such factors have not been identified and, if any, the modes of transport and perception of these signals between the radicle tip and ME need to be explained.
In the traditional hypothesis, GA was assumed to affect both the embryo (growth potential increase) and endosperm (ME gene expression) in parallel (Groot and Karssen, Reference Groot and Karssen1987). However, it is possible that GA is primarily responsible for changes in the embryo and affects ME gene expression indirectly or secondarily. The latter contention is well integrated with an alternative new hypothesis of mechano- or touch-sensing into a comprehensive scheme, in which the embryonic (growth potential increase) and endospermic (ME gene expression) events are placed sequentially (GA biosynthesis/signalling in the embryo → embryo growth → touch ME → ME gene expression → ME weakening → germination). The traditional view of GA dependency of ME gene expression and the new hypothesis of mechano- or touch-sensing are not mutually exclusive, because GA is still necessary for the embryo to ‘touch’ ME and cause ME-specific gene expression in the new hypothesis also. The new hypothesis also explains why ethylene signalling, which is typical of wounding, touch or thigmotropism, is important for ME gene expression. Probably, it is a logical next step to test this emerging hypothesis for comprehensive understanding of the biological significance of ME events during seed germination, while other possibilities, including direct stimulation of ME gene expression by GA or secondary messengers from the embryo should not be excluded.
Perspectives
This opinion paper focused only on the ‘touch ME’ mechanism. However, there were more discoveries from the high-throughput transcriptomes mentioned in this paper. For example, the Arabidopsis transcriptome (Dekkers et al., Reference Dekkers, Pearce, Van Bolderen-Veldkamp, Marshall, Widera, Gilbert, Drost, Bassel, Muller, King, Wood, Grosse, Quint, Krasnogor, Leubner-Metzger, Holdsworth and Bentsink2013) identified INFLORESCENCE DEFICIENT IN ABSCISSION (IDA)-LIKE1 (IDL1) as one of the highest differentially expressed genes in MCE (>20-fold). This gene encodes a peptide ligand, which interacts with receptor kinases (RLKs). This peptide–RLK interaction is important for cell separation during lateral root emergence (Kumpf et al., Reference Kumpf, Shi, Larrieu, Sto, Butenko, Peret, Riiser, Bennett and Aalen2013), which requires penetration of cell layers covering the lateral root primordia and somewhat mimics endosperm rupture by the radicle in seeds (Belin and Lopez-Molina, Reference Belin and Lopez-Molina2010). Cell separation may be an important final determinant of ME rupture (Linkies et al., Reference Linkies, Muller, Morris, Tureckova, Wenk, Cadman, Corbineau, Strnad, Lynn, Finch-Savage and Leubner-Metzger2009). The discovery of IDL induction in ME after TR has provided a good target of research to elucidate the mechanisms of cell separation in ME, an uncharacterized, but possibly the most important, last step of seed germination. Furthermore, IDL1 identification in ME also opened a whole new area of research – peptide–RLK signalling in ME, which could also mediate communication between ME and the radicle tip. IDA and IDL share sequence and functional similarities with CLAVATA3 (CLV3), one of the best-characterized signalling peptides in plants (Kondo et al., Reference Kondo, Sawa, Kinoshita, Mizuno, Kakimoto, Fukuda and Sakagami2006), a modified form of which (MCLV3) suppresses plant stem cell fate ((Ito et al., Reference Ito, Nakanomyo, Motose, Iwamoto, Sawa, Dohmae and Fukuda2006). CLV3 was originally identified as EMBRYO SURRONDING REGION (ESR), which is specifically expressed in ME of developing maize seeds (Opsahl-Ferstad et al., Reference Opsahl-Ferstad, Deunff, Dumas and Rogowsky1997). It is conceivable that peptide–RLK signalling is involved in the biology of ME. Thus, recent data have opened possibilities of new research development in ME and seed germination biology.
Acknowledgements
I am grateful to Henk Hilhorst, Editor-in-Chief, for providing an opportunity for me to submit this opinion paper to Seed Science Research.
Financial support
This work was supported by US–Israel Binational Science Foundation grant number 2009173 to H.N.
Conflicts of interest
None.